From oxygenated monomers to well-defined low-carbon polymers
2024-04-06YnniXiChengjinZhngYongWngShunjieLiuXinghongZhng
Ynni Xi ,Chengjin Zhng,∗ ,Yong Wng ,Shunjie Liu ,Xinghong Zhng,d,∗
a National Key Laboratory of Biobased Transportation Fuel Technology,International Research Center for X Polymers,Department of Polymer Science and Engineering,Zhejiang University,Hangzhou 310027,China
b School of Chemistry and Chemical Engineering,Huazhong University of Science and Technology,Wuhan 430074,China
c Key Laboratory of Polymer Ecomaterial,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
d Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering,Hangzhou 310027,China
Keywords: Polymer synthesis Degradable polymer Low-carbon polymer Catalysis Ring-opening polymerization
ABSTRACT The synthesis of degradable polymers with easy-to-break in-chain carbon-oxygen bonds has attracted much attention.This minireview introduces the synthesis of a variety of degradable polymers from the(co)polymerizations of several typical oxygenated monomers such as epoxides,cyclic carbonates,cyclic esters,carbon dioxide (CO2),carbonyl sulfide (COS),and cyclic anhydrides.We highlight the catalysts and mechanisms for these (co)polymerizations.The ring-opening copolymerization of five-membered carbonate with cyclic anhydride or COS has been introduced.We also highlight the synthesis of block copolymers and cyclic copolymers with well-defined sequences by the method of growing center switching.We hope that these new polymerization systems can provide new ideas for the development of degradable low-carbon polymers in the future.
1.Introduction
Plastics,with an annual global production of approximately 368 million tons,have demonstrated desirable properties and affected all aspects of our social society [1].However,more than 80%of plastic products end up in the natural environment as waste that is difficult to degrade naturally,causing serious impacts on the ocean,ecological environment,and human health [1,2].As an important class of environmentally friendly and degradable polymer materials,polymers containing easy-to-break carbon-oxygen bonds in the main chain,such as polyesters,poly(thio)esters,poly(thio)carbonates [3],have received extensive attention from academia and industry in recent years [4–9].
The composition and structure of a polymer have a great impact on its properties.Cellulose is the main component of plants,in which the ratio of oxygen to carbon is 5/6 and the content of oxygen and hydrogen in cellulose accounts for 55.5% of the total weight.The synthesis of polymers with a ratio of oxygen,carbon,and hydrogen close to that of cellulose,that is,oxygenrich polymers (or "low-carbon" polymers),should be an interesting synthetic route worth trying.However,it remains a longterm challenge to synthesize low-carbon polymers with comparable properties to traditional carbon chain polymers.Selecting a suitable catalytic system is an effective method for the synthesis of high-molecular-weight low-carbon polymers with different structures.Wanget al.developed a “two-in-one” porphyrin photothermal catalyst with both photothermal effect and catalytic ability,and realized the copolymerization of carbon dioxide (CO2) and epoxides [10].Li and co-workers used organophosphazenes combined with triethylborane (TEB) as binary organocatalysts for the copolymerization of CO2and cyclohexene oxide(CHO) under ambient conditions,resulting in high-molecularweight polycarbonates [11].Zhao and co-workers reported the synthesis of polyester–polyether block copolymers by one-pot copolymerization of phthalic anhydride (PA) and epoxides [12].However,there is still a big gap between the performance of the above-mentioned polymers and traditional polyolefin.And it might be a good way to introduce other chain segments by copolymerization.Meng and co-workers synthesized diblock and triblock copolymers with a high ultimate tensile strength (σB) of 54.8 MPa by one-pot selective copolymerization of CHO/propylene oxide (PO)/PA/CO2[13].Tanget al.toughened a biodegradable isotactic poly(3-hydroxyburtyrate)-based copolyester by selective copolymerization of octa-membered dimethyldimethylglycol ester withε-caprolactone andγ-butyrolactone [14].It is also a good idea to develop new monomers.Liet al.reported the living ring-opening polymerization (ROP) of 1,4-oxathiepan-7-one catalyzed by diphenyl phosphate to obtain poly(ε-caprolactone) (PCL)analogues containing thioether groups in the main chain [15].Zhuet al.developed a class of 1,4-dithian-2-one (DTO) with thioether and thioester functionalities to prepare chemically recyclable polymers by ROP,which exhibited polyolefin-like mechanical properties [16].In addition to the chain polymerization approach,Zhanget al.synthesized chemically recyclable polyethylene-like sulfurcontaining plastics from sustainable feedstocks by stepwise polymerization [17].
