Molecular mechanisms of ferroptosis and its role in the treatment of breast cancer
2024-03-26JINGBoCHENHengyuWUHuangfu
JING Bo, CHEN Heng-yu, WU Huang-fu✉
1. The Second Clinical College of Hainan Medical University, Haikou 571199, China
2. Department of Breast & Thyroid Surgery, the Second Affiliated Hospital of Hainan Medical University, Haikou 570311, China
Keywords:
ABSTRACT Breast cancer is a critical threat to women around the globe.Current radio- and chemotherapy regimens can induce multiple drug-resistant effects, e.g., anti-apoptosis, anti-pyroptosis,and anti-necroptosis, causing a poor clinical response to therapy.Ferroptosis is a newly programmed cell death characterized by iron overload, the massive production of reactive oxygen species (ROS), and membrane lipid peroxidation.The occurrence of ferroptosis results from an imbalance between peroxidation mechanisms (execution systems) and antioxidation mechanisms (defense systems), including the iron metabolism pathway, amino acid metabolism pathway, and lipid metabolism pathway.Recently, the vital role of ferroptosis in various diseases, including cancer, hypertension, diabetes, and Alzheimer's, has been identified.Specifically, triggering ferroptosis in breast cancer cells can inhibit their proliferation and invasion, and improve the chemoradiotherapy sensitivity, which makes it a potential strategy for breast cancer therapy.This review summarizes the definition and features of ferroptosis,as well as its role in the treatment of breast cancer, aimed at providing a theoretical basis for future drug development.
Breast cancer is the most common malignant tumor in women with a high fatality rate.According to the study of Harbeck et al., there are about 17 million newly diagnosed cases of breast cancer worldwide every year, and 5 million women die from this disease[1].Clinically, the main therapeutic methods for this disease include surgical resection, chemoradiotherapy, endocrine therapy,immunotherapy and targeted therapy.However, due to its high degree of malignancy and susceptibility to drug resistance, the prognosis of breast cancer patients is generally not ideal[2].In recent years, programmed cell death modes, such as apoptosis, necrosis,autophagy, pyrodeath and necrotic apoptosis, have been found to be closely related to the proliferation, migration and drug resistance of breast cancer cells[3].ferroptosis, a concept proposed by Dixon et al in 2012, is an iron-dependent, non-apoptotic programmed cell necrosis, mainly manifested by reactive oxygen species (ROS)accumulation and membrane lipid peroxidation damage, which ultimately leads to cell death[4].ferroptosis has been confirmed to enhance the sensitivity of breast cancer to radiotherapy and chemotherapy, inhibit the growth of breast cancer tissues, distant metastasis and recurrence of cancer tissues, etc., which makes the induction of ferroptosis a potential strategy for the treatment of breast cancer[5].This paper focuses on the mechanism of ferroptosis and its role in the treatment of breast cancer to provide theoretical support for further improvement of breast cancer treatment and targeted drug development.
1.Characteristics of ferroptosis
Ferroptosis is different from the known programmed cell death in morphological, biochemical and genetic aspects.Morphologically,the most prominent feature was the change of mitochondrial ultrastructure, which was manifested as obvious atrophy of mitochondria, increased membrane density and decreased or disappeared mitochondrial cristae, while the nucleus was normal in size and the nuclear chromatin was not condensed.In terms of biochemical characteristics, when ferroptosis occurs, intracellular iron and ROS levels are significantly increased, glutathione (GSH)is consumed, glutathione peroxidase 4 (GPX4) is inactivated and mitochondrial membrane potential is reduced[6].From the perspective of mechanism, several previously discovered programmed cell death are execution-centered.For example,apoptosis is mainly executed by the caspases family, pyroptosis is mainly executed by the gasdermins family, and necroptosis is mainly mediated by mixed-lineage kinase domain-like protein (MLKL)[7].As a unique mode of cell death, ferroptosis depends on the confrontation between the execution system and the defense buffer system[8].Its implementation mechanisms include iron accumulation,Fenton reaction, synthesis and peroxidation of Polyunsaturated fatty acid-phospholipid (PUFA-PLs), etc.The defense mechanism is mainly System Xc-/GSH/GPX4 system[9, 10].The mechanism by which ferroptosis occurs is shown in Figure 1.
