Inhibitory Effect of Flavonoid Glycosides from Chlorophytum comosum on Nasopharyngeal Carcinoma 5-8F Cells and Its Mechanism
2024-03-07ChenliangCHUXinchenWANGKuanLULiangQINLuJIN
Chenliang CHU, Xinchen WANG, Kuan LU, Liang QIN, Lu JIN
1. College of Food and Pharmaceutical Engineering, Zhaoqing University, Zhaoqing 526061, China; 2. School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China
Abstract [Objectives] To study the inhibitory activity of two flavonoid glycosides isolated from Chlorophytum comosum Laxum R. Br on human nasopharyngeal carcinoma (NPC) cell line 5-8F in vitro and its mechanism. [Methods] The flavonoid glycosides were isolated and purified from the ethanol alcoholic extract of the roots of Liliaceae plant Chlorophytum comosum by silica gel column chromatography, macroporous resin column chromatography, Sephadex LH-20, and reverse column chromatography (ODS). The inhibitory activity of flavonoid glycosides on human nasopharyngeal carcinoma cells was analyzed by CCK-8 method, and the potential mechanism was preliminarily analyzed by molecular docking. [Results] Two flavonoid glycosides were identified as isovitexin 2″-0-rhamnoside and 7-2″-di-O-β-glucopyranosylisovitexin. Two flavonoid glycosides showed promising inhibitory effect on human nasopharyngeal carcinoma cell line 5-8F, with IC50 values of 24.8 and 27.5 μmol/L, respectively. Molecular docking results showed that the potential targets of two flavonoid glycosides include CyclinD1, Bcl-2 β-Catenin, ILK, TGF-β, in addition, two glycosides showed higher predicted binding affinity towards CyclinD1, which verifies the cytotoxicity of the two compounds on human nasopharyngeal carcinoma cell line 5-8F in vitro. [Conclusions] Two flavonoid glycosides are the active molecules in Chlorophytum comosum that can inhibit the proliferation of human nasopharyngeal carcinoma cells, and have the potential to be used in the research and development of anti nasopharyngeal carcinoma drugs.
Key words Chlorophytum comosum Laxum R. Br., Flavonoid glycosides, 5-8F cells, Antitumor mechanism
1 Introduction
Nasopharyngeal Carcinoma (NPC) is one of the malignant tumors of human head and neck, which showed high incidence rate in many southern provinces of China. In clinic, the disease is mainly treated with combined radiotherapy[1], however, the commonly used chemotherapy drugs in clinical practice have many side effects, leading to the decline of human immune function and poor therapeutic effect[2-3]. In recent years, the development of traditional Chinese medicine has made great progress, and the research on anti-tumor activity of traditional Chinese medicine has also made great breakthroughs. Its efficacy and mechanism of action have been experimentally verified[4-6]. With the improvement and development of network pharmacology and molecular docking technology, the research on molecular mechanism of Chinese medicine to exert clinical efficacy has become visual, efficient and standardized[7-8], providing a guarantee for the research on anti-tumor mechanism of Chinese medicine.
ChlorophytumcomosumLaxum R. Br is a plant belonging toChlorophytumcomosum, Liliacea, also known as Triangulum, has been listed into the Flora of China ofChlorophytumcomosumlaxiflorum,GuangxiPlantListofChineseChives, and Main Poisonous Plants in the South of China ofTuophiopogonjaponicus, mainly grown in southern Guangdong, with the functions of clearing heat, detoxification, swelling and pain[9]. It is mainly used in clinical treatment of snakebite[10], swelling and pain after tibial fracture, adhesion after hand tendon exercise. Its main chemical components include steroids and their glycosides, alkanes, flavonoid glycosides,etc.[11]. Studies have shown that flavonoid glycosides have a strong inhibitory effect on the activity of human nasopharyngeal carcinoma cell line 5-8F[12]. Wangetal.[13]found that flavonoid glycosides can inhibit autophagy and reverse the radioresistance of nasopharyngeal carcinoma cells. Li Jingetal.[14]confirmed that flavonoid glycosides can inhibit the tumor development of nasopharyngeal carcinoma bearing mice. Pyruvoside is a potentially valuable drug molecule in the treatment of nasopharyngeal carcinoma. This study is mainly to isolate flavonoid glycosides from the ethanol extract ofChlorophytumcomosumgrandiflorum, and carry out targetedinvitrocytotoxic activity research, explore and analyze its activity mechanism using molecular docking technology, and increase the active ingredients available for screening for the research and development of nasopharyngeal cancer drugs.