This paper reviews the recent works by three young scientists just entering their careers.Dr.Chengjian Zhang had paid attention to the development of sustainable polymers in recent years,developed the organocatalytic synthesis of high-molecular-weight polyester and polythiocarbonate,and introduced sulfur atoms into the polymer main chain with carbonyl sulfide (COS) as a monomer.Dr.Yong Wang focused on the precise synthesis of biodegradable polymers,used living polymerization methods to accurately synthesize biodegradable polymers such as carbon dioxide-based polycarbonate,and developed a "one-pot" efficient synthesis method for various topological polymers,especially cyclic polymers.And Dr.Shunjie Liu focused on the controllable ring-opening copolymerization of five-membered cyclic carbonates with low ring tension,and developed a new polymerization method to realize its high-value utilization.These works present a bright future of polymer design and new polymerization methods,as well as the authors’concerns on the topic.This review paper summarizes the synthesis of polyesters,poly(thio)esters,and poly(thio)carbonates developed by the authors’groups in very recent years (Fig.1).
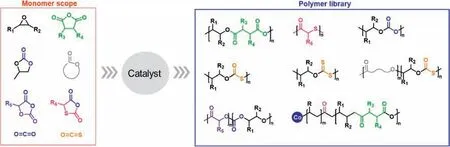
Fig.1.Selected monomers and low-carbon polymers in this minireview.
2.Polyester synthesis by ring-opening copolymerization
Polyesters are considered as a potentially sustainable alternative to petroleum-based plastics.The copolymerization of epoxides and cyclic anhydrides is one of the most attractive techniques for the synthesis of polyesters,owing to the atom-economical approach and a wide range of raw material sources including biomass [18–22].In Zhang and co-workers’recently published work,the synthesis of polyesters by the ring-opening copolymerization (ROCOP) of PO and various cyclic anhydrides,affording polyesters withMns up to 35.8 kDa and dispersity (Đ=Mw/Mn) of 1.06.The triethylamine(TEA)/TEB pair exhibited relatively high activity in the copolymerization of PO and maleic anhydride (MA);while 7-methyl-1,5,7-triazabicyclo[4.4.0]dec–5-ene (MTBD)/TEB exhibited the relatively high activity in the copolymerization of PO and PA.The content of head-to-tail (H-T) linkages of the resultant polyesters was up to 98% [21,23].Meanwhile,the copolymerization of isobutylene oxide (IBO) and various anhydrides into semi-crystalline polyesters was disclosed for providing copolymers with>99% alternating degree,>90% head-to-tail linkages,andMnup to 42.3 kDa (Fig.2).These semi-crystalline polyesters have melting temperatures (Tms)of 67°C to 141°C due to their high regioregularity.The heterogeneous catalyst,a zinc-cobalt(III) double metal cyanide complex(Zn-Co DMCC),was used to suppress the isomerization of IBO,and had productivity up to 680 g polyester/g catalyst.By the addition of water as the chain transfer agent,telechelic polyesters(3.9–7.1 kDa) with two hydroxyl ends were obtained.Interestingly,the degree ofcis-transisomerization of the C–C double bonds in the poly(IBO-alt-MA) backbone can be regulated by diethylamine in solution,resulting in the transition from one semi-crystalline state (Tm=72°C) to amorphous state and then to another semicrystalline state (Tm=153°C) [24].
