Metformin: A promising clinical therapeutical approach for BPH treatment via inhibiting dysregulated steroid hormones-induced prostatic epithelial cells proliferation
2024-03-21TingtingYngJiyuYunYutingPngJilPngZhnQiuShngxiuChnYuhnHungZhnzhouJingYilinFnJunjiLiuToWngXuynZhouSitongQinJinngSongYiXuQinLuXioxingYin
Tingting Yng ,Jiyu Yun ,Yuting Png ,Jil Png ,Zhn Qiu ,Shngxiu Chn ,,Yuhn Hung ,, Zhnzhou Jing , Yilin Fn , Junji Liu , To Wng , Xuyn Zhou ,Sitong Qin , Jinng Song ,g, Yi Xu , Qin Lu ,**, Xioxing Yin ,*
a Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy, Xuzhou Medical University, Xuzhou, Jiangsu, 221004, China
b Department of Pharmacy, Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, Jiangsu, 222061, China
c Department of Pharmacy, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221006, China
d New Drug Screening Center, Jiangsu Center for Pharmacodynamics Research and Evaluation, China Pharmaceutical University, Nanjing, 210009, China
e School of Life Sciences, University of Essex, Essex CO4 3SQ, United Kingdom
f Department of Urology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221006, China
g Department of Pharmacy, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu, 214000, China
Keywords:Metformin Benign prostatic hyperplasia Sex steroid hormones homeostasis Proliferation DHT YAP1-TEAD4 heterodimer
A B S T R A C T The occurrence of benign prostate hyperplasia(BPH)was related to disrupted sex steroid hormones,and metformin (Met) had a clinical response to sex steroid hormone-related gynaecological disease.However, whether Met exerts an antiproliferative effect on BPH via sex steroid hormones remains unclear.Here, our clinical study showed that along with prostatic epithelial cell (PEC) proliferation, sex steroid hormones were dysregulated in the serum and prostate of BPH patients.As the major contributor to dysregulated sex steroid hormones, elevated dihydrotestosterone (DHT) had a significant positive relationship with the clinical characteristics of BPH patients.Activation of adenosine 5'-monophosphate(AMP)-activated protein kinase (AMPK) by Met restored dysregulated sex steroid hormone homeostasis and exerted antiproliferative effects against DHT-induced proliferation by inhibiting the formation of androgen receptor (AR)-mediated Yes-associated protein (YAP1)-TEA domain transcription factor(TEAD4) heterodimers.Met’s anti-proliferative effects were blocked by AMPK inhibitor or YAP1 overexpression in DHT-cultured BPH-1 cells.Our findings indicated that Met would be a promising clinical therapeutic approach for BPH by inhibiting dysregulated steroid hormone-induced PEC proliferation.
1.Introduction
The emergence of benign prostatic hyperplasia(BPH),a bladder outlet obstruction (BOO) caused by epithelial and stromal benign hyperplasia in the transitional zone of the prostate,could result in a series of lower urinary tract symptoms (LUTS).Epidemiological studies have identified that the incidence of BPH is approximately 70%for males at 70 years of age and becomes more prevalent with advancing age [1].Current clinical medical treatments for BPH consist of high-affinity α1-receptor blockers and 5α-reductase inhibitors (5ARIs),such as terazosin,finasteride,and dutasteride[2].Nevertheless, the side effects of these drugs, including dizziness,loss of libido,and erectile dysfunction,have restricted their clinical applications.Therefore,it is of great significance to further explore the pathogenesis of BPH and more effective treatments.
The prostate is highly reactive to sex steroid hormones,including androgens and oestrogens.As potential promoting factors, sex steroid hormones play an important role in BPH development by activating sex steroid hormone receptors, resulting in prostate proliferation of the transitional zone of stromal and epithelial cells [3-5].Testosterone (T), dihydrotestosterone (DHT),androstenedione (A4), dehydroepiandrosterone (DHEA) and androsterone (A) participate in the first phases of prostate development,and higher levels of T might be involved in BPH occurrence[6].Tcould also be metabolized into oestradiol-17β(E2),and E2 was considered to be the most potent oestrogen in men.Estrone(E1),as a weak oestrogen, is considered to have minimal influence on oestrogenic signalling within the prostate.In addition to androgens, the prostate is also a key target of oestrogens, and the overproduction of E2 in the prostate is a powerful inducer of prostatic proliferation.All of these findings indicated that sex steroid hormone profiles had a close relationship with BPH, and targeting pathways mediated by sex steroid hormones may be a great potential treatment and prevention of BPH.
Metformin (Met) is a widely known first-line hypoglycaemic agent for type 2 diabetes (T2DM).Clinical randomized controlled trials have demonstrated that Met has a clinical response to sex steroid hormone-related gynaecological diseases,such as polycystic ovary syndrome (PCOS) and breast cancer (BC) [7,8].A Canadian cancer trial showed that Met improved outcomes and lowered oestradiol levels in postmenopausal hormone receptor-negative BC subjects[9].Twenty-four months of Met treatment improved most hormonal profiles in PCOS patients, and T levels could be used as patient selection criteria or treatment prognostics[10].Other clinical studies confirmed that Met could reduce T and E2 levels in nondiabetic women with BC,and three months of Met therapy could reduce blood glucose and T levels in men with T2DM[11,12].All this clinical evidence indicated that Met had a certain ability to regulate sex steroid hormone profiles.In addition,clinical trials have also proven that Met can improve endometrial hyperplasia (EH), endometrial cancer(EC),bladder cancer,prostate cancer(PCa),and so on[13-15].Related preclinical studies have shown that Met inhibits cellular proliferation and blocks the cell cycle in the G0/G1 stage of atypical EH and endometrioid EC and PCa cell lines, resulting in its antiproliferative effect [16].However, only a nationwide populationbased cohort study showed that Met could reduce the risk of PCa in men with BPH and diabetes.Meanwhile,it is still unknown whether this reduction effect of Met is related to sex steroid hormone profiles.
Thus,the aim of this study was twofold:(1)Screening the most sensitive sex steroid hormones that affect prostate pathologies by revealing the characteristics of the disrupted sex steroid hormones in BPH patients; (2) Assessing Met might represent a novel antiproliferative agent for BPH patients by exploring its antiproliferative effect and mechanism on prostatic epithelial cells(PEC).Our results highlighted the antiproliferative effect and underlying mechanism of Met against BPH, and the current study provided strong evidence that Met might be a promising clinical therapeutic approach for the treatment of BPH.
2.Materials and methods
2.1.Reagents
Met was purchased from J&K Chemical Ltd.(Beijing,China)and Solarbio(Beijing,China).Testosterone propionate(TP)and olive oil were supplied by Aladdin(Shanghai,China).5-Aminoimidazole-4-carboxamide1-β-D-ribofuranoside (AICAR) (S1515) was purchased from Beyotime (Shanghai, China).Compound C (CC; HY-13418A)was purchased from MedChemExpress (Monmouth Junction, NJ,USA).Radioimmunoprecipitation assay (RIPA) lysis buffer and NP-40 were purchased from Beyotime.The primary antibodies against cell cycle protein 1 (CyclinD1; 26939-1-AP), TEA domain transcription factor 4(TEAD4;12418-1-AP)and lamin B1(12987-1-AP)were from Proteintech Inc.(Rosemont,IL,USA).Antibodies against yes-associated protein (YAP1; 14074), activation of adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK; 5831S),and p-AMPK (2535S) were purchased from Cell Signalling Technology (Danvers, MA, USA).Antibodies against androgen receptor(AR) (ab133273) were purchased from Abcam (Fremont, CA, USA).Antibodies against Ki67 (AF0198) and proliferating cell nuclear antigen (PCNA) (AF0239) were obtained from Affinity Biosciences(Changzhou, China).An antibody against β-actin (AP0060) was purchased from Bioworld (St.Louis Park, MN, USA).Antibodies against goat anti-rabbit IgG (H + L) DyLight 488 and goat antirabbit IgG (H + L) DyLight 594 were purchased from Bioworld (St.Louis Park, MN, USA).
DHEA, A4, T, and A were purchased from Aladdin.DHT, hydroxylamine hydrochloride, E1, E2, dansyl chloride, and vitamin C were supplied by Sigma-Aldrich (St.Louis, MO, USA).D5-Estradiol(D5-E2) and D4-Estrone (D4-E1) were purchased from C/D/N ISOTOPES (Montreal, QC, Canada).High-performance liquid chromatography (HPLC)-grade methanol, acetonitrile, water, formic acid, acetic acid, and ethyl acetate were obtained from Merck(Branchburg,NJ,USA).Ultrapure water was purified using a Milli-Q system by Millipore (St.Louis, MO, USA).