2 Materials and methods
2.1 Materials
2.1.1Instruments. rotary evaporator RE-2000 (Shanghai Yarong Instrument Co., Ltd.), X-4 digital display micro-melting point tester (Beijing Tec Instrument Co., Ltd.), IR tester (EQUZNOXTM55-A590/3 F type), ESI-MS (A.B. Sicence), NMR (Bruker 400 ASCENDTM).
2.1.2Reagents. petroleum ether (60-90 ℃), ethyl acetate, ethanol (95%), Dichloromethane, n-butanol, methanol (all analytical alcohols, purchased from Tianjin Fuyu Fine Chemical Co., Ltd.), silica gel (200-300 mesh Qingdao Marine Chemical Plant), ODS column chromatographic filler (Merck, Germany), Sephadex LH-20 column (Pharmacia Biotech AB, Uppsala, Switzerland), DMEM medium (Hyclone, USA), fetal bovine serum (FBS, Hyclone, USA), TransDetect Cell Counting Kit (CCK), Article No.: L0001, Shanghai Yuchun Biotechnology Co., Ltd.
2.1.3Medicinal materials. Dried roots of small flowerChlorophytumcomosum. The original plant was identified by Teng Xifeng, associate professor of Guangdong Pharmaceutical University, asChlorophytumLaxumR.Br, a small flower of LiliaceaeChlorophytumcomosum, and stored in Room 608, Science and Engineering Building, Zhaoqing University (CCL-220712).
2.1.4Cells. Human nasopharyngeal carcinoma cells 5-8F were provided by the Cancer Hospital affiliated to Sun Yat-sen University.
2.2 Methods
2.2.1Extraction and separation. The root ofChlorophytumcomosum(2.0 kg) was cut into 1 cm segments, heated and refluxed with 95% ethanol for three times, each time for 3 h, combined with ethanol extract, recovered ethanol, concentrated to obtain ethanol extract (265 g), then dispersed with 700 mL of water to form a suspension, extracted with petroleum ether, dichloromethane, ethyl acetate, and n-butanol in turn (1:1V/V), extracted three times separately, combined with solutions, concentrated to obtain petroleum ether part (18.4 g), Dichloromethane (39.0 g) ethyl acetate (47.5 g), n-butanol (60.0 g). Took the n-butanol part (58 g), passed through the macroporous resin column, and eluted with (20%, 50%, 75%, and 100%) ethanol: water gradient to obtain four parts (Fr.1-Fr.4). Fr; Fr. 4.1 was separated by silica gel column chromatography again. The sample was separated by silica gel (200-300 mesh) column chromatography. The sample was mixed with silica gel (1:1). The column was packed with sample: silica gel 1:30, eluted with dichloromethane: ethyl acetate 10:1 equivalency, TLC detection, the same components were combined, and a total of 6 parts (Fr. D1.1-1.6) were obtained. Fr. D1.3 was eluted with sephadex LH-20 (MeOH) equivalency, and purified to obtain compound 1 (20.0 mg); Fr. D1.4 was eluted and purified with sephadex LH-20 (MeOH) to obtain a compound, which was purified by ODS column chromatography, and then eluted with methanol: water (70:30) to obtain compound 2 (28.5 mg)
2.2.2Detection of cell proliferation by CCK8 method. The inhibition rate of two flavonoid glycosides on human nasopharyngeal carcinoma cell line 5-8F was determined by CCK-8 methodinvitro. 1640 medium for human nasopharyngeal carcinoma cell line 5-8F (with 10% fetal bovine serum, 100 μg/L penicillin and 100 μg/L streptomycin) was cultured in an incubator with 5% CO2and 37 ℃. Inoculate 100 μL cell suspension (2 per well×103cells), pre-cultured for 24 h in a 37 ℃, 5% carbon dioxide incubator according to the experimental requirements. Add different concentrations [(5, 12.5, 25, 50, and 100 μL. The cells were treated with compounds (1-2) of M/L)] and cultured for 12 h. 11 μL of CCK solution was added to each well. The cells were cultured for 4 hours in a carbon dioxide incubator. TheODat 450 nm was measured with an enzyme marker, and repeated for 6 times. TheODvalue was recorded and the inhibition rate of each compound to be tested was calculated. TheIC50was calculated using SPSS software, and the cell control and basic culture control were designed.