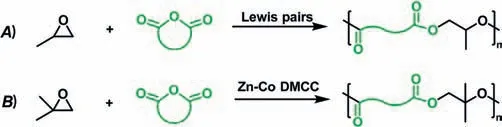
Fig.2.Controlled alternating copolymerization of epoxides and cyclic anhydrides.(A) High-Mn polyesters obtained from copolymerization of PO and cyclic anhydrides catalyzed by organic Lewis pairs;(B) semi-crystalline polyesters with high melting temperatures from copolymerization of IBO and cyclic anhydrides catalyzed by Zn-Co DMCC.
Cyclic carbonate is an ideal cyclic monomer for the synthesis of polymers due to its low toxicity,high solubility in a variety of solvents,high boiling point,etc.[25].With the application of CO2and the mass production of cyclic carbonate,there has been increasingly more attention to the use of cyclic carbonate to make polymers for economic and ecological considerations.However,the most common cyclic carbonates,five-member cyclic carbonates,such as ethylene carbonate (EC) and propylene carbonate (PC),are hardly homopolymerized on account of thermodynamic stability.The ROP of cyclic monomers is based primarily on both thermodynamic and kinetic factors.The Gibbs free energy (ΔGp) should be negative in thermodynamics,whereas the enthalpy changes (ΔHp)of polymerization of a five-membered cyclic carbonate are positive.Along with the decarboxylation during polymerization,the entropy changes (ΔSp) could be positive resulting inΔGp<0,thus making high-temperature polymerization thermodynamically feasible.It has been reported that the polymerization does not take place for EC at below 110°C and no polymer was observed for PC at 140°C within several days [26,27].The pioneering work of Ikeda and co-workers indicated that 170°C and 180°C are the most suitable polymerization temperatures for EC and PC,respectively [27].Heitzet al.employed a variety of catalysts for the ROP of EC,ranging from dibutyl(ethylenedioxy)tin to butyllithium,finding that the carbonate unit content is not more than 50% under any conditions and the retention of CO2decreased as the relative basicity of the catalyst increased [28].Using KOH as an initiator,Leeet al.studied the ROP of EC,assuming a ring-opening mechanism in which alkyl and carbonyl attacks occur in parallel [29].Zsugaet al.analyzed the position of carbonate groups in oligomers by MALDI-TOF and “post-source” decay technique PSD techniques,and once again proved that alkoxide anion attacks both the carbonyl carbon and the alkylene carbon [30].Although many efforts on homopolymerization of five-membered carbonates have analyzed the structure of the polymer,the hydrolysates and the key intermediates of the polymerization,the small molecular by-products generated by ROP have been ignored,which is of great significance for understanding the potential mechanism of five-membered ROP [28,31–33].
In Liu and co-workers’recently published work (Fig.3),they have meticulously analyzed the composition and structure of oligomers and the small molecule by-products produced by ROP of PC,demonstrating for the first time that PO generatedin situby PC decarboxylation is a key intermediate for polymerization [34].The PO generatedin-situcould participate in PC copolymerization as monomers and eventually generate to give oligo(propylene oxideco-propylene carbonate).On the other hand,PO can be converted into some small molecular by-products by proton elimination reaction or hydrolysis reaction,which can participate in polymerization as a chain transfer agent.Based primarily on the mechanism ofinsitugeneration of PO,they proposed a novel strategy of the ROCOP of PC/cyclic anhydrides,realizing the quantitative transformation of PC into polyesters.The catalyst of PPNCl converted a mixture of PC and PA into an alternating poly(PO-alt-PA) (Mn=13.0 kDa)within one hour at 180°C.Furthermore,the polymerization could be carried out efficiently at 140–220 °C.Noticeably,the CO2released by the polymerization,through the phenomenon that the CO2pressure increases as the reaction proceeds,provided a convenient means to visualize the polymerization process in real time.Further investigation of ROCOP of various cyclic carbonates/cyclic anhydrides revealed the universality of our synthetic methodology.