In addition, two non-classical antioxidant systems, FSP1-CoQH2 system and DHODH-CoQH2 system, are also generally recognized[11].
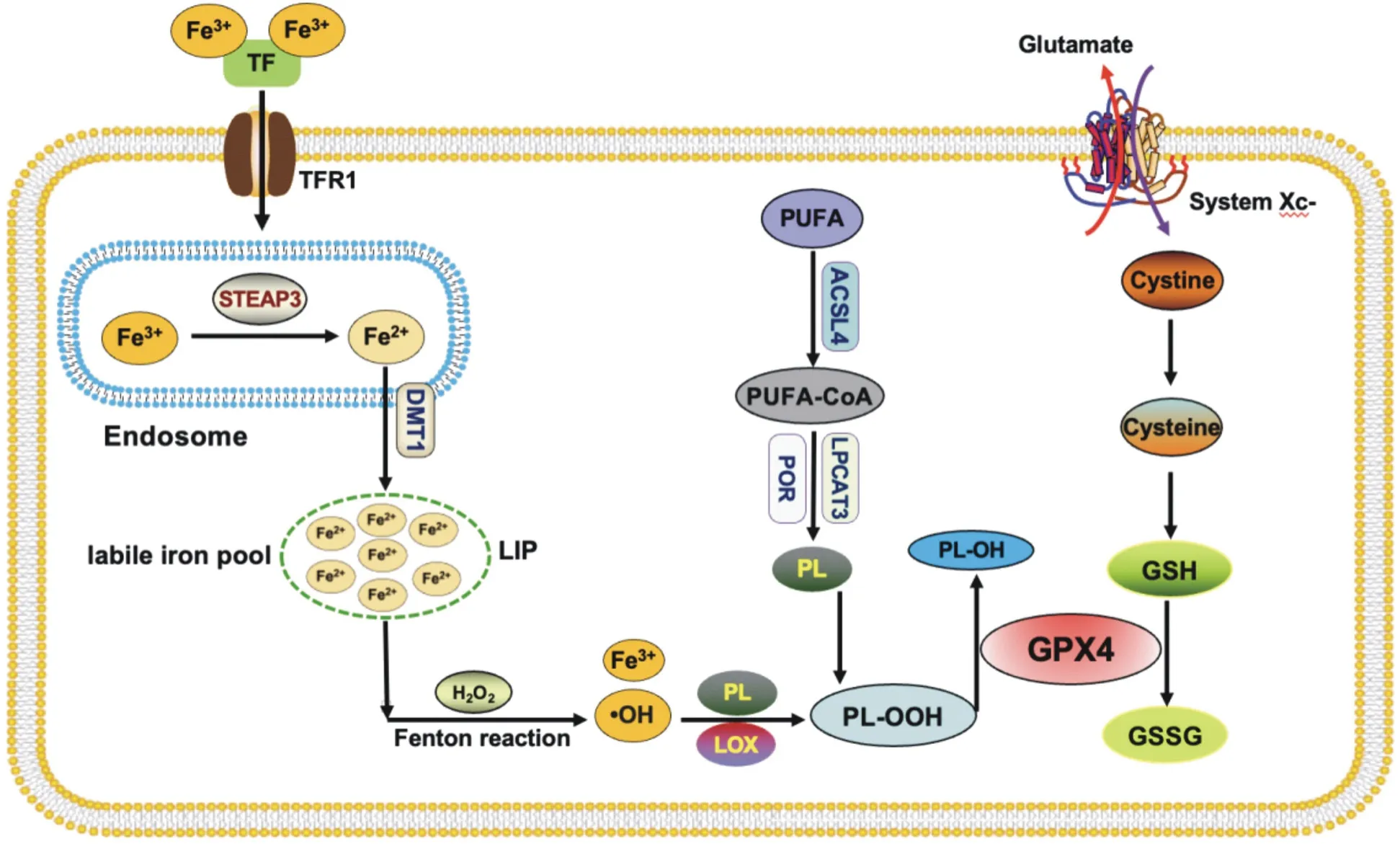
Fig 1 Mechanisms of ferroptosis
2.Ferroptosis regulates pathways for breast cancer therapy
Breast cancer is the most common malignant tumor in Chinese women.At present, the treatment methods for breast cancer mainly include surgery, radiotherapy and chemotherapy, endocrine therapy and targeted therapy.Although some achievements have been made,drug resistance and radiotherapy insensitivity often occur in the later stage, making the prognosis of breast cancer patients not ideal.In recent years, studies have confirmed that a variety of drugs, active compounds and related nanomaterials can inhibit the growth of breast cancer tissues, enhance the sensitivity of cancer tissues to radiotherapy and chemotherapy, and inhibit the distant metastasis and recurrence of breast cancer by inducing ferroptosis, which makes the induction of ferroptosis a potential strategy for the treatment of breast cancer[11].The pathways by which ferroptosis regulates breast cancer treatment and drug resistance are shown in Table 1
2.1 Accumulation of iron
Circulating Fe3+can combine with transferrin (TF) to form TFFe3+complex, which is endocytosed to the cell through transferrin receptor 1 (TFR1) on the cell membrane surface and localized tothe endosome.In the endosome, Fe3+is reduced to Fe2+by the sixtransmembrane epithelial antigen of prostate 3 (STEAP3), which is released into the labile iron pool (LIP) of the cytosol with the help of divalent metal ion transporter 1 (DMT1).A part of intracellular Fe2+can combine with ferritin heavy chain (FTH1), be oxidized to Fe3+, and then bind to ferritin light chain (FTL) to form ferritin complex stored in the cell.When the intracellular Fe2+content is significantly increased, too much iron will combine with hydrogen peroxide (H2O2) to produce Fenton reaction.The generation