Cellular preconditioning and mesenchymal stem cell ferroptosis
2024-03-24DoaaHusseinZineldeenMazharMushtaqKhawajaHusnainHaider
Doaa Hussein Zineldeen,Mazhar Mushtaq,Khawaja Husnain Haider
Abstract In this editorial,we comment on the article published in the recent issue of the World Journal of Stem Cells.They focus on stem cell preconditioning to prevent ferroptosis by modulating the cystathionine γ-lyase/hydrogen sulfide (H2S)pathway as a novel approach to treat vascular disorders,particularly pulmonary hypertension.Preconditioned stem cells are gaining popularity in regenerative medicine due to their unique ability to survive by resisting the harsh,unfavorable microenvironment of the injured tissue.They also secrete various paracrine factors against apoptosis,necrosis,and ferroptosis to enhance cell survival.Ferroptosis,a regulated form of cell death characterized by iron accumulation and oxidative stress,has been implicated in various pathologies encompassing degenerative disorders to cancer.The lipid peroxidation cascade initiates and sustains ferroptosis,generating many reactive oxygen species that attack and damage multiple cellular structures.Understanding these intertwined mechanisms provides significant insights into developing therapeutic modalities for ferroptosisrelated diseases.This editorial primarily discusses stem cell preconditioning in modulating ferroptosis,focusing on the cystathionase gamma/H2S ferroptosis pathway.Ferroptosis presents a significant challenge in mesenchymal stem cell(MSC)-based therapies;hence,the emerging role of H2S/cystathionase gamma/H2 S signaling in abrogating ferroptosis provides a novel option for therapeutic intervention.Further research into understanding the precise mechanisms of H2Smediated cytoprotection against ferroptosis is warranted to enhance the therapeutic potential of MSCs in clinical settings,particularly vascular disorders.
Key Words: Cell survival;Cell therapy;Hydrogen sulfide;Ferroptosis;Preconditioning;Stem cells;Umbilical cord
INTRODUCTION
The current understanding of stem cell biology,refinement of cell culture techniques,the progress in tissue engineering,and data emanating from clinical trials have provided new momentum to regenerative medicine and tissue repair.Notwithstanding the encouraging results and progress made therein,many issues in stem cell-based therapy necessitate resolution to ensure it can be established as a routine treatment option in clinical settings.
Massive donor cell death post-engraftment due to the uncongenial cytokine-rich host tissue microenvironment infiltrated by inflammatory cells is one of these issues that severely reduces the efficacy of the treatment.In some of the published studies,the reported survival of the transplanted cells is so low that the whole exercise of cell-based therapy becomes futile[1].Hence,massive donor cell death is one crucial reason underlying the mixed data from clinical trials,leading to diverse approaches to enhance their survival post-engraftment[2].Dominated by apoptosis,necrosis,and autophagy,donor cell death post-engraftment has multifarious mechanisms,with ferroptosis emerging as a novel programmed mechanism of cell death,morphologically and biochemically distinct from other cell death pathways[3].
Ferroptosis is a biological process that plays a vital role in the metabolism of amino acids,iron,and polyunsaturated fatty acids,as well as the production of glutathione (GSH),phospholipids,NADPH,and coenzyme Q10.It has been implicated in a subset of pathologies,including neurodegenerative diseases,cancer and stroke,vascular disorders,and pulmonary hypertension in mammals[4-7].It is an iron-dependent form of regulated cell death that involves the accumulation of lipid peroxides and reactive oxygen species (ROS)[8,9].
The research interest in ferroptosis is gaining momentum as it is implicated in the poor engraftment of donor mesenchymal stem cells (MSCs),leading to the reduced efficiency of MSC-based treatment[6,10].Ferroptosis can occur in response to diverse stimuli,such as starvation,exposure to toxins,ROS,etc,leading to intracellular iron accumulation[11].Mechanistically,iron can react with hydrogen peroxide and lipid peroxides to form ROS with heightened lipid peroxidation and cell membrane injury death[11].