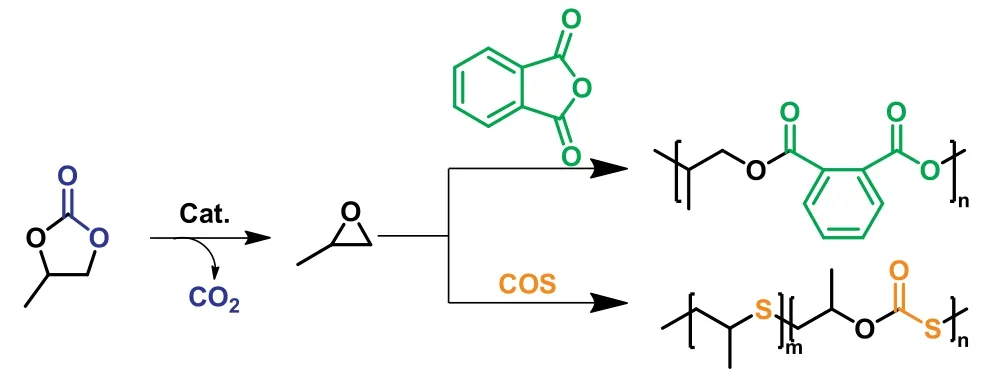
Fig.3.Copolymerization of PC with cyclic anhydrides or COS.
Similarly,the copolymerization of PC with carbonyl sulfide(COS) to prepare polythioethers was reported by Zhanget al.in a one-pot protocol (Fig.3) [35].The polymerization involves several sequential steps includingin-situdecarboxylation of PC to generate PO,coupling of COS/PO to cyclic thiocarbonate (CTC),decarboxylation of CTC to generate propylene sulfide (PS),and the ROP of PS.The resulting polymer contained mainly thioether units (65–100 mol%) and some thiocarbonate units.By adjusting the polymerization temperature,time,and catalyst,copolymers with different thioether/thiocarbonate unit contents could be obtained,which helped to adjust the degradation performance of the polymer.
3.Polythioester synthesis by ring-opening polymerization
Polythioesters possess attractive properties such as metal coordination ability and high refractive index due to the introduction of sulfur atoms.The ROP of thiolactones is an effective method for the synthesis of polythioesters [36,37].However,some challenges remain,including the minimal functional diversity of available thiolactone monomers and the side reaction of transthioesterification.Tao and co-workers recently reported the ROP of amino acid-derivedS-carboxylic acid anhydrides (SCA) to synthesize polythioesters within 2 min in air [38].Compared with thiolactones,the ROP of SCA showed significantly high reactivity and fast reaction rate,which was the key to achieve controllable polymerization and suppress various side reactions (transthioesterification and epimerization).Hong and co-workers replaced the carbonbased oxygen atoms of lactones with sulfur atoms and synthesized high-molecular-weight polythioesters through isomerizationdriven irreversible ROP of five-membered thiolactones [39,40].
4.Polycarbonate synthesis by ring-opening copolymerization of CO2 and epoxide
CO2,regarded as the main gas causing the greenhouse effect,has a wide range of sources and is nontoxic.The copolymerization of CO2and epoxides,with 100% atomic utilization,can not only obtain degradable polycarbonate,but also turn CO2waste into treasure,effectively reducing carbon emissions.Owing to the precisely designed ligands bound to transition metals,metal organic catalysts simultaneously exhibit high activity and high selectivity for the alternating insertion of CO2and epoxides.Zhang and coworkers reported the copolymerization of CO2and IBO into crystalline polycarbonate,catalyzed by a heterogeneous Zn-Co DMCC,providing alternating copolymer with>95% alternating degree andMnof 19.6 kDa [41].Especially,poly(isobutylene carbonate) (PIBC)hadTmof 94°C owing to high regioregularity,aσBof 4.4 MPa,and an elongation at break (εB) of 350%.