2.2.Clinical study
All clinical samples were obtained from the Affiliated Hospital of Xuzhou Medical University (Xuzhou, China).We retrospectively reviewed data from one hundred male patients pathologically diagnosed with BPH with no history of prior anti-prostate hyperplasia medication between May 2017 and Dec 2018.The clinical serum samples of BPH patients were enrolled from the Department of Urology, whereas the healthy subjects were enrolled from the Health Screening Center of the Affiliated Hospital of Xuzhou Medical University.All subjects were evaluated through medical history,physical examination,and routine clinical laboratory tests.We assessed serum prostate-specific antigen(PSA)levels in all BPH patients.Patients with increased PSA levels (4.0 ng/mL or more)underwent transrectal sextant biopsies to rule out the presence of prostate cancer.Men were excluded from analysis if they had a history of prostate or bladder cancer, neurogenic bladder dysfunction, bladder calculi or clinically significant bladder diverticulum, active infection, treatment for chronic prostatitis, diagnosis of urethral stricture, meatal stenosis or bladder neck contracture, a damaged external urinary sphincter, stress urinary incontinence, postvoid residual urine greater than 300 mL or urinary retention, self-catheterization use or prior prostate surgery.Men receiving anticoagulants or bladder anticholinergics and those with severe cardiovascular disease were also excluded.The inclusion criteria and exclusion criteria for this study population are described in Fig.S1.Serum was separated by centrifugation at 4000 r/min for 10 min at 4°C and stored at -80°C until analysis.
Between March 2020 and December 2021, para-cancerous tissues as control subjects from eight PCa patients (mean age 71.75±2.06 years)were collected before radical prostatectomy and any therapeutic treatment.Nineteen patients with BPH (mean age 72.89±1.16 years)and eight controls were selected among subjects admitted to the same hospital during the same period, who were matched for ethnicity and age and were from the same geographical regions.All the patients signed the informed consent, and the demographic and clinical characteristics of the two groups are detailed in Table S1.Inclusion criteria and exclusion criteria for BPH samples have been described previously,and detailed information is provided in Fig.S2.The criteria for recruiting PCa patients were as follows: suspected to have PCa based on elevated serum PSA of 4 ng/mL and/or abnormal digital rectal examination (DRE).These subjects underwent systematic ultrasound-guided needle biopsies.
2.3.Clinical assessment
Weight and height were measured in lightly clad participants,and body mass index (BMI) was calculated.BMI = weight (kg)/height2(m).Prostate size at biopsy was recorded in the pathology records in several ways,prostate volume(PV),prostate dimensions in three axes, or prostate weight; in some instances, no data on prostate size were recorded.When PV data were unavailable, PV was estimated from the reported prostate dimensions as (dimension 1 × dimension 2 × dimension 3) × (3.14/6).
2.4.Clinical laboratory test
Total prostate-specific antigen(TPSA)and free prostate-specific antigen(FPSA)were measured using an automated analyser at the Affiliated Hospital of Xuzhou Medical University.Age, weight, and BMI were obtained from medical records.
2.5.Cell culture and transfection
The human prostate hyperplasia cell line BPH-1 was a kind gift from Nanjing Normal University (Nanjing, China).Cells were seeded into tissue culture dishes in Roswell Park Memorial Institute(RPMI)1,640 medium(normal glucose,11.2 mmol/L)with 10%heatinactivated fetal bovine serum(FBS)and 1%penicillin/streptomycin and were placed in a humidified incubator at 37°C and 5%CO2.The BPH cell model was induced using a previous method with minor modifications.BPH-1 cells were allowed to attach for 12 h and were then serum-starved for 8 h.BPH-1 cells were incubated with fresh medium containing 1% FBS and 5 nM DHT for 24 h.Subsequently,the medium was exchanged with medium containing 1% FBS,0.5-2 mM Met[17]and AICAR(1 mM)or CC(5 μM)[18].Cells were incubated in this medium for 24 h.
Specifically, targeted human YAP1 expression and scrambled control sequences were purchased from Gene Pharma Inc.(Shanghai,China).shRNA was used to decrease YAP1 expression in BPH-1 cells cultured with DHT.Prior to transfection, BPH-1 cells(30%-40%confluent)were washed with phosphate buffered saline(PBS),and the medium was replaced with serum-free medium.The shRNA-specific or control lentivirus vector for YAP1 was added to the cells.For YAP1 overexpression experiments, BPH-1 cells were divided into five groups,and the cells were transfected with a YAP1 overexpression lentivirus vector before stimulation with DHT and Met.More specifically, BPH-1 cells were grown to 30%-40%confluence and then infected for 48 h at 37°C in a humidified atmosphere of 5% CO2with lentivirus carrying the YAP1 gene.Lipofectamine 3000 (Thermo Fisher, Waltham, MA, USA) was used in lentivirus transfection, and a vector was used as a control.Following infection for 48 h, cells were selected with 2.0 μg/mL puromycin (Sigma-Aldrich).The shRNA sequences are shown in Table S2.The fluorescence intensity was observed under a fluorescence microscope, and the transfection efficiency was detected by western blotting.
2.6.Animals and treatments
Male Sprague-Dawley(SD)rats(150-180 g)were obtained from SIPPR-BK Lab Animal Co.,Ltd.(Shanghai,China).The rats were kept on a standard laboratory diet and water ad libitum and were adapted to the laboratory environment (20-26°C) under a 12 h light-dark cycle for one week before the experiment.The SD rat model of BPH was established by previous studies [19,20].After adaptation to the environment for 2 weeks, all rats weighed 250-300 g and were randomly divided into three groups (n = 6)that received the respective treatments daily for 4 weeks.The effective dosages of TP and Met were selected in accordance with reference [21-23].Normal control (NC) group: rats were administered sodium carboxymethylcellulose (CMC-Na) orally and injected with olive oil subcutaneously(s.c.)for 4 weeks.BPH group:rats were orally injected with CMC-Na and subcutaneously injected with TP (3 mg/kg, s.c.) dissolved in olive oil.Met group: rats were given 250 mg/kg Met by oral administration following the injection of TP.
The body weights(BWs)of the rats were recorded every week.Following the final injection and overnight fasting, animals were sacrificed under anaesthesia by intraperitoneal injection of chloral hydrate to collect blood samples from the aorta abdominalis.The tubes containing blood remained at room temperature for 0.5 h,and the blood was separated by centrifugation at 4,000 r/min for 10 min at 4°C.The supernatant was stored at -80°C for further assays.The intact prostate tissues were carefully harvested and measured immediately.The prostate weight index (PI) was calculated as the ratio of prostate weight (mg) to body weight (g).The prostate tissue was mainly divided into two parts:one was fixed in 10% neutral formalin and embedded in paraffin; the others were stored at -80°C for further analysis.
2.7.Prostate morphology assessment
The ventral prostate tissues were fixed in 4% formaldehyde immediately for 24 h and embedded in paraffin.Prostate samples were then paraffin-embedded and sectioned at 4 μm thickness.After dewaxing and rehydration, prostate sections were mounted on slides and stained with hematoxylin and eosin(H&E)for routine histological examination under a light microscope (Olympus,Tokyo, Japan).
2.8.Calculation of basic rat parameters
The prostate index (mg/g) was calculated as the ratio of the weight of the prostate and the body weight of the rats.Increase in PI(%)=100-(PI of treated group×100/PI of BPH group);recovery rate(%)=100-((PI of BPH group-PI of treated group)×100/PI of BPH group); growth inhibition (%) = 100 - ((PI of treated group-PI of control group)×100/(PI of BPH group-PI of control group)).These biochemical indices were measured to estimate the progression of BPH.
2.9.Cell counting kit-8 (CCK-8) assay
Cell viability was detected by CCK-8(DoJinDo,Shanghai,China)[24].BPH-1 cells were cultured in 96-well microplates at a density of 500 cells per well.Cells were incubated in 10 μL CCK-8 for 2 h.The absorbance value at 450 nm was read using a Varioskan LUX Multi-Mode Microplate Reader (Thermo Fisher).The experiment was repeated in triplicate to determine the mean optical density(OD) value.
2.10.5-Ethynyl-2′-deoxyuridine (EdU) assay
BPH-1 cells were cultured in 96-well plates at 2 ×104cells per well.According to the manual of the EdU labelling/detection kit(Ribobio, Guangzhou, China), 50 μM EdU labelling medium was added to the cell culture to allow incubation for 2 h at 37°C under 5%CO2after different treatments.The EdU assay was carried out as described previously.
2.11.Immunofluorescence (IF) analysis
Parts of the prostate samples were fixed in 4% paraformaldehyde and embedded in paraffin.BPH-1 cells cultured on glass coverslips were washed three times with cold PBS and fixed with cold methanol at -20°C for 20 min.Immunofluorescence staining was performed as described previously.The slides were incubated overnight at 4°C with the following primary antibodies:YAP1 (1:100 dilution), PCNA (1:200 dilution), and Ki67 (1:100 dilution).Subsequently,the cells were stained with 4',6-diamidino-2-phenylindole (DAPI; Beyotime) to detect cell nuclei.The coverslips were mounted onto glass slides, the tissue images were captured by confocal microscopy (Leica Stellaris 5, Leica Microsystems,Deerfield,IL,US),and the cell images were viewed with an Olympus BX43F fluorescence microscope.
2.12.Immunohistochemistry (IHC)
Prostate tissue sections of 4 μm thickness were deparaffinized in xylene, hydrated in graded alcohol and water, and subsequently placed in 3% H2O2for 20 min to eliminate endogenous peroxidase activity.After 30 min of pepsin antigen retrieval,the sections were washed with PBS for 3 min three times, blocked with 2% bovine serum albumin(BSA)for 0.5 h at room temperature,and incubated with primary antibodies against PCNA (1:600 dilution) and Ki67(1:100 dilution)at 37°C for 2 h or 4°C overnight.IHC was carried out as described previously[25].