Signaling pathways involved in ferroptosis are complex and need to be better understood.However,recent research has shed light on some of the critical signaling pathways,such as p53,nuclear factor erythroid 2-related factor 2 (Nrf2),protein kinase R-like endoplasmic reticulum kinase[12],phospholipid remodeling pathway,GSH peroxidase 4 (GPX4),ferroptosis suppressor protein 1 and other emerging signaling pathways are considered to play a role therein[13,14].This editorial aims to shed light on the pivotal role of the novel cystathionine γ-lyase (CSE)/hydrogen sulfide (H2S) pathway in ferroptosis,supported by the recent scientific evidence by Huet al[10].
CSE/H2S pathway in cellular functions
The CSE is an enzyme primarily expressed in the liver,kidney,and brain that critically regulates H2S production in the body[15].It is a known cardio-protective enzyme detoxifying sulfur-containing amino acids,i.e.homocysteine,and requires pyridoxal phosphate as a prosthetic group.CSE has gained immense interest in the research as it produces a potent cell signaling gasotransmitter,H2S,an essential signaling molecule involved in several physiological and pathological processes such as inflammation,cell cycle,cell metabolism,cell death,and autophagy[15,16].
The CSE/H2S pathway has been implicated in various cellular events,including vasodilation,anti-proliferative,antiinflammatory,and redox homeostasis[17,18].Invariably,H2S production requires an appropriate concentration of cysteine and homocysteine in the presence of cystathionine-β-synthase and CSE,albeit CSE produces about 90% of the total H2S.H2S signalsviaprotein sulfur hydration impart its pathologic effects on ischemia,myocardial fibrosis,metabolic disorder,traumatic brain injury,and bowel diseases[19,20].In response to many stimuli,H2S orchestrates diverse molecular pathways to exert its effects on apoptosis and ferroptosis.The CSE/H2S pathway has been explicitly implicated in human melanoma progression and modulates tissue homeostasis implications in ophthalmic disease,angiogenesis,multiple sclerosis,and Parkinson’s disease[16,21,22].
H2S has been identified as a potent signaling molecule in various physiological processes,including neuromodulation within the brain and smooth muscle relaxation in the vascular system[20].Additionally,it exhibits a remarkable cytoprotective effect by shielding neurons against oxidative stress and cardiac muscle from ischemia-reperfusion injury.Moreover,H2S can influence inflammation,insulin secretion,and angiogenesis[20].The CSE/H2S pathway has also yielded novel biomarkers like homolanthionine and lanthionine,reported in the urine of homocystinuria patients and cardiovascular pathology[23].
The crucial role of the CSE/H2S pathway in ferroptosis
Mechanistically,CSE catalyzes the cleavage of L-cysteine into pyruvate and NH3and eliminates H2S,which plays a role in cellular signaling and has a cardiovascular protective role.Moreover,CSE is the critical enzyme for L-cysteine generation,a precursor of the antioxidant GSH[24].
Recently,gas-transmitters’ role in various physiological and pathological processes has intensified.H2S has become a key player in cellular signaling and regulation[16,24].One of the critical mechanisms by which H2S regulates ferroptosis is its ability to scavenge ROS.ROS are known to induce lipid peroxidation,a hallmark of ferroptosis.H2S reacts with ROS to attenuate their damaging effects on lipids,preventing the initiation of a ferroptosis cascade[16,25].In addition,H2S can indirectly activate antioxidant machinery,such as the Nrf2 pathway,which plays a crucial role in maintaining cellular redox homeostasis[25].Furthermore,H2S has been shown to regulate iron metabolism by controlling cellular iron homeostasis through the modulation of ferroportin-1 and transferrin receptor-1 expression[26].