However,the copolymerization of CO2with epoxidesviaorganocatalysis remains a great challenge [42,43].For the first time,Feng and co-workers successfully realized the highly active anionic copolymerization of CO2and epoxides under metal-free conditions,providing polycarbonates with highMn[44].Recently,Zhang and co-workers reported a zwitterionic method for the selective copolymerization of CO2and PO,providing poly(propylene carbonate) (PPC) with>99% alternating degree,around 80% headto-tail linkages,Mnup to 56.0 kDa,and narrow dispersity (below 1.2) [45].The trialkylboron/tertiary amine catalytic system has extremely high catalytic efficiency and regioregularity.The sequential insertion of PO and CO2into the Lewis pair formed an endto-end zwitterion featuring a TEB-masked anion and an onium cation,making it highly selective for alternating copolymerization.The organocatalytic system composed of TEA and TEB had productivity up to 171 g PPC/g while the double-site tertiary amines,N,N,N′,N′-tetraethyl ethylenediamine (TEED),paired with TEB exhibited higher activity and productivity (up to 216 g PPC/g catalyst).It should note that bifunctional organoboron catalysts having B-N (P) centers is an effective and selective catalyst system for the copolymerizations of epoxides and CO2[46].
5.Block polymer synthesis from switchable polymerization
As elegantly exemplified by biopolymers such as DNA,proteins,and polysaccharides,the property and functionality of polymers are closely related to their composition,sequence,and topology.Whereas nature exhibits exceptional sophistication and efficiency in synthesizing biopolymers with an extremely high degree of structure complexity,polymer chemistry is still in its infancy.The synthesis of polymers with a high degree of structural complexity typically involves multiple-step procedures,orthogonal catalysis,and painstaking purification processes.Toward this end,Williams and co-workers pioneered the switchable polymerization from ROCOP of epoxide/cyclic anhydride to ROP of lactones,which enables the straightforward synthesis of polyester-b-polyesters from mixtures of monomers [47].Since then,the self-switchable polymerization has emerged as the most powerful tool to achieve block copolymers in a single efficient procedure.
Zhang and co-workers reported the one-pot synthesis of polyester-b-polythiocarbonate block copolymers by the copolymerization of commercially available lactones,epoxides,and COS (Fig.4) [48].1,5,7-Triazabicyclo[4.4.0]dec–5-ene (TBD) can activate the cyclic ester through hydrogen bonds and deprotonate alcohol protons to initiate polymerization,forming an alkoxy anion chain growth center.After the introduction of COS,due to the coordination of TEB with the chain anion,the active center switched to the active center bound by TEB [49,50].And then the alternating copolymerization of COS with epoxides was selectively catalyzed to yield polythiocarbonate blocks.By sequential addition of monomers,nine-block copolymers were synthesized.However,the switching of the growth chain center was irreversible,that is,it was necessary to synthesize the polyester block first,and then synthesize the polythiocarbonate block.Tao and co-workers recently reported the one-pot synthesis of poly(ester-b-carbonate) by the switchable polymerization ofO-carbonyl anhydrides (OCAs) and epoxides catalyzed by TEB/onium salt Lewis acid-base pair [51].
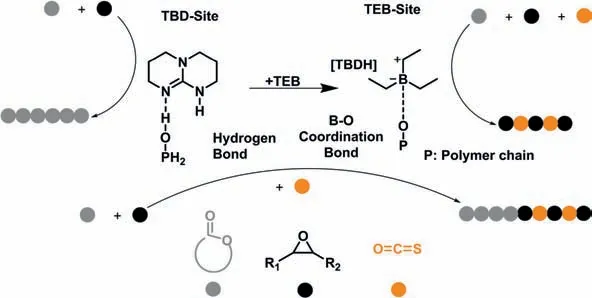
Fig.4.Illustration of the “dual active sites” for the synthesis of polyester-polythiocarbonate block copolymers (color online).