2.13.Western blotting analysis
The preserved prostate tissue was weighed and homogenized using an electronic tissue homogenizer in RIPA lysis buffer(1:9)in an ice bath.BPH-1 cells were harvested in NP40 lysis buffer.Proteins were quantified and then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as previously described[26-28].The membranes were blocked in PBS containing 3% BSA for 1 h at room temperature and further incubated with special antibodies (1:1000) at 4°C overnight.After transferring proteins to a nitrocellulose transfer membrane, samples were incubated first with primary antibodies at 4°C for overnight and then with secondary antibodies at room temperature for 1 h.After scanning the blots, target bands were analysed using Image J software (National Institutes of Health,Bethesda, MD, USA).
2.14.Nuclear and cytoplasmic extraction
Nuclear extracts were prepared using an NE-PER Nuclear Cytoplasmic Extraction Reagent kit(Thermo-Scientific)according to the manufacturer's instructions [29].The resulting supernatant,constituting the nuclear extract, was used for subsequent western blot analysis.
2.15.Co-immunoprecipitation (Co-IP) assay
Co-IP analysis was carried out as described previously[28].Cell or tissue lysates adjusted to 1 mg/mL protein was precleared by IPgrade antibodies against YAP1 or TEAD4 or IgG (1:50 dilution).After gentle rocking at 4°C overnight, Protein A/G PLUS-agarose(Santa Cruz Biotechnology, Paso Robles CA, USA) was added to the lysate/antibody mixture and incubated with gentle agitation at 4°C overnight.The immunoprecipitants were collected by centrifugation, washed five times with cell lysis buffer, and then boiled for 5 min with the same volume of 2×loading buffer.Proteins were resolved by 10% SDS-PAGE and subjected to western blotting.
2.16.Ingenuity Pathway Analysis (IPA)
Based on the known interactions between genes and proteins collected by the Ingenuity Knowledge Base, IPA software (QIAGEN,Frederick, MD, USA), as an all-in-one integrated online analytics software,was used to predict putative gene networks and construct pathway analyses of BPH and AMP-activated protein kinase(AMPK).
2.17.Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of oestrogen
The abundance of oestrogens(E1 and E2)was assayed by LC-MS/MS as previously described [30].LC separation was performed using an AB Sciex ExionLC™100 HPLC System,and mass analysis was carried out with an AB Sciex 5,500 triple quadrupole mass spectrometer(Foster City,CA,USA).Data acquisition and quantification were conducted with AB Sciex Analyst® software.Chromatographic separation was performed at 40°C using a ZOEBAX Eclipse Plus C18column (2.1 mm × 100 mm, i.d.; 1.8 μm), which was protected by a Security Guard (ZOEBAX Eclipse Plus C18,2.1 mm × 5 mm, i.d.; 1.8 μm) (Agilent Technologies, Folsom, CA,USA).The mobile phase flow rate was 0.3 mL/min, and the mobile phases were 0.1% formic acid in water (mobile phase A) and 0.1%formic acid in acetonitrile (mobile phase B).The LC gradient conditions were as follows: 70% B for 0-2.0 min, 70%-80% B for 2.0-3.5 min,80%B for 3.5-11.0 min,80%-70%B for 11.0-11.5 min,and 30% B for 11.5-17.0 min.D5-E2 was used as the internal standard (IS) for the measured oestrogens.The autosampler temperature was 4°C, and the injection volume was 20 μL.
Mass detection was carried out using positive ionization in multiple reaction monitoring mode(MRM).The nebulizer gas(GS1)was nitrogen at 55 psi, and the auxiliary gas (GS2) was nitrogen maintained at 55 psi.The collision (CAD) and curtain gas (CUR)values were set to 8 and 35 psi,respectively.The ion spray voltage was set to 5,500 V in positive mode, and the heater temperature was 550°C.The system control and data analysis were performed by AB Sciex Analyst®software.E1 and E2 were resolved by the established LC-MS/MS method (Fig.S3A).
For preparation of serum, aliquots of 400 μL of human or rat serum and 10 μL of IS(D5-E2,0.3 μmol/L)were vortexed for 1 min and extracted in 1.2 mL ethyl acetate for 10 min.Then,the samples were centrifuged for 5 min at 13,000 r/min and 4°C.The supernatant was spun and dried at 40°C in a vacuum dryer, and the remaining samples in microcentrifuge tubes were extracted again.The upper layer was pipetted carefully into a microcentrifuge tube,dried using a vacuum dryer, and redissolved in 0.1 mol/L Na2CO3/NaHCO3buffer solution (50 μL) and 1 g/L dansyl chloride solution(50 μL).The samples were then heated at 70°C for 15 min and centrifuged at 13,000 r/min at 4°C for 15 min.The supernatant was transferred to a vial for LC-MS/MS detection.
For preparation of tissue, rat or human prostate samples were homogenized in cold PBS buffer (10 mg tissue/100 μL PBS) on ice.Samples were stored at-80°C until use.Aliquots of 400 μL of tissue mixture and 10 μL of IS were transferred to microcentrifuge tubes and vortexed for 1 min.The remaining steps were the same as the preparation of serum.
2.18.High performance liquid chromatogram-tandem mass spectrometry (HPLC-MS/MS) analysis of androgen
For HPLC-MS/MS analysis of androgen,please see the extended methods in Tables S3-8 and Figs.S3 and 4 [31].
2.19.Targeted metabolomics analysis
Principal component analysis (PCA) was used to obtain the overview changes in metabolic patterns of prostate tissues among BPH and normal men using SIMCA 14.0 software (Umetrics, San Jose,CA,USA).Moreover,orthogonal projection to latent structures discriminant analysis (OPLS-DA) was employed to maximize separations between the two groups and identify important metabolites contributing to the separations using SIMCA 14.0.The variable importance for the projection(VIP)values summarized the overall contribution of each X-variable and estimated the importance of each variable.The variables with a VIP value exceeding 1.0 were considered significant metabolites.

Fig.1.Disruption of sex steroid hormones in clinical samples of benign prostate hyperplasia(BPH) patients.(A) Hematoxylin and eosin (H&E) staining of prostate sections from BPH patients(n=6).(B)The relative protein levels of proliferating cell nuclear antigen(PCNA)in clinical prostate tissue by immunohistochemistry(IHC)staining and western blot analysis(n=6).(C)The relative protein levels of Ki67 in clinical prostate tissue by IHC staining(n=6).(D)The relative protein levels of cell cycle protein 1(CyclinD1)in clinical prostate tissue by western blot analysis(n=6).(E)Hierarchical clustered heat map of steroid hormones in the serum of normal men and BPH patients(n=100).(F)3D scatter plot of steroid hormones in the serum of normal men and BPH patients.(G)Variable importance for the projection(VIP)value of steroid hormones in the serum of normal men and BPH patients.(H)Hierarchical clustered heat maps of steroid hormones in the prostate tissue of normal men(n=8)and BPH patients(n=19).(I)3D scatter plot of steroid hormones in the prostate tissue of normal men and BPH patients.(J)VIP value of steroid hormones in the prostate of normal men and BPH patients.Arrows indicate anatomical regions:prostatic epithelium or the acini(yellow arrow) and prostatic stroma (blue arrow).Data are mean ± standard error of mean (SEM).**P <0.01, compared to N as indicated.A: androsterone; A4: androstenedione; DHEA:dehydroepiandrosterone; DHT: dihydrotestosterone; E1: estrone; E2: estradiol; T: testosterone.
2.20.Ethics
The human study was registered in the Chinese Clinical Trial Register(No.ChiCTR1800020339)and was performed in accordance with the Helsinki Declaration; The protocol was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Approval number: XYFY2018-KL093).Animal experiments were conducted in accordance with the principles provided by the National Institutes of Health(NIH)Guideline for the Care and Use of Laboratory Animals.Approval to proceed with this experiment was issued by the Animal Ethics Committee of Xuzhou Medical University(Approval number:201808A168),and protocols also conformed to the Guidelines for Ethical Conduct in the Care and Use of Animals.
2.21.Statistical analysis
All experimental data are expressed as the means ± standard error of means (SEMs).Differences between the two groups were tested by Student's t-test.Significance among multiple groups was tested using one-way analysis of variance (ANOVA).Differences were considered significant if P <0.05.The statistical analyses were performed using GraphPad Prism software version 9.0 (GraphPad Software, Inc., San Diego, CA, USA) for Windows.The obtained figures were analysed using Origin 2022 software(Electronic Arts,Redwood City, CA, USA).Hierarchical clustered heatmap analysis and heatmap analysis via Pearson correlation were completed using Wekemo BioinCloud (https://www.bioincloud.tech).Heatmap analysis via Pearson correlation was plotted by https://www.bioinformatics.com.cn (last accessed on Oct 31, 2022), an online platform for data analysis and visualization.Simple linear regression was performed using GraphPad Prism software version 9.0.