2.2.3Study on the mechanism of anti-nasopharyngeal carcinoma cell line 5-8F. Literature studies show that the proteins or targets related to the proliferation of human nasopharyngeal carcinoma 5-8F cells include TGF-β1 (PDB:ID 1PY5)[12], Bcl-2(PDB:ID 2XA0)[15], β-Catenin (PDB: ID 2GL7)[15], ILK (PDB: ID 3KM W)[16], CyclinD1 (PDB: ID 6P8G) protein[17], respectively, can inhibit the growth of 5-8F in human nasopharyngeal carcinoma cells by inhibiting these protein targets. This experiment will take these six protein targets as the research object, and use Autodock software to dock two flavonoid glycosides components respectively, and preliminarily analyze the molecular mechanism of flavonoid glycosides inhibiting the activity of human nasopharyngeal carcinoma cells.
3 Results and analysis
3.1 Structure identificationCompound 1: yellow powder (methanol), mp.214-215 ℃; HR-ESI-MSm/z595.166 4[M+H]+, molecular formula C27H30O15,1HNMR (400 MHz C5D5N) δ (ppm): 7.83 (2H, d, J=8.3Hz, H-2′, 6′), 7.13 (1H,s, H-8), 7.08 (2H, m, H-3′, 5′), 6.89 (1H,s, H-3), 5.81(1H, d, J =7.4 Hz,H-1 of 2″-O-glc), 5.62 (1H, d, J=6.4Hz, H-6 of 6-C-glc), 5.21 (1H, d, J=9.3Hz, H-1 of 6-C-glc), 4.05 (1H, d, J=9.1Hz, H-6 of 2″-O-glc), 4.11-4.50(7H, m);13CNMR(100 MHz C5D5N) δ(ppm): 160.9 (C-2), 103.8 (C-3), 182.8 (C-4), 163.6 (C-5), 105.9 (C-6), 164.6 (C-7), 94.5 (C-8), 157.1 (C-9), 111.7 (C-10), 121.4 (C-1′), 128.8 (C-2′, 6′), 116.4 (C-3′, 5′), 162.6 (C-4′), 6-C-glc:74.5 (C-1), 80.8 (C-2), 79.0 (C-3), 71.0 (C-4), 82.3(C-5), 62.3 (C-6), 2″-O-glc: 102.9(C-1), 77.5(C-2), 74.9 (C-3), 72.9 (C-4), 70.8 (C-5), 61.7 (C-6). Combined with the spectral data of the literature, compound 1 was identified as isovitexin 2″-0-rhamnoside
Compound 2: yellow powder (methanol), mp.217-219 ℃; HR-ESI-MSm/z757.213 0[M+H]+, molecular formula C33H40O20,1HNMR (400 MHz MeOD) δ (ppm): 7.86(2H, d, J=8.6 Hz, H-2′, 6′), 6.90(2H, d, J=8.3Hz, H-3′, 5′), 6.65 (1H,s, H-3), 5.08 (1H, d, J =7.2 Hz,H-1of 7-O-glc), 5.03 (1H, d, J =10 Hz,H-1 of 6-C-glc), 4.34 (1H, d, J =7.0 Hz, H-1 of 2″-O-glc);13CNMR (100MHz MeOD) δ (ppm):166.1 (C-2), 103.6 (C-3), 183.3 (C-4), 160.8 (C-5), 110.3 (C-6), 163.4 (C-7), 93.8 (C-8), 158.2 (C-9), 106.1 (C-10), 122.2 (C-11), 129.1 (C-2′, 6′), 116.5 (C-3′, 5′), 162.4 (C-4′), 6-C-glc: 72.1(C-1), 81.4 (C-2), 79.4 (C-3), 70.7 (C-4), 80.4 (C-5), 62.0 (C-6), 7-O-glc:101.7 (C-1), 74.4 (C-2), 77.1 (C-3), 70.5 (C-4), 77.9 (C-5), 61.9 (C-6), 2″-O-glc:104.7 (C-1), 75.2 (C-2), 76.8 (C-3), 70.2 (C-4), 76.7 (C-5), 61.3 (C-6). Combined with literature spectral data, compound 2 was identified as 7,2″-di-O-β-glucopyranosyliso-vitexin[7]
3.2 Antitumor activity screeningThe CKK-8 method was used to test the inhibitory activity of two flavonoid glycosides on nasopharyngeal carcinoma cell 5F-8invitro, and camptothecin was used as the control drug. The results showed that the two flavonoid glycosides showed inhibitory activity on nasopharyngeal carcinoma cell 5F-8, withIC50values of 24.8 and 27.5 μM. These two flavonoid glycosides are potentially active molecules for the treatment of rhinitis and cancer.