of hydroxyl free radicals ( OH) with strong oxidation ability increases the intracellular ROS level, and further promotes the peroxidation of PUFA-PLs under the synergistic effect of ester oxygenase (LOX),leading to cell membrane rupture and ferroptosis[12].Tyrosine kinase inhibitor neratinib is a commonly used chemotherapeutic drug for the treatment of HER-2+ breast cancer patients.It can increase the expression of TFR1 in cancer cells, induce iron accumulation and ferroptosis, and inhibit brain metastasis of cancer tissues in vivo[13].Similarly, the synergistic anticancer effect of chemotherapy drugs siramesine and lapatinib can increase TF content, promote intracellular iron accumulation, and induce ferroptosis in TNBC cells to exert synergistic anticancer effect[14].

Tab 1 Pathways by which ferroptosis regulates Breast cancer therapy
Liu et al.found that transmembrane protein 189 (TMEM189) can inhibit autophagy of TNBC cancer cells, reduce TFR1 expression and intracellular lipic-ROS content, alleviate ferroptosis, and promote the growth of breast cancer in vivo[15], suggesting that TMEM189 inhibitors may have a good therapeutic effect on TNBC.Iron-saturation lactoferrin (Holo-Lf) combined with radiotherapy at a dose of 4 Gy can significantly enhance the ferroptosis induced by Erastin in TNBC, inhibit the proliferation and migration of cancer cells, and enhance the radiosensitivity[16].Shuganning injection(SGNI), a Chinese patent drug, can selectively up-regulate the expression of heme oxygenase-1 (HO-1) in TNBC cells, promote iron accumulation and ferroptosis in LIP, and inhibit the growth of breast cancer tissues in vitro and in vivo[17].Artemisinin can promote ferritinophagy, increase intracellular Fe2+content and ferroptosis in TNBC cells, enhance the sensitivity of cancer tissue to ferroptosis inducer RSL3, and inhibit the development of breast cancer in vivo[18].Holo-Lf, SGNI and artemisinin are promising candidates for the treatment of TNBC.