H2S and its effects on ROS and iron metabolism can also impact signaling pathways involved in ferroptosis.It can suppress MAPK signaling linked to ferroptosis activation[27].H2S can also regulate the expression and activity of key molecules involved in ferroptosis,such as the cystine/glutamate antiporter system Xc-,GPX4,and the lipid repair enzyme GSH-S-transferase alpha 4[24,27].The solute carrier family seven-member 11 (SLC7A11)/GPX4 pathway is a crucial defense mechanism against ferroptosis.SLC7A11 is a protein that helps synthesize reduced GSH and import cystine,which is then converted to cysteine for GSH synthesis[28].
GSH,in the presence of GPX4,converts toxic lipid peroxides to harmless lipid alcohols.Inhibiting SLC7A11 leads to GSH depletion,downregulation of GPX4,and the accumulation of damaging lipid peroxides.The SLC7A11/GPX4 axis is a target for potentially treating ferroptosis-related diseases[29,30].Bioinformatic studies revealed multiple genes expressed in ferroptosis and other genes,and microRNAs can regulate ferroptosis and disease progression[30,31].Given the significant role of the CSE/H2S pathway in cellular function,recent studies have revealed its crucial role in regulating ferroptosis in stem cells[10,32].
In their comprehensive investigation,Huet al[10] investigated the therapeutic potential of human umbilical cord MSCs(hUCMSCs) in treating hypoxia-induced pulmonary arterial hypertension (PAH) in a murine model.They explored the impact of modulating the CSE/H2S pathway in preventing ferroptosis in hUCMSCs.Furthermore,the study demonstrated the capacity of hUCMSCs to homing towards injured lung tissue and protecting against pulmonary artery remodeling.
Remarkably,preconditioning hUCMSCs with a ferroptosis inhibitor,Fer-1,exhibited enhanced survival and efficacy in managing PAH,while ferroptosis induced low cellular viability and therapeutic effectiveness.These compelling findings imply a potential association between ferroptosis and the survival of hUCMSCs in hypoxia-induced PAH[10].
In this study,Huet al[10] have shown that treatment of hUCMSCs with erastin,a ferroptosis inducer,significantly increases cell apoptosis and mitochondrial changes,with concomitant expression of ferroptosis markers,i.e.Fe2+,ROS,lipid peroxidation,and malondialdehyde,besides 4-hydroxynonenal,Fe3+-bound transferrin receptor,and nuclear receptor coactivator 4.
Conversely,the GSH/oxidized GSH ratio levels,cystine uptake,GPX4,ferritin heavy chain 1,and SLC7A11 expressions were significantly decreased[10].These molecular changes were reversed when the cells were treated with Fer-1,a ferroptosis inhibitor,and further aggravated when CSE was inhibited,thus supporting the role of CSE/H2S signaling in preventing ferroptosis in hUMSCs and its efficiency as a therapeutic tool.
These data were further supported by a CSE-dependent decrease in H2S production in hUCMSCs while overexpressing CSE in the cells increased H2S levels[10].CSE inhibition also reduced Nrf2 activation induced by Fer-1,while CSE overexpression upregulated Nrf2 inactivation caused by erastin.The expression of Nrf2 was also affected,with erastin treatment inhibiting its nuclear translocation.CSE inhibition upregulates Nrf2 expression in the cytoplasm and downregulates in the nucleus[10].CSE overexpression-induced ferroptosis inhibition,which was eliminated by an Nrf2 inhibitor.Kelch-like ECH-associated protein 1 (Keap1) is the primary negative regulator of Nrf2 and mediates ubiquitylation and degradation of Nrf2[33,34].
Huet al[10] have also reported that increased H2S-mediated sulfhydration of Keap1 in the hUCMSCs overexpressing CSE and decreased S-sulfhydration of CSE knockout cells treated with Fer-1,highlighting the role of CSE/H2S signaling in modulating ferroptosis in hUCMSCs.