The limitation of self-switchable polymerization is that only AB and ABA block copolymers with linear architecture and oxygenated composition are typically accessible.Toward this end,Wang and co-workers developed the switchable polymerization between the ROCOP of epoxide/cyclic anhydride/CO2,and organometallic mediated radical polymerization (OMRP) of vinyl monomers based on the quantitative transformation of Co-O and Co-C bondsviagas-switching carbon monoxide (Fig.5).This new switchable catalytic system allows the facile synthesis of block copolymers connecting polyacrylate,poly(vinyl acetate) with oxygenated blocks,which permits significant potential in constructing highvalue-added nanomaterials based on the ideal phase separation behavior [52].Inspired by various radical organic transformations mediated by organocobalt complexes,they successfully synthesized cyclic polyesters and cyclic CO2-based polycarbonatesviavisible light regulated switchable catalytic from ROCOP of epoxide/cyclic anhydride/CO2to highly regioselective radical cyclization process [53].They used a double Schiff base cobalt complex (complex3) with an axial group of pentenoate as a catalyst for the ring-opening copolymerization.Through the quantitative insertion of carbon monoxide,the active species of ROCOP are quantitatively transformed into linear precursors with double bonds and cobalt carbonyl bonds as end groups.Under the action of visible light,the carbonyl cobalt bond of the linear precursor is homogenously cleaved to generate carbonyl radicals,and undergoes a highly regioselective radical addition reaction with the double bond (the Markov addition product is greater than 99%),resulting in cyclic polyester and cyclic polycarbonate.Compared with linear polyesters and polycarbonates,cyclic polyesters and polycarbonates without end groups have better stability,less chain entanglement,and higher glass transition temperatures.
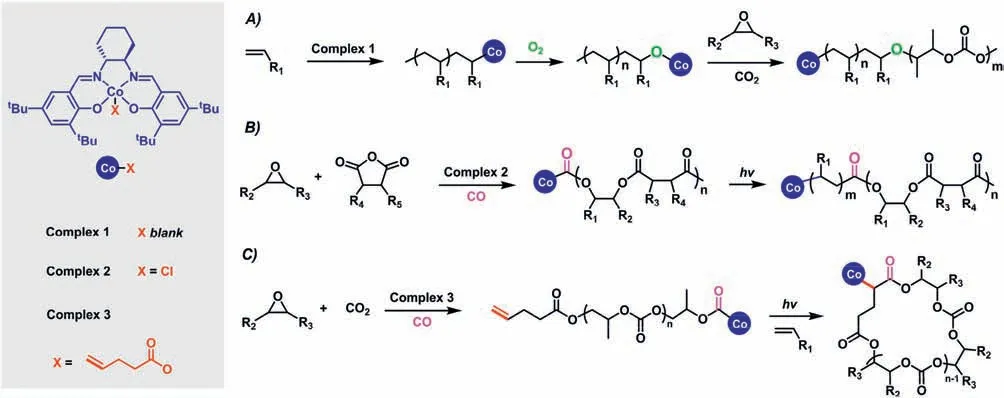
Fig.5.Cobalt-mediated switchable catalysis for one-pot synthesis of biodegradable and renewable polymers with different topologies: (A) From OMRP to ROCOP for synthesizing polyacrylate-b-polycarbonates;(B) from ROCOP to OMRP for obtaining polyacrylate-b-polyesters;(C) from ROCOP to radical cyclization reaction for constructing cyclic polymers.
6.Conclusion and perspective
The synthesis of degradable polymers with easy-to-break carbon-oxygen bonds in the main chain is the requirement of social green and sustainable development.It is an effective method to synthesize poly(thio)ester and poly(thio)carbonate through the(co)polymerization of monomers such as epoxides,cyclic carbonate,cyclic esters,carboxyanhydride,CO2,COS,and cyclic anhydrides.However,there are still many challenges to moving this aggregation technology toward large-scale applications.First,the control of sequence,regioselectivity,and stereoselectivity in the current system is still insufficient.Second,compared with the traditional polyolefin,the polymer obtained by the current method has a lower molecular weight,resulting the gaps in thermal and mechanical properties.Last,although the polymers can be degraded,they are difficult to depolymerize into monomers or other cyclic small molecules that can be polymerized again,which cannot achieve the purpose of chemical recycling.Further research efforts shall be directed to the development of new catalytic methodologies that enable the one-pot/one-step construction of renewable and biodegradable copolymers with diverse compositions (new monomers) and topology (linearly multi-block,brush,branched,and star).The synthesis of chemically recyclable polymers with practical application prospects by developing novel catalytic systems is also an important development direction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support of the National Science Foundation of China (Nos.52203129,51973190) and Zhejiang Provincial Department of Science and Technology (No.2020R52006).
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- Doping-induced charge transfer in conductive polymers
- Quinoline-based anti-MRSA agents: Current development,structure-activity relationships,and mechanisms