3.Results
3.1.Sex steroid hormone compositions were dysregulated in the serum and prostate of BPH patients
We quantitatively profiled sex steroid hormones in the prostate of BPH patients (n = 19) and matched healthy men (NC group,n = 8), including five androgens and two oestrogens.The demographic and clinical characteristics of these two groups are detailed in Table S8.Pathological analysis showed that compared with healthy men, BPH patients presented with significant thickening, hypertrophy, and hyperplasia with papillary projections in the lining epithelium or the acini (yellow arrow) and prostatic stroma (blue arrow) (Fig.1A).Significantly increased protein expression of PCNA and Ki67 could be observed in the PEC(yellow arrow)and prostatic stroma(blue arrow)of BPH patients compared with healthy men (yellow arrow, Figs.1B and C).As an important regulatory protein of the cell cycle, the protein expression of CyclinD1 also increased in the prostate tissue of BPH patients,suggesting that there was prostate epithelial proliferation in BPH patients (Fig.1D).
Hierarchical clustered heatmap analysis of sex steroid hormones in clinical plasma showed that the BPH group and NC group formed an independent cluster (Fig.1E).The 3D scatter plot of OPLS-DA showed that the sex steroid hormone metabolic profile of the NC group was clearly separated from that of the BPH group, and DHT(VIP value >1, P <0.05) was the major contributor to intergroup differentiation and was selected as the most sensitive sex steroid hormone in the plasma of BPH patients(Figs.1F and G).Hierarchical clustered heatmap analysis and OPLS-DA of the prostate collectively showed that there was a clear separation of metabolic profiles of sex steroid hormones between the NC group and BPH group,and DHT(VIP value >1,P <0.05)was also the major contributor to the dysregulated sex steroid hormones in the prostate of BPH patients (Figs.1H-J).All these data demonstrated that BPH patients had disrupted sex steroid hormone compositions in the serum and prostate, and DHT was responsible for a large proportion of the dysregulated sex steroid hormones.
3.2.DHT levels had a close association with the clinical characteristics of BPH patients
The relationship between clinical markers of BPH and sex steroid hormones was examined by using a multivariate correlation analysis.The results showed that sex steroid hormones were closely associated with BPH, and DHT had the strongest positive correlation with FPSA, TPSA, and PV (Fig.2A).Based on the abovementioned findings, the levels of DHT were simultaneously quantified in the serum and prostate of BPH patients.Compared with healthy men, DHT levels were markedly increased in the serum of BPH patients, and they also showed significantly increased DHT levels in the prostate(Figs.2B and C).Along with the elevated DHT levels in the prostate,upregulated protein expression of AR could be observed in the PEC of BPH patients, as demonstrated by IHC staining and western blot analysis(Fig.2D).Further correlation analysis showed that DHT levels had significant positive relationships with FPSA(R2=0.344,P <0.01),TPSA(R2=0.337,P <0.01),and PV(R2=0.358,P <0.01)in BPH patients by using linear regression analysis, suggesting that DHT might positively participate in the process of BPH (Fig.2E).Taken together, these results suggested that DHT might be the most important promoter in the pathological process of BPH.
3.3.Met reversed the dysregulated steroid hormone-induced PEC proliferation of BPH in vivo and in vitro
To explore the effect of Met on BPH in vivo, experiments were carried out in TP-treated rats.The basic parameters of the rats are shown in Table 1.Met treatment decreased the rat weight of the BPH group,and the significantly greater PW,PV,and PI of BPH rats were markedly reduced by Met treatment.Compared with the BPH group, the inhibition percentage of PI was 24% in the Met group,and the alleviation of the increased prostate weight index (%) and recovery rate (%) was 92.64% and 7.36%, respectively, after Met treatment.H&E staining showed that the obvious thickening, hypertrophy, and hyperplasia with papillary projections of PEC were all ameliorated by Met in BPH rats(Arrow,Fig.3A).Met treatment reduced the elevated protein expression of PCNA and Ki67 in the PEC of BPH rats (Arrow, Figs.3B and C).
The LC-MS/MS data showed significant changes in sex steroid hormone metabolic profiles among the NC,BPH and Met groups in the serum and prostate of rats.Hierarchical clustered heatmap analysis of serum showed that the NC group clustered with the Met treatment groups, while the BPH group formed an independent cluster (Fig.3D).According to OPLS-DA, BPH caused a significant perturbation of sex steroid hormone profiles in rat serum, which were restored by Met treatment to a certain extent (Fig.3E).The simultaneous quantification of DHT showed that its elevated levels were downregulated by Met treatment in the serum of BPH rats(Fig.3F).Subsequently,hierarchical clustered heatmap analysis and OPLS-DA of the prostate collectively suggested that Met could recover the disrupted sex steroid hormones via the facilitation of sex steroid hormone profile reprogramming (Figs.3G and H).Consistent with serum,Met treatment decreased DHT levels in the prostate of BPH rats,and it also decreased AR protein expression in the PEC of BPH rats(Figs.3I and J).
Along with the upregulated protein expression of AR,our in vitro study showed that DHT dose-dependently upregulated the protein expression of PCNA and Ki67 in BPH-1 cells (Figs.4A-C).EdU and CCK-8 assays showed that DHT could induce BPH-1 cell proliferation to varying degrees (Figs.4D and E).In addition, increased protein expression of CyclinD1 was observed in DHT-treated BPH-1 cells compared with normal cells (Fig.4F).Accompanying the increased phosphorylation of AMPK and decreased protein expression of AR,Met downregulated the increased expression of PCNA and Ki67 in DHT-cultured BPH-1 cells (Figs.4G-J).Met treatment could also inhibit the proliferation of DHT-induced BPH-1 cells, as demonstrated by EdU assay and CCK-8 assay(Figs.4K and L).In addition,the elevated protein expression of CyclinD1 induced by DHT was also downregulated by Met (Fig.4M).Taken together, our in vivo and in vitro data demonstrated that Met inhibited the dysregulated steroid hormone-induced PEC proliferation of BPH,especially DHT.
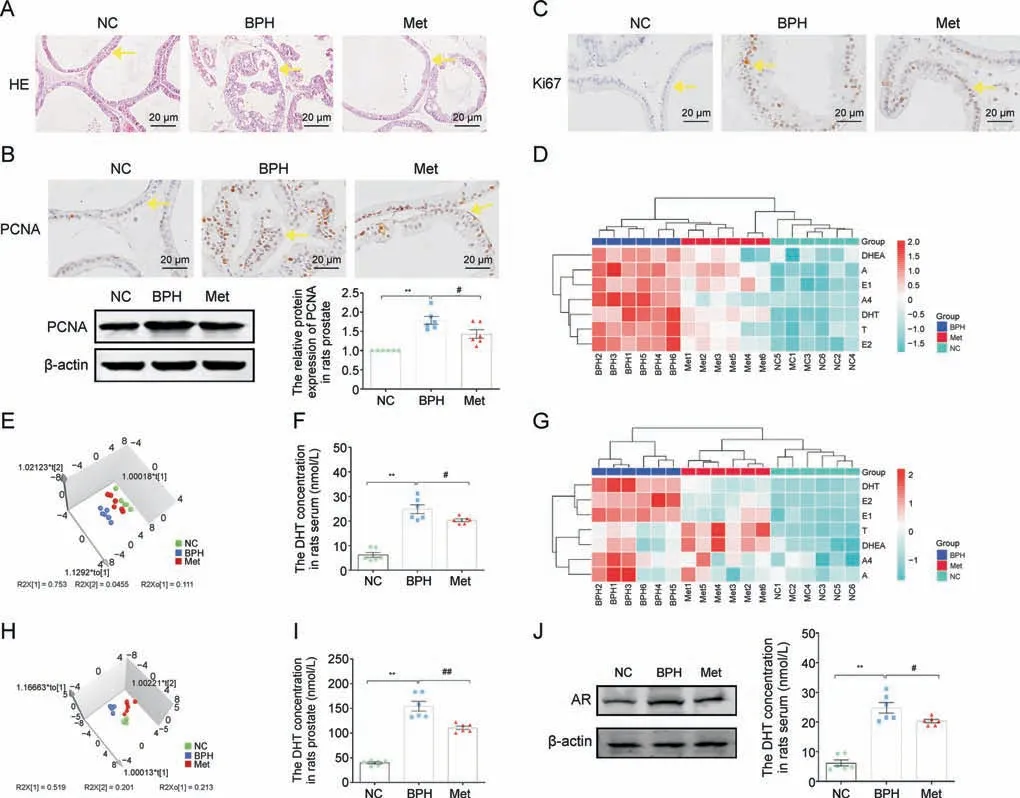
Fig.3.Metformin (Met) inhibited prostatic epithelial cell (PEC) proliferation and restored disrupted steroid hormones in benign prostate hyperplasia (BPH) rats.(A) Hematoxylin and eosin(H&E)staining of rat prostate tissue.(B)The relative protein levels of proliferating cell nuclear antigen(PCNA)in the tissue of rat prostate by immunohistochemistry(IHC)staining and western blot analysis.(C)The relative protein levels of Ki67 in rat prostate tissue by IHC staining.(D)Hierarchical clustered heat map of steroid hormones in the serum of rat.(E)3D scatter plot of steroid hormones in the serum of rats.(F)Quantitative analysis of dihydrotestosterone(DHT)levels in the serum of rats.(G)Hierarchical clustered heat map of steroid hormones in the tissue of rat prostate.(H)3D scatter plot of steroid hormones in the tissue of rat prostate.(I)Quantitative analysis of DHT levels in the prostate tissue of rats.(J)The relative protein levels of androgen receptor(AR)in rat prostate.Arrows indicate anatomical regions:prostatic epithelium or the acini(yellow arrow).Data are mean±standard error of mean (SEM) for rat groups of six.**P <0.01, compared to normal control (NC) group as indicated; #P <0.05, ##P <0.01, compared to BPH as indicated.A: androsterone; A4: androstenedione; DHEA: dehydroepiandrosterone; E1: estrone; E2: estradiol; T: testosterone.