In recent years, there have also been many reports on nanoparticles targeting breast cancer therapy by iron accumulation and ferroptosis mechanism.Xu et al.developed a Fe2+-based nanoorganic metal framework (MOF-Fe2+), which can efficiently deliver Fe2+to breast cancer cells, promote the Fenton reaction and the generation of large amount of ROS, induce ferroptosis, and effectively inhibit the growth of breast cancer tissues in vivo[19].Zhu et al.constructed a Fe3+ cross-linked nanoparticle loaded with trans-azo-conpratin(trans-Azo-CA4), which can specifically enter breast cancer cells.After being excited by infrared light, the loaded Fe3+is converted to Fe2+,triggering Fenton reaction and lipid peroxide accumulation,and inducing ferrodeath of cancer cells.After the application of this particle in mice, the volume of breast cancer was significantly reduced and the anti-tumor effect was good[20].Zhang et al.designed a cascade of heparanase-driven release nanoparticles loaded with doxorubicin (Dox), ferrocene (Fc), and TGF-β receptor inhibitor SB431542.After entering breast cancer cells, the nanoparticles can increase intracellular iron accumulation and ROS content through Dox and Fc, activate ferroptosis, and successfully prevent cancer tissue metastasis in mice [21].The efficacy of these nanoparticles in clinical application needs to be further confirmed.
2.2 System Xc-/GSH/GPX4
The System Xc-/GSH/GPX4 system is the most important antioxidant defense mechanism for ferroptosis[22].GPX4 is a GSHdependent key enzyme that cleans intracellular lipid-ROS, converts reduced GSH to oxidized GSH (GSSG), and degrades phospholipid hydroperoxides (PLOOH) to non-toxic fatty alcohols to maintain the homeostasis of membrane Lipid bilayer, which is a key step to inhibit ferroptosis[23].The vast majority of intracellular GSH is synthesized from glutamate, cysteine and glycine by catalytic enzymes.The glutamate-cystine reverse transport system (system Xc-) is the way for most cells to obtain cysteine.This system is composed of disulfide bonded heterodimers SLC7A11 and SLC3A2,which can transport intracellular glutamate to the outside of the cell and extracellular cystine to the inside of the cell at a ratio of 1:1[24].Inhibiting the activity of System Xc-/GSH/GPX4 axis is an effective way to prevent the growth of breast cancer and treat breast cancer.
2.2.1 GPX4
Clinical studies have found that dihydroisotanshinone Ⅰ, the active component of salvia miltiorrhiza, can inhibit the expression of GPX4 in breast cancer cells, promote ferroptosis, and inhibit the growth of tumor tissues in mice without showing significant side effects[25].DMOCPTL, a derivative of natural product parthenolide, can directly bind to GPX4 protein to promote GPX4 ubiquitination and induce ferroptosis in breast cancer cells.In vivo, DMOCPTL can effectively inhibit the growth of mouse mammary tumors and significantly prolong the life span of mice without obvious toxicity[26].Wen et al.extracted the active compound glycyrrhetinic acid from the traditional herb licorice, and found that it could reduce the activities of GSH and GPX4, aggravate lipid peroxidation, and induce ferroptosis in breast cancer cells[27].Metformin can up-regulate the level of miR-324-3p in TNBC cells, target inhibition, promote ferroptosis, and significantly reduce the growth of cancer tissues in vivo[28].In addition, Jaggupilli et al.found that V9302, a small molecule compound that inhibits glutamine uptake, induces lipid peroxidation and promotes ferroptosis in TNBC cells by significantly inhibiting GSH and GPX4 activities in SUM159 and MDA-MB-231 cells.In vitro and in vivo combined with paclitaxel can significantly inhibit the growth of breast cancer[29].Dihydroisotanshinone Ⅰ, DMOCPTL, glycyrrhetinic acid, metformin and V9302 may be effective potential drugs for the treatment of breast cancer.
Yao et al.reported that simvastatin (SIM) can attenuate 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) activity to inhibit mevalonate (MVA) pathway and GPX4 expression, thereby inducing ferroptosis in TNBC cancer cells.They further loaded SIM into zwitzwitzonic polymer-coated magnetic nanoparticles (Fe3O4@PCBMA) and achieved good anti-breast cancer effect in vivo.At present, Fe3O4 has been proved to be clinically available by the US Food and Drug Administration (FDA), and Fe3O4@PCBMA-SIM nanosystems may have great potential for clinical application[30].