Exploiting CSE/H2S signaling pathway to precondition MSCs
MSCs hold immense potential in regenerative medicine,as they can differentiate into various cell types and replenish damaged tissues[35].Stem cell preconditioning can effectively alleviate ferroptosisviaseveral approaches.Hypoxic conditions increase the expression of heme oxygenase-1,which regulates iron metabolism and ferroptosis.Intriguingly,molecules like hydrogen peroxide or lipopolysaccharide can stimulate the secretion of protective paracrine factors from stem cells and modulate the expression of crucial ferroptosis genes,such as GPX4 and transferrin receptor 1,thereby mitigating ferroptosis-induced cell death[36].
Consequently,stem cell preconditioning has been extensively employed for therapeutic purposes in clinical settings and has progressed to advanced phases of clinical trials[37].Recent research has shed light on the role of ferroptosis as a form of regulated cell death in stem cell biology,focusing on CSE/H2S signaling due to its cardinal physiological and pathological roles in cell death.The CSE/H2S pathway modulates and increases the viability of transplanted MSCs[38].
The underlying mechanism of improved survival has been attributed to the PI3K/Akt pathway activation to reduce cell death by mitochondrial and endoplasmic reticulum stress alleviation[19,38].Moreover,ferroptosis of hUCMSCs is reduced by regulating the CSE/H2S pathway,which improves vascular remodeling in mice with hypoxia-induced PAHviamaintaining the balance between stem cell maintenance and differentiation[10].While genetic modification of stem cells for CSE expression is an efficient way of preconditioning the cells against ferroptosis,alternative preconditioning strategies may also be exploited[29,39,40].
These anti-ferroptosis strategies underscore their significance for potentially targeting ferroptosis signaling for therapeutic interventions in various diseases,including metabolic,inflammatory,and cancerous disorders[14].As mitochondrial stress and ROS are integral parts of the molecular mechanism underlying ferroptosis,it would be interesting to assess the effectiveness of anti-apoptotic preconditioning strategies,i.e.physical,chemical,mechanical,genetic modulation,and growth factor treatment[41-47],either alone or in combination with anti-ferroptosis intervention to support improved cell survival.On the same note,combining sub-cellular preconditioning,i.e.mitochondrial targeting of Connexin-43[48] with anti-ferroptosis strategies to achieve their synergistic cytoprotective effect would be exciting.