3.4.Activated AMPK was critical for Met's anti-proliferation in DHT-cultured BPH-1 cells
Met, a small molecule antihyperglycaemic agent, is an AMPK activator [32].To identify the antiproliferative effect of AMPK in DHT-treated BPH-1 cells,we first selected the AMPK agonist AICAR in the following studies.AICAR blocked the increased protein expression of AR in DHT-treated BPH-1 cells(Fig.5A).The elevated protein expression of PCNA and Ki67 was downregulated by AICAR in DHT-cultured BPH-1 cells(Figs.5B and C).The results of the EdU assay and CCK-8 assay demonstrated that AICAR exerted an antiproliferative effect against DHT-cultured BPH-1 cells (Figs.5D and E).Meanwhile, AICAR also downregulated the increased protein expression of CyclinD1 in DHT-cultured BPH-1 cells (Fig.5F).
Subsequently,the AMPK antagonist CC was used to identify the indispensable role of activated AMPK in Met's antiproliferative effects against DHT-cultured BPH-1 cells.CC blocked the downregulated protein expression of AR in Met-treated BPH-1 cells cultured with DHT(Fig.5G).The downregulatory effects of Met on proliferation markers were blocked by the follow-up treatment of CC in DHT-cultured BPH-1 cells,regardless of PCNA or Ki67(Figs.5H and I).In addition, the antiproliferative effect of Met against DHTcultured BPH-1 cells was also inhibited by CC, which also upregulated the protein expression of CyclinD1 compared with that in the Met + DHT group, suggesting that inactivated AMPK blocked the antiproliferative effect of Met on DHT-cultured BPH-1 cells (Figs.5J-L).These results indicated that activated AMPK was indispensable for Met's antiproliferative effect in DHT-cultured BPH-1 cells by inhibiting AR.
3.5.Identification of yes-associated protein 1 as a candidate mediator of prostate epithelial proliferation in BPH
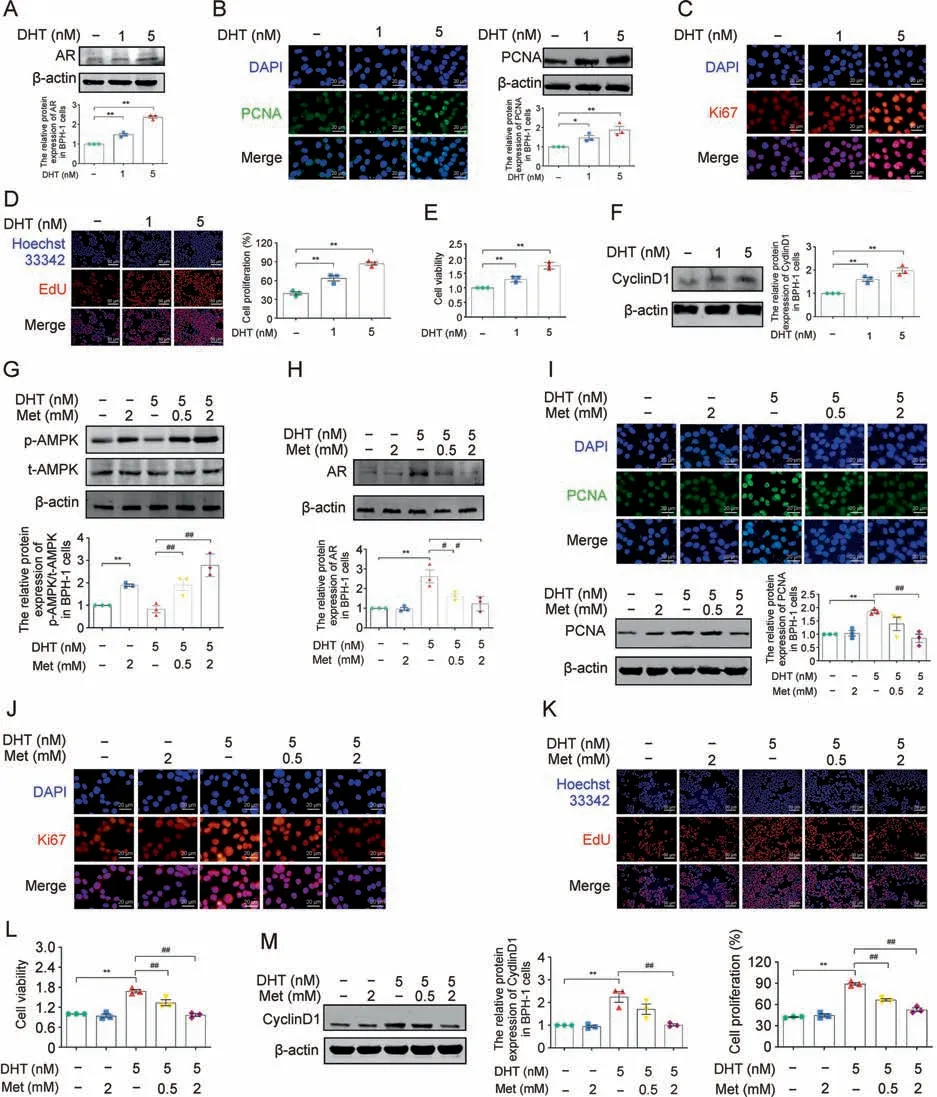
Fig.4.Effects of metformin (Met) on dihydrotestosterone (DHT)-induced proliferation in vitro.(A) The relative protein levels of androgen receptor (AR) in benign prostate hyperplasia(BPH)-1 cells cultured with DHT.(B)The relative protein levels of proliferating cell nuclear antigen(PCNA)in BPH-1 cells cultured with DHT by immunofluorescence(IF)and western blot analysis.(C)The relative protein levels of Ki67 in BPH-1 cells cultured with DHT by IF.(D)BPH-1 cell proliferation activity detected by 5-Ethynyl-20-deoxyuridine(EdU) assay.(E) Cell viability in DHT treated BPH-1 cells.(F) The relative protein levels of cell cycle protein 1(CyclinD1) in BPH-1 cells cultured with DHT.(G)The relative protein levels of phosphorylation-AMP-activated protein kinase(p-AMPK)in BPH-1 cells cultured with DHT and Met.(H)The relative protein levels of AR in BPH-1 cells cultured with DHT and Met.(I) The relative protein levels of PCNA in BPH-1 cells co-cultured with DHT and Met by IF and western blot analysis.(J) The relative protein levels of Ki67 in BPH-1 cells cultured with DHT and Met by IF.(K)BPH-1 cell proliferation activity detected by EdU assay.(L)Cell viability in DHT and Met cultured BPH-1 cells.(M)The relative protein levels of CyclinD1 in BPH-1 cells cultured with DHT and Met.Data are mean±standard error of mean(SEM)for cell groups of three.*P <0.05, **P <0.01,compared to normal control(NC)as indicated; #P <0.05, ##P <0.01, compared to DHT as indicated.t-AMPK: total-AMP-activated protein kinase.
We classified AMPK into genes and chemicals based on ingenuity pathway analysis (IPA, Ingenuity Systems).IPA revealed that AMPK positively regulated multiple proteins and negatively regulated yes-associated protein 1 (YAP1) (Fig.6A).YAP1 plays a central role in regulating cell growth and tissue homeostasis by binding to the transcription factor TEA/ATS domain 4(TEAD4),and our previous study found that the activated YAP1/TEAD4 signaling pathway is involved in mesangial cell proliferation in early diabetic nephropathy(DN)[25].It was also reported that AR regulated YAP1 nuclear entry in human prostate cancer cell lines,and the activated AMPK induced by Met could inhibit cell proliferation via the YAP1/TEAD4 axis in bladder cancer cells.The results of disease and functions of IPA revealed that proliferation of multiple cells could be regulated by YAP1, which is negatively regulated by AMPK,including epithelial cell lines (Fig.6B).The regulatory networks of IPA showed that although multiple proteins and factors strung BPH and AMPK together, the relevance of YAP1 and BPH was not revealed (Figs.6C and D).

Fig.5.Inhibition of 5'-monophosphate (AMP)-activated protein kinase (AMPK) blocked anti-proliferative effects of metformin (Met) in vitro.(A) The relative protein levels of androgen receptor(AR)in benign prostate hyperplasia(BPH)-1 cells cultured with dihydrotestosterone(DHT)and 5-Aminoimidazole-4-carboxamide1-β-D-ribofuranoside(AICAR).(B)The relative protein levels of proliferating cell nuclear antigen(PCNA)in BPH-1 cells cultured with DHT and AICAR by immunofluorescence(IF)and western blot analysis.(C)The relative protein levels of Ki67 in BPH-1 cells cultured with DHT and AICAR by IF.(D) BPH-1 cell proliferation activity detected by 5-ethynyl-2′-deoxyuridine (EdU) assay.(E) Cell viability in BPH-1 cells cultured with DHT and AICAR.(F)The relative protein levels of cell cycle protein 1(CyclinD1)in BPH-1 cells cultured with DHT and AICAR.(G)The relative protein levels of AR in BPH-1 cells cultured with DHT, Met and compound C (CC).(H) The relative protein levels of PCNA in BPH-1 cells cultured with DHT, Met and CC by IF and western blot analysis.(I) The relative protein levels of Ki67 in BPH-1 cells cultured with DHT, Met and CC by IF.(J) BPH-1 cell proliferation activity detected by EdU assay.(K) Cell viability after treated with CC in BPH-1 cells.(L)The relative protein levels of CyclinD1 in BPH-1 cells cultured with DHT,Met and CC.Data are mean±standard error of mean(SEM)for cell groups of three.**P <0.01,compared to normal control(NC)as indicated; #P <0.05, ##P <0.01,compared to DHT as indicated; &P <0.05, &&P <0.01,compared to DHT+Met as indicated.