2.2.2 GSH
Li et al.found that tumor-associated macrophages (TAMs)can secrete transforming growth factor β1 (TGF-β1) and upregulate the expression of hepatic leukemia factor (HLF) in TNBC cells, which makes breast cancer tissue larger and less sensitive to chemotherapy drug cisplatin.Mechanistically, HLF transcriptionally activates γ-glutamyl transpeptidase (GGT1), which catalyzes the cleavage of extracellular GSH and increases GSH content in cancer cells, thereby inhibiting ferroptosis and enhancing the proliferation and invasion ability of TNBC cells and the resistance of cancer tissues to cisplatin[31].Zou et al.identified fibroblast growth factor receptor 4 (FGFR4) as an essential gene for the acquisition of drug resistance in HER2+ breast cancer, and inhibition of FGFR4 in mice in vivo reduced GSH synthesis and Fe2+efflux efficiency through the β-catenin/TCF4-SLC7A11/FPN1 axis.It leads to excessive ROS production, iron accumulation and ferroptosis in LIP, and enhances the sensitivity of drug-resistant HER2+ breast cancer to chemotherapeutic drugs[32].HLF and FGFR4 may be the targets of drug development for the prevention and treatment of breast cancer.New breast cancer prevention and treatment materials targeting GSH and ferroptosis have also been reported in recent years.A sonodynamic sensitizer containing platinum (ii) -indocyanine complex can enhance the sensitivity of cancer tissue to ultrasound radiation therapy by reducing GSH content in TNBC cell line 4T1,promoting ROS generation and ferroptosis[33].Zhou et al.used cinnamaldehyde as a material to make a dimer capable of consuming GSH.When combined with sorafenib, this dimer significantly enhanced ferroptosis of 4T1 cancer cells and successfully cured breast cancer in mice in vivo by promoting dendritic cell maturation and CD8+T cell priming[34].
2.2.3 System Xc-
Actin-binding protein 1 (fascin) can directly bind to System Xc-,degrade System Xc- through the ubiquitin-proteasome system,enhance the sensitivity of MCF7 cells to Erastin, and inhibit the growth of breast cancer in vivo[35].The chemotherapeutic drug sulfasalazine can promote ferroptosis of breast cancer cells by specifically inhibiting the function of SLC3A1, and the use of this function to treat breast cancer has been in phase II clinical trials[36].Metformin can inhibit the UFMylation modification of SLC7A11,down-regulate the expression of SLC7A11, promote the ferroptosis of TNBC cancer cells, reduce the volume of breast cancer in vivo,and the effect is better when combined with sulfasalazine[37].Isoglycyrrhizin can inhibit the NF-κB signaling pathway in MDAMB-231 and MCF-7 cells, reduce the expression of System Xc-,promote ferroptosis, and reduce the resistance of breast cancer tissue to doxorubicin[38].Trastuzumab can increase the expression of circular RNA BGN (circ-BGN) in breast cancer cells BT474 and SKBR3, enhance the deubiquitination and expression of SLC7A11,inhibit ferroptosis, and induce drug resistance in cancer tissues,which can be reversed by Erastin[39].
2.3 ACSL4
The synthesis and peroxidation of PUFA-PLs is a prerequisite and key step for ferroptosis.Compared with monounsaturated fatty acids(MUFA), there are easily oxidized diallyl groups in PUFA, especially PUFA-PLS (PUFA-PE) containing phosphatidylethanolamine (PE)are most likely to undergo peroxidation under the catalysis of iron,which leads to the accumulation of a large amount of lipid peroxides in the cell membrane, cell membrane rupture and ferrodeath[40].Acyl-coa synthetase long-chain family member 4 (ACSL4) and lyso phosphatidylphosphatidyltransferase 3 (LPCAT3) are key enzymes in the synthesis of PUFA-PE[41, 42].ACSL4 can combine free PUFAs with coenzyme A (CoA) through acylation to form PUFA-CoA,which then occurs esterification under the action of LPCAT3 and reacts with PE to form PUFA-PE[43].Sha et al.collected biopsy specimens from 199 breast cancer patients who received paclitaxelcisplatin chemotherapy, and found that both ACSL4 expression and ACSL4/GPX4 combination status were independent predictors of achieving a pathological complete response (PCR) by rank test and Cox proportional regression.Moreover, ACSL4 expression is positively correlated with the overall survival rate of patients[44].The increased expression of ACSL4 in TNBC cell line MDA-MB-157 can enhance the sensitivity of cancer tissues to the ferroptosis inducer RSL3, while after knocking out ACSL4, cancer cells will not be induced to ferroptosis by RSL3, and the volume of cancer tissues in vivo becomes larger[45].These clues suggest that ACSL4 is a target for drug development in the treatment of TNBC.