CONCLUSION
In conclusion,and as depicted in Figure 1,the emerging role of the CSE/H2S pathway in ferroptosis advocates that targeting this pathway could be a promising therapeutic approach for multiple diseases associated with ferroptosis.Developing CSE/H2S activators or H2S-releasing therapeutics may help attenuate ferroptosis and provide protective effects.Furthermore,understanding the specific mechanisms by which CSE/H2S signals in stem cells could enhance its therapeutic potential in various pathologies.
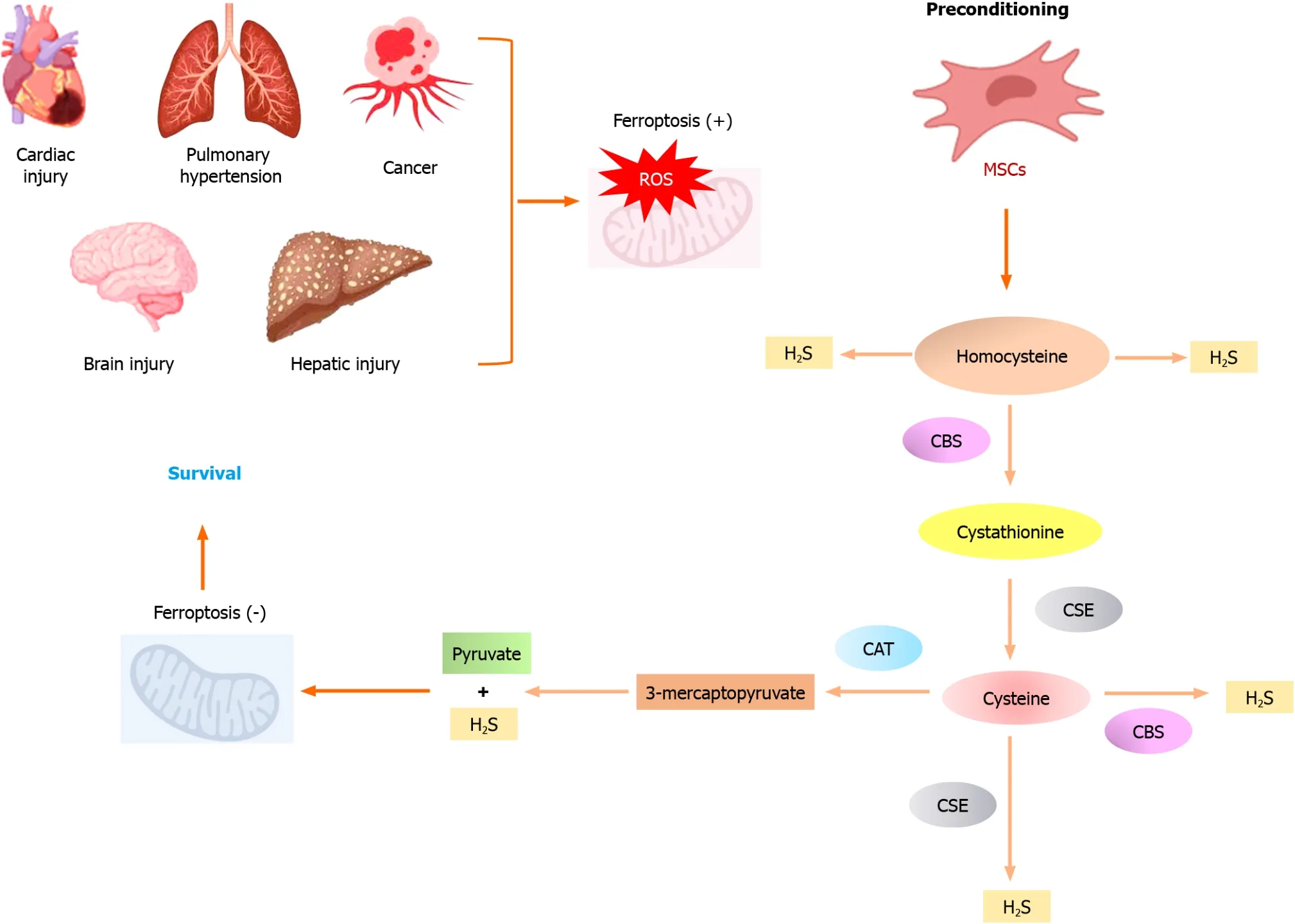
Figure 1 Preconditioned mesenchymal stem cells with high therapeutic potential in various pathologies. Cystathionine γ-lyase/H2S signaling pathway attenuates oxidative stress and alleviates ferroptosis.CAT: Cysteine aminotransferase;CBS: Cystathionine-β-synthase;CSE: Cystathionine γ-lyase;H2S:Hydrogen sulfide;MSCs: Mesenchymal stem cells.
FOOTNOTES
Co-first authors:Doaa Hussein Zineldeen and Mazhar Mushtaq.
Author contributions:Zineldeen DH contributed to the writing and generating of the visual abstract of this manuscript;Mushtaq M was involved in the writing and revisions of this article;Haider KH participated in the writing,finalizing,and submission of the manuscript.
Conflict-of-interest statement:All the authors report having no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Saudi Arabia
ORCID number:Khawaja Husnain Haider 0000-0002-7907-4808.
S-Editor:Wang JJ
L-Editor:Filipodia
P-Editor:Yuan YY
杂志排行
World Journal of Stem Cells的其它文章
- Multiple pretreatments can effectively improve the functionality of mesenchymal stem cells
- Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects
- Unlocking the versatile potential: Adipose-derived mesenchymal stem cells in ocular surface reconstruction and oculoplastics
- Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation
- Human pluripotent stem cell-derived kidney organoids: Current progress and challenges
- Recent progress in hair follicle stem cell markers and their regulatory roles