To investigate whether YAP1 is a candidate mediator of AMPK in prostate epithelial proliferation in BPH, we first examined the protein expression of YAP1 in BPH patients.Compared with healthy men,the protein expression of total YAP1 increased in the prostate of BPH patients, who also showed a significant redistribution of YAP1 from the cytoplasmic to the nuclear fraction(Figs.6E and F).Obvious increased TEAD4 expression was also observed in the prostate of BPH patients compared with healthy men(Fig.6G).Further in vitro studies showed that the increased total YAP1 expression and its nuclear translocation were enhanced with the dosage of DHT in BPH-1 cells (Figs.6H and I).DHT treatment also upregulated TEAD4 expression in BPH-1 cells(Fig.6J).All these data suggested that YAP1/TEAD4 signaling might be a candidate mediator of DHT/AR axis-induced PEC proliferation in BPH.

Fig.6.The expression of yes-associated protein 1(YAP1)in the prostate tissue of benign prostate hyperplasia(BPH)patients and dihydrotestosterone(DHT)-cultured BPH-1 cells.(A)The predicted downstream molecule of 5'-monophosphate(AMP)-activated protein kinase(AMPK)mechanistic networks based on ingenuity pathway analysis(IPA)analysis.(B)The proliferation regulation effect of AMPK and YAP1.(C) Illustration of the “BPH” and “AMPK” functional networks based on IPA analysis.Figure legend illustrates the relationship between molecules within the network and their activation state.Orange color indicates activation while blue color indicate suppression.(D)The relationship of YAP1 and AMPK on BPH.(E)The relative protein levels of total YAP1 in the prostate tissue of normal men and BPH patients by immunofluorescence(IF)and western blot analysis.(n=6).(F)The relative protein levels of cytoplasm(c)-YAP1 and cytoplasm(n)-YAP1 in the prostate tissue of normal men and BPH patients(n=6).(G)The relative protein levels of TEA domain transcription factor 4(TEAD4)in the prostate tissue of normal men and BPH patients(n=6).(H)The relative protein levels of total YAP1 in BPH-1 cells cultured with DHT by IF and western blot analysis.(I)The relative protein levels of c-YAP1 and n-YAP1 in BPH-1 cells cultured with DHT.(J)The relative protein levels of TEAD4 in BPH-1 cells cultured with DHT.Data are mean± standard error of mean (SEM) for cell groups of three.*P <0.05, **P <0.01, compared to N or normal control (NC) as indicated.t-YAP1: total-yes-associated protein 1.
3.6.Met suppressed DHT-induced formation of the YAP1-TEAD4 heterodimer via AMPK
To confirm whether the YAP1/TEAD4 signaling pathway is involved in Met's antiproliferative effect against DHT-cultured BPH-1 cells, the expression of the YAP1/TEAD4 signaling pathway was examined first in Met-treated BPH rats.Compared with the NC group, upregulated protein expression of total YAP1 could be observed in the prostate of BPH rats, while this uptrend was alleviated by Met (Fig.7A).Meanwhile, the promoted nuclear translocation of YAP1 and the increased protein expression of TEAD4 were all inhibited by Met in the prostate of BPH rats(Figs.7B and C).Furthermore, co-IP was used to detect the binding of YAP1 and TEAD4,and the results showed that compared with normal control rats,the increased formation of the YAP1-TEAD4 heterodimer was reduced by Met treatment in the prostate of BPH rats, which also showed decreased protein expression of CyclinD1 after Met treatment (Figs.7D and E).Further in vitro studies showed that as the dose increased, Met significantly decreased the expression of total YAP1 and blocked the redistribution of YAP1 from the cytoplasmic to the nuclear fraction in DHT-cultured BPH-1 cells(Figs.7F and G).Met also downregulated the protein expression of TEAD4 and inhibited the formation of the YAP1-TEAD4 heterodimer in DHTcultured BPH-1 cells (Figs.7H and I).
AICAR downregulated total YAP1 expression and its nuclear translocation in DHT-cultured BPH-1 cells, and it also decreased TEAD4 protein expression in DHT-cultured BPH-1 cells (Figs.8A-C).The increased formation of the YAP1-TEAD4 heterodimer induced by DHT was inhibited by AICAR in BPH-1 cells (Fig.8D).However,the inhibitory effect of Met on total YAP1 expression and subsequent nuclear translocation induced by DHT were all blocked by CC compared with those in the Met+DHT group(Figs.8E and F).Compared with the Met + DHT group, the CC + Met + DHT group showed a significant increase in TEAD4 expression (Fig.8G).The inhibited formation of the YAP1-TEAD4 heterodimer induced by Met was restored by follow-up treatment with CC in DHT-cultured BPH-1 cells, indicating that Met downregulated the DHT/AR axismediated YAP1/TEAD4 signalling pathway and suppressed the formation of the YAP1-TEAD4 heterodimer in DHT-cultured BPH-1 cells by activating AMPK (Fig.8H).

Fig.7.Effects of metformin (Met) on yes-associated protein 1 (YAP1) expression in the prostate tissue of benign prostate hyperplasia (BPH) rats and dihydrotestosterone (DHT)cultured BPH-1 cells.(A) The relative protein levels of total YAP1 in the prostate of rats by immunofluorescence(IF) and western blot analysis.(B) The relative protein levels of c-YAP1 and nuclear(n)-YAP1 in the tissue of rat prostate.(C)The relative protein levels of TEA domain transcription factor 4(TEAD4)in rat prostate by IF and western blot analysis.(D)The interactions between YAP1 and TEAD4 were detected by co-immunoprecipitation (Co-IP) in rat prostate.(E) The relative protein levels of cell cycle protein 1(CyclinD1) in rat prostate.(F)The relative protein levels of total YAP1 in BPH-1 cells cultured with DHT and Met by IF and western blot analysis.(G)The relative protein levels of cell(c)-YAP1 and n-YAP1 in BPH-1 cells cultured with DHT and Met.(H)The relative protein levels of TEAD4 in BPH-1 cells cultured with DHT and Met.(I)The interactions between YAP1 and TEAD4 were detected by Co-IP in BPH-1 cells.Data are mean±standard error of mean(SEM)for rat groups of six and cell groups of three.*P <0.05, **P <0.01,compared to normal control(NC) as indicated; #P <0.05, ##P <0.01, compared to BPH or DHT as indicated.t-YAP1: total-yes-associated protein 1.
3.7.The YAP1-TEAD4 heterodimer is involved in the antiproliferative effect of Met in vitro
Concerning the involvement of the YAP1-TEAD4 heterodimer in Met's antiproliferative effect on DHT-cultured BPH-1 cells,the role of the YAP1-TEAD4 heterodimer requires further study.First,small interfering RNA was used to decrease YAP1 expression in BPH-1 cells cultured with DHT.YAP1 knockdown blocked its nuclear translocation in DHT-cultured BPH-1 cells,together with decreased TEAD4 expression (Figs.9A and B).Further, Co-IP assays were conducted to confirm the exact mechanism of this phenotype.YAP1 is directly bound to TEAD4, while YAP1 knockdown decreased the binding of YAP1 and TEAD4 in DHT-cultured BPH-1 cells (Fig.9C).YAP1 knockdown not only resulted in the decreased expression of PCNA and Ki67 but also inhibited the proliferation of DHT-cultured BPH-1 cells (Figs.9D-G).After silencing YAP1, the expression of CyclinD1 was significantly decreased in DHT-treated BPH-1 cells(Fig.9H).These results indicated that YAP1 knockdown inhibited the formation of the YAP1-TEAD4 heterodimer and subsequently inhibited the proliferation of DHT-cultured BPH-1 cells.