2.4 ACSL3/SCD1
In contrast to ACSL4, MUFas such as octadecenoic acid mediated by ACSL3 or stearoyl-coa desaturase 1 (SCD1) competitively inhibit PUFA-related ferroptosis by substituting PLs in the cell membrane to form MUFA-PL.Studies have shown that inactivation of ACSL3 or SCD1 enhances the sensitivity of breast cancer cells to ferroptosis[46, 47].Luis et al.reported that SCD1 and fatty acid binding protein 4 (FABP4) were significantly up-regulated in human breast cancer specimens and were associated with poor prognosis in different types of breast cancer.Mechanistically, SCD1 can catalyze fatty acid desaturation and cooperate with FABP4 in the breast cancer microenvironment to promote lipid droplet formation,reduce hypoxia-induced ferroptosis, and promote cancer regrowth and recurrence.Down-regulation of SCD1 and FABP4 expression in vivo can significantly inhibit lipid transport in the cancer microenvironment and induce ferroptosis.Reduce the recurrence and metastasis of breast cancer[48].Cannabinoid receptor 1 (CB1)inhibitor rimonabant can activate PI3K and MAPK signaling pathways, reduce SCD1 and fatty acyl dessaturated enzyme 2(FADS2) -mediated MUFA production, promote ferroptosis of TNBC cells and the sensitivity of cancer tissues to Erastin and RSL3,and limit the growth of breast cancer tissues in vivo[49].Rimonabant may be an effective drug for the treatment of breast cancer, and the specific mechanism needs to be further confirmed.
2.5 Nrf2
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a very important transcription factor for oxidative stress response, which can induce the expression of HO-1 and inhibit the expression of ROS, and exert anti-inflammatory and anti-ferroptosis effects.In recent years, it has been found that Nrf2 plays an important role in the progression,treatment and drug resistance of breast cancer.Jiang et al.found that TYRO3 expression in breast cancer tissues was associated with poor prognosis in breast cancer patients treated with anti-PD-1 /PD-L1 therapy.Mechanistically, anti-PD-1 /PD-L1 can induce a significant increase in TYRO3 expression in breast cancer tissues,further increase intracellular Nrf2 level, reduce ROS generation,and create a microenvironment conducive to tumor growth by inhibiting ferroptosis, making them resistant to anti-PD-1 /PDL1[50].Wu et al.reported that the expression of glycogen synthase kinase-3β (GSK-3β) was decreased, while Nrf2 was significantly overexpressed in breast cancer tissues.Overexpression of GSK-3β in TNBC cells inhibited Nrf2 expression, increased ROS and MDA content, and enhanced erastin-triggered ferroptosis.In the breast cancer xenograft model in vivo, overexpression of GSK-3β enhanced the effect of erastin-induced inhibition of tumor growth[51].Levistilide A, an active compound extracted from Ligusticum chuanxiong, can damage the mitochondrial structure and function of breast cancer cells by activating the Nrf2/HO-1 signaling pathway,and enhance ROS-induced ferroptosis in TNBC cells.Levistilide A may be a potential lead compound for breast cancer treatment[52].BET inhibitor (BETi) is a commonly used chemotherapeutic drug in the treatment of breast cancer, and its resistance is mainly mediated by NR5A2 and NCOA3.Mechanistically, NR5A2 interacts with NCOA3 to increase Nrf2 expression and inhibit ferroptosis in cancer cells.Inhibition of NR5A2 or NCOA3 using small molecule inhibitors can significantly enhance the anti-cancer effect of BETi on breast cancer in vitro and in vivo[53].