Fig.8.Inhibition of 5'-monophosphate(AMP)-activated protein kinase(AMPK)blocked anti-proliferative effects of metformin(Met)in vitro.(A)The relative protein levels of total yes-associated protein 1(YAP1)in benign prostate hyperplasia (BPH)-1 cells cultured with dihydrotestosterone (DHT) and 5-Aminoimidazole-4-carboxamide1-β-D-ribofuranoside(AICAR) by immunofluorescence (IF) and western blot analysis.(B) The relative protein levels of cytoplasm (c)-YAP1 and nuclear (n)-YAP1 in BPH-1 cells cultured with DHT and AICAR.(C)The relative protein levels of TEA domain transcription factor 4(TEAD4)in BPH-1 cells cultured with DHT and AICAR.(D)The interactions between YAP1 and TEAD4 were detected by co-immunoprecipitation (Co-IP) in BPH-1 cells cultured with DHT and AICAR.(E) The relative protein levels of total YAP1 in BPH-1 cells cultured with DHT, Met and compound C(CC)by IF and western blot analysis.(F)The relative protein levels of c-YAP1 and n-YAP1 in BPH-1 cells cultured with DHT,Met and CC.(G)The relative protein levels of TEAD4 in BPH-1 cells cultured with DHT,Met and CC.(H)The interactions between YAP1 and TEAD4 were detected by Co-IP in BPH-1 cells cultured with DHT,Met and CC.Data are mean ± standard error of mean (SEM) for cell groups of three.*P <0.05, **P <0.01, compared to normal control (NC) as indicated; #P <0.05, ##P <0.01, compared to DHT as indicated; &P <0.05, &&P <0.01, compared to DHT + Met as indicated.t-YAP1: total-yes-associated protein 1.
To confirm the indispensable role of the YAP1-TEAD4 heterodimer in the antiproliferative effect of Met,a YAP1 overexpression lentiviral vector was used to increase YAP1 expression in DHT-cultured BPH-1 cells.The blocking effect of Met on YAP1 nuclear translocation was resisted by YAP1 overexpression in DHTcultured BPH-1 cells, along with the upregulated protein expression of TEAD4(Figs.10A and B).Meanwhile,the decreased binding of YAP1 and TEAD4 induced by Met was also upregulated by YAP1 overexpression in DHT-cultured BPH-1 cells when compared with the cotransfected empty vector plasmid group (Fig.10C).The results of IF and western blotting showed that the downregulation effect of Met on the protein expression of PCNA and Ki67 was interrupted by YAP1 overexpression in DHT-cultured BPH-1 cells,while the regulatory effect of Met on the protein expression of these two proliferation markers was unaffected by cotransfection with empty vector plasmid(Figs.10D and E).However,although the antiproliferative effect of Met on DHT-cultured BPH-1 cells was not affected by cotransfection with empty vector plasmid, it was blocked by YAP1 overexpression, as demonstrated by EdU assay and CCK-8 assay (Figs.10F and G).The downregulated protein expression of CyclinD1 induced by Met was significantly increased by YAP1 overexpression in DHT-cultured BPH-1 cells, indicating that YAP1 overexpression recovered the formation of the YAP1-TEAD4 heterodimer and subsequently inhibited the antiproliferative effect of Met in DHT-cultured BPH-1 cells (Fig.10H).Taken together, the YAP1-TEAD4 heterodimer is involved in Met's antiproliferative effect on DHT-cultured BPH-1 cells.
4.Discussion
In addition to migration, uncontrolled proliferation of PEC increases the risk of development and progression of BPH[33].It was reported that the proliferation of PEC was influenced by sex hormones, resulting in prostate enlargement, especially the dysregulated plasma levels of T and E2 [34].The human body has sex hormone metabolic profiles, and the ratio of E2 to T was used to reflect the balance of androgens and oestrogens in vivo.However,the effects of BPH on sex hormone metabolic profiles have not been fully disclosed.We first demonstrated that dysregulated sex hormones, especially DHT might participate in the pathological process of BPH by using self-established LC-MS/MS and targeted metabonomic analysis.
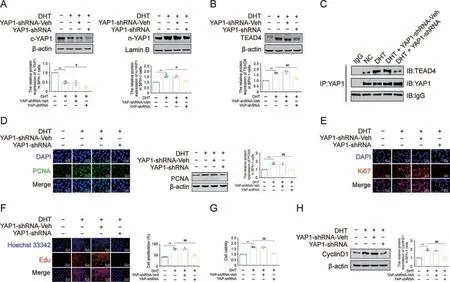
Fig.9.Knocking down yes-associated protein 1(YAP1)inhibited dihydrotestosterone(DHT)-induced proliferation in vitro.(A)The relative protein levels of cytoplasm(c)-YAP1 and nuclear (n)-YAP1 in benign prostate hyperplasia (BPH)-1 cells.(B) The relative protein levels of TEA domain transcription factor 4 (TEAD4) in BPH-1 cells.(C) The interactions between YAP1 and TEAD4 were detected by co-immunoprecipitation(Co-IP)in BPH-1 cells.(D)The relative protein levels of proliferating cell nuclear antigen(PCNA)in BPH-1 cells by immunofluorescence (IF) and western blot analysis.(E) The relative protein levels of Ki67 in BPH-1 cells by IF.(F) BPH-1 cell proliferation activity detected by 5-ethynyl-2′-deoxyuridine (EdU) assay.(G) Cell viability of BPH-1 cells.(H) The relative protein levels of cell cycle protein 1(CyclinD1) in BPH-1 cells.Data are mean ± standard error of mean(SEM), for cell groups of three.**P <0.01, compared to normal control (NC) as indicated; #P <0.05, ##P <0.01, compared to DHT as indicated.Met: metformin; t-YAP1: total-yesassociated protein 1; YAP1-shRNA, yes-associated protein 1 knockdown; YAP1-shRNA-Veh, yes-associated protein 1 knockdown virus empty.
The main drugs approved for the current treatment of BPH symptoms are alpha-blockers, 5ARIs, PDE5 inhibitors, β3-receptor agonists and so on [35].However, a significant proportion of men go on to need surgical intervention because of the most important adverse effects of these drugs [36].A study showed that Met treatment might be beneficial for PCOS patients by improving serum steroid hormones in DHT-treated PCOS mice, including progesterone, oestradiol and DHT [37].Met could reduce T and oestradiol levels in the serum of nondiabetic women with breast cancer [12].There is a molecular link between androgen and Met activities in the PCOS endometrium, and Met inhibits the proliferation of multiple tumor cells[38].Based on the above findings,we carried out pharmacodynamic evaluation of Met in BPH rats.Previous studies have confirmed the antiproliferative effects of Met on colorectal tumorigenesis by using a dosage of 250 mg/kg in rodents [23].It was determined that the plasma concentration of Met by administration of a dose of 250 mg/kg Met in rodents was achieved in routine diabetes treatment [39,40].Thus, we selected 250 mg/kg Met in our in vivo studies.Consistent with previous reports,our study identified that activating AMPK by Met inhibited PEC proliferation in BPH rats (Fig.S5) [41].At the same time, our data showed that the dysregulated sex steroid hormone was recovered both in the serum and prostate of BPH rats,in which the increased DHT levels were downregulated by Met treatment.Androgens exert their function by binding to AR, and pharmacodynamic studies of BPH have shown that the protein expression of AR is downregulated in the prostate of BPH rats and DHT-treated BPH-1 cells after drug treatment [42-44].In our study, along with the downregulated levels of DHT,the upregulated protein expression of AR was also decreased by Met in BPH rats and DHT-treated BPH-1 cells,indicating that the levels of DHT might be crucial in the antiproliferative effect of Met against BPH via AR.
DHT might promote prostate cell growth, resulting in hyperplasia.BPH-1 cells were stimulated by DHT to verify the role of DHT in PEC proliferation.Met(0.5-10 mM)inhibited the proliferation of insulin-like growth factor 1 (IGF-1)-stimulated BPH-1 cells and P69 cells in a dose-dependent manner, and 5 mM abrogated the proliferation of BPH-1 cells induced by IGF-1 [17].Treatment with 20 mM Met suppressed the proliferation of PCa cells[45].Thus,we chose 0.5 mM,2 mM and 5 mM Met in the following study.In this study, 0.5 mM and 2 mM Met treatment had no effect on normal BPH-1 cells, while 5 mM Met could reduce BPH-1 cell viability(Fig.S6).Based on these findings, 0.5 mM and 2 mM Met were chosen in the follow-up studies.
After identifying that Met could inhibit the proliferation of DHTcultured BPH-1 cells, we further determined the mechanism by which Met exerted its antiproliferative effect in vitro.Met exerts its hypoglycaemic effect by activating AMPK, and Met inhibits the proliferation of multiple tumor cells by targeting AMPK.Studies have also demonstrated that activated AMPK subsequently targets several cellular pathways involved in the cell growth and proliferation of cancer stem cells [46].Our study showed that activating AMPK by AICAR not only downregulated AR protein levels in DHTcultured BPH-1 cells but also inhibited proliferation and stimulated cell cycle arrest (Fig.S7A).To confirm the indispensable role of activated AMPK in Met's antiproliferative effects against DHTcultured BPH-1 cells, CC was used in the following experiments.Along with the inactivation of AMPK by CC, the blocked antiproliferative effect suggested that Met exerted antiproliferative effects against DHT-cultured BPH-1 cells by activating AMPK(Fig.S7B).