2.6 FSP1-CoQH2
Ferroptosis inhibitor protein 1 (FSP1), which inhibits ferroptosis independently of GPX4, is a flavoprotein encoded by a gene thought to be a p53-responsive gene[54], which is anchored to the cell membrane after N-terminal acylation and oxidize NAD(P)H to NAD(P)+ using FAD as a cofactor.At the same time, the generated reduced coenzyme Q (CoQH2) can reduce toxic lipid peroxides to non-toxic lipid alcohols[54], thereby inhibiting the process of lipid peroxidation and playing the role of anti-ferroptosis.FSP1 is a glutathione independent iron repressor[55] and a novel anti-ferroptosis biomarker[56].Silk fibroin nanoparticles coated with rosuvastatin can effectively inhibit the oxidoreductase activity of FSP1 and slow down the malignant progression of triple negative breast cancer[57].
2.7 DHODH-CoQH2
Dihydroorotate dehydrogenase (DHODH), which is present in the mitochondrial inner membrane, participates in the synthesis of pyrimidine and can reduce CoQ to CoQH2 in the mitochondrial inner membrane.When DHODH is activated, the production of CoQH2 increases, thereby inhibiting the process of lipid peroxidation in mitochondria[58], thus exerting an anti-ferroptosis effect.The DHODH inhibitor Breqinar selectively inhibits tumor growth with low GPX4 expression by inducing ferroptosis, while combination therapy with Breqinar and sulfasalazine synergistically induces ferroptosis and inhibits tumor growth with low GPX4 expression[58].DHODH inhibitor siR/IONs@LDH can induce ferroptosis in breast tumors[59].
3.Summary and Prospect
At present, targeted ferroptosis has been recognized as an effective method for the treatment of breast cancer, but its clinical application still faces many challenges.Firstly, most of the studies only carried out in vitro experiments, and the in vivo experiments were relatively ignored, so it is difficult to measure the effect of ferroptosis inducers on the growth and invasion of breast cancer tissues at the overall level.Moreover, the optimal dose of ferroptosis inducers in the treatment of breast cancer is rarely studied.Secondly, ferroptosis occurs not only in breast cancer tissues, but also in normal tissues.Therefore, ferroptosis inducers can not only kill tumor cells, but also cause harm to normal cells.TNBC is a type of breast cancer with the most difficult to treat and the highest recurrence rate in clinical practice.It has not been reported whether TNBC can be reedited to make it more sensitive to ferroptosis.Similar to the role of caspases, MLKL, GSDMs in other programmed death, what is the most representative marker of ferroptosis? Like other types of programmed cell death, ferroptosis has been found to play a doubleedged sword role in cancer, which depends on the release of damprelated molecular patterns in the tumor microenvironment and the activation of immune response triggered by ferroptosis damage.Therefore, how to reduce the side effects of ferroptosis inducers in the treatment of breast cancer is also an important problem.To solve these problems, future research should focus on both basic experiments and clinical trials, not only to explore the regulatory mechanism of ferroptosis, but also to pay attention to the changes in the function of various organs.It is possible to envisage that new treatment schemes designed for ferroptosis will be an important direction for the prevention and treatment of breast cancer in the future.
杂志排行
Journal of Hainan Medical College的其它文章
- Mechanism of stilbene glycosides on apoptosis of SH-SY5Y cells via regulating PI3K/AKT signaling pathway
- Protective effect of camellia oil on H2O2-induced oxidative stress injury in H9C2 cardiomyocytes of rats
- Mechanism of Yanghe Pingchaun granules on airway remodeling in asthmatic rats based on IL-6/JAK2/STAT3 signaling axis
- Ghrelin regulates insulin resistance by targeting insulin-like growth factor-1 receptor via miR-455-5p in hepatic cells
- Pharmacodynamic study and mechanism of action of Linggui Zhugan Decoction in the intervention of Nonalcoholic fatty liver disease
- Bioinformatics and network pharmacology identify the therapeutic role and potential targets of diosgenin in Alzheimer disease and COVID-19