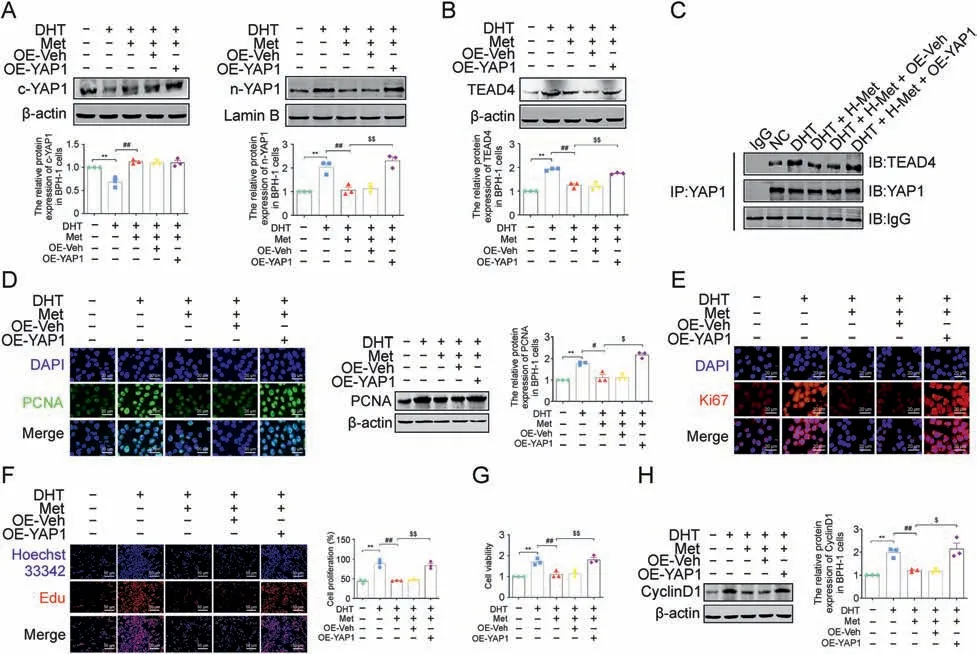
Fig.10.Overexpression of yes-associated protein 1(YAP1)blocked the anti-proliferative effect of metformin(Met)in vitro.(A)The relative protein levels of cytoplasm(c)-YAP1 and nuclear (n)-YAP1 in benign prostate hyperplasia (BPH)-1 cells.(B) The relative protein levels of TEA domain transcription factor 4 (TEAD4) in BPH-1 cells.(C) The interactions between YAP1 and TEAD4 were detected by co-immunoprecipitation(Co-IP)in BPH-1 cells.(D)The relative protein levels of proliferating cell nuclear antigen(PCNA)in BPH-1 cells by immunofluorescence (IF) and western blot analysis.(E) The relative protein levels of Ki67 in BPH-1 cells by IF.(F) BPH-1 cell proliferation activity detected by 5-Ethynyl-2′-deoxyuridine (EdU)assay.(G)Cell viability in BPH-1 cells.(H) The relative protein levels of cell cycle protein 1(CyclinD1) in BPH-1 cells.Data are mean ± standard error of mean(SEM),for cell groups of three.**P <0.01,compared to normal control(NC)as indicated; #P <0.05, ##P <0.01,compared to dihydrotestosterone(DHT)as indicated; $P <0.05, $$P <0.01, compared to DHT + Met as indicated.t-YAP1: total-yes-associated protein 1; OE-YAP1: yes-associated protein 1 overexpression; OE-Veh: yes-associated protein 1 overexpression virus empty.
Substantial evidence has revealed that androgen/AR signalling regulates YAP1 nuclear entry and exerts biological functions in human cancer cell lines [47].There is a crosstalk between YAP1/TEAD4 signalling and metabolism, and AMPK can modulate the YAP1 signaling pathway [48].As the major effectors of the Hippo pathway, activated YAP1/TEAD4 signaling participates in cell proliferation during multiple diseases.Downregulated YAP1 could inhibit the proliferation of PC-3 cells (a prostate cancer cell line),and YAP1 promoted the proliferation of colorectal cancer cells through AMPK [49].Met treatment inhibited the growth and viability of melanoma cells by downregulating YAP1 and TEAD4 expression in vitro [50].Nevertheless, whether YAP1/TEAD4 signalling is involved in PEC proliferation has not been reported either in BPH patients or in DHT-cultured BPH-1 cells.Meanwhile,there is a gap in our knowledge about the role of YAP1/TEAD4 signalling in Met's antiproliferative effects against DHT-cultured BPH-1 cells.In this study, based on the results of IPA analysis, our clinical and in vitro results first showed that YAP1/TEAD4 signalling was activated both in the PEC of BPH patients and in DHT-cultured BPH-1 cells,and the follow-up Met treatment downregulated YAP1/TEAD4 signalling,indicating that this signalling might participate in Met's antiproliferative effects against DHT-cultured BPH-1 cells.Then,the regulatory effect of CC and AICAR on YAP1/TEAD4 signalling suggested that Met downregulated YAP1/TEAD4 signalling in DHTcultured BPH-1 cells by activating AMPK.
Our previous study demonstrated that quercetin inhibited high glucose (HG)-induced glomerular mesangial cell proliferation by reversing the increased YAP1-TEAD heterodimer induced by HG[51].In this study, after finding that Met could decrease the formation of the YAP1-TEAD4 heterodimer in DHT-cultured BPH-1 cells by activating AMPK,RNA silencing and overexpression were performed by using a YAP1 knockdown/expression lentivirus to investigate the role of the YAP1-TEAD4 heterodimer in the antiproliferative effects of Met against DHT-cultured BPH-1 cells.The success of gene editing in DHT-cultured BPH-1 cells was proven by the decreased protein expression of YAP1 in the DHT+YAP1 shRNA group compared with the DHT + Vehicle group, as demonstrated by western blot and immunofluorescence(Fig.S8).In contrast,the increased protein expression of YAP1 in the DHT+Met+OE-YAP1 group proved the success of gene editing in DHT-cultured BPH-1 cells compared with the DHT+Met+Vehicle group(Fig.S9).Our results identified that the YAP1-TEAD4 heterodimer is involved in the antiproliferative effect of Met in DHT-cultured BPH-1 cells.
In this study,our results highlighted the antiproliferative effects and underlying mechanism of Met against BPH.A clinical study showed that women with PCOS had higher levels of T and E1, and Met treatment reduced E2 content[52].In fact,our results revealed that although the levels of E1 and E2 were significantly lower than those of DHT,their levels also showed significant increasing trends in the serum and prostate tissue of BPH patients, and Met treatment reduced E1 and E2 levels in the serum and prostate of BPH rats, in addition to DHT (Fig.S10).However, it is still unclear whether E1 and/or E2 are involved in the anti-proliferative effects of Met on BPH, which needs to be investigated in future studies.Another limitation of this study was that we could not carry out clinical studies to further verify our findings of Met in simple BPH patients.Our previous study demonstrated that insulin and its analogues should be used very carefully for the clinical antihyperglycaemic treatment of BPH complicated with T2DM patients[53].Based on the beneficial effect of Met on simple BPH, we hypothesized that Met might be the recommended clinical antihyperglycaemic treatment strategy for BPH complicated with T2DM patients, which was our other ongoing project.
5.Conclusions
In summary,our results demonstrated that owing to the highest contribution to the dysregulated sex steroid hormone homeostasis of BPH patients, DHT dose-dependently induced BPH-1 cell proliferation by upregulating AR, which could be inhibited by Met treatment.Further mechanistic studies showed that targeting AMPK by Met exerted antiproliferative effects on DHT-cultured BPH-1 cells by inhibiting the formation of the AR-mediated YAP1-TEAD4 heterodimer.Our study highlighted that Met may be a novel and potential clinical therapeutic strategy for BPH.
CRediT author statement
Tingting Yang:Conceptualization, Investigation, Funding acquisition, Supervision, Data curation, Writing - Original draft preparation, Reviewing and Editing;Jiayu Yuan:Data curation,Investigation, Project administration, Writing - Original draft preparation;Yuting Peng:Formal analysis, Data curation, Investigation, Project administration;Jiale Pang:Software, Investigation,Project administration,Validation;Zhen Qiu:Investigation,Project administration, Validation;Shangxiu Chen:Investigation, Project administration, Validation;Yuhan Huang:Software, Project administration;Zhenzhou Jiang:Investigation, Writing - Reviewing and Editing;Yilin Fan:Investigation,Methodology;Junjie Liu:Project administration;Tao Wang:Validation;Xueyan Zhou:Methodology, Visualization;Sitong Qian: Software, Visualization;Jinfang Song:Resources, Investigation, Project administration;Yi Xu:Project administration,Resources;Qian Lu:Conceptualization,Funding acquisition,Supervision,Writing-Reviewing and Editing;Xiaoxing Yin:Conceptualization,Funding acquisition,Supervision,Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant Nos.: 81973377, 81903689, 82073906 and 82273987), the Key Natural Science Foundation of Jiangsu Higher Education Institutions of China (Grant Nos.: 19KJB350006 and 19KJA460008), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the initializing Fund of Xuzhou Medical University (Grant No.: D2018011), and Postgraduate Research Practice Innovation Program of Jiangsu Province(Grant Nos.:KYCX21-2733 and KYCX22-2966).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.08.012.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Lipid metabolism analysis in esophageal cancer and associated drug discovery
- Push forward LC-MS-based therapeutic drug monitoring and pharmacometabolomics for anti-tuberculosis precision dosing and comprehensive clinical management
- The role of innate immunity in diabetic nephropathy and their therapeutic consequences
- Epimedin B exhibits pigmentation by increasing tyrosinase family proteins expression, activity, and stability
- Hydralazine represses Fpn ubiquitination to rescue injured neurons via competitive binding to UBA52
- Distinct molecular targets of ProEGCG from EGCG and superior inhibition of angiogenesis signaling pathways for treatment of endometriosis