3.3 Molecular docking resultsWhen the binding free energy is less than 0 kcal/mol, it indicates that compounds with biological activity can spontaneously combine with the target. The smaller the binding free energy, the stronger the binding ability of the compound with the target[18]. In this study, the binding free energy ≤-5.0 kcal/mol is used as the screening criteria[18]. Two flavonoid glycosides and Bcl-2 β-Catenin, ILK, TGF-β1. The results of molecular docking of the five targets of CyclinD1 are shown in Table 1 and Fig.1-5. The binding free energy is between (between -5.1 and -8.1) kcal/mol, indicating that the two flavonoid glycosides have strong affinity with the five target proteins.
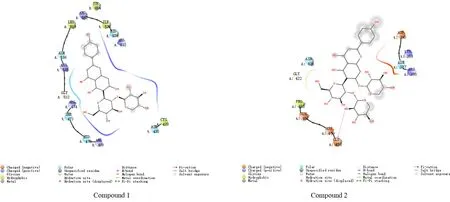
Fig.2 Schematic diagram for the docking of compounds 1 and 2 with 2GL7

Fig.3 Schematic diagram for the docking of compounds 1 and 2 with 3KMW
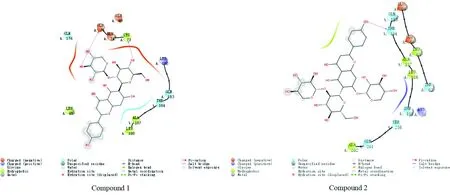
Fig.4 Schematic diagram for the docking of compounds 1 and 2 with 6P8G
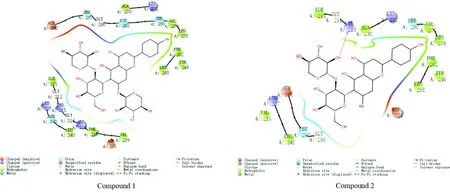
Fig.5 Schematic diagram for the docking of compounds 1 and 2 with 1PY5

Table 1 Binding free energy of two flavonoid glycosides and five targets
3.4 Analysis of anti-nasopharyngeal carcinoma cell 5-8F mechanismMolecular docking results showed that the binding free energy of the two flavonoid glycosides with BCL-2 and CyclinD1 of human nasopharyngeal carcinoma cell line 5-8F was between (-7.0 and -8.1 kcal/mol), indicating that the compounds may process good affinity with the target, suggesting that the two flavonoid glycosides might up-regulate the activity of Bax protein by inhibiting the activity of BCL-2 and CyclinD1 of human nasopharyngeal carcinoma cell line 5-8F, thus affecting the cell cycle of 5-8F, Inducing apoptosis[19]; Inhibition of BCL-2 of 5-8F cells may also up-regulate the expression of pro-apoptotic protein NoxAd to promote the apoptosis of nasopharyngeal carcinoma cells[20].
β-Catenin is a key signal molecule in the Wnt signal transduction pathway.The expression of Catenin can inhibit the activity of Wnt pathway, inhibit the proliferation of human nasopharyngeal carcinoma cell line 5-8F, and induce its apoptosis[21]. Two flavonoid glycosides and β-Catenin protein has good affinity, and its binding free energy is -7.3 and -7.1 kcal/mol respectively, suggesting that the two compounds may inhibit the proliferation of human nasopharyngeal carcinoma cell line 5-8F and induce its apoptosis through this pathway.
The binding free energies of the two flavonoid glycosides and ILK were -6.7 and -7.6 kcal/mol, respectively, indicating that the affinity between the compounds and the target was good. By inhibiting the expression of integrin-linked kinase (ILK), it can inhibit the proliferation of human nasopharyngeal carcinoma cells[16], suggesting that the two compounds may also achieve the purpose of inhibiting the proliferation of nasopharyngeal carcinoma cells through this pathway.
4 Conclusions
In this study, two flavonoid glycosides were isolated and identified fromChlorophytumlaxumR. Br, namely Apigenin-6-C-glucosylglucoside and 7-2″-di-O-β-Glucopyranosylisovitexin, antitumor activity studyinvitroshowed that the two flavonoid glycosides showed a certain inhibitory effect on human nasopharyngeal carcinoma cell line 5-8F. Through molecular docking technology, it was found that the two compounds could interact with Bcl-2 β-Catenin, ILK, TGF-β1, and CyclinD1 (PDB: ID 6P8G) with good affinity, which verified the cytotoxicity of two compounds on human nasopharyngeal carcinoma cell line 5-8Finvitro. Combined with the research of literature, the cytotoxicity mechanism of two flavonoid glycosides on human nasopharyngeal carcinoma cell line 5-8F was discussed and analyzed, suggesting that the two flavonoid glycosides are active molecules inChlorophytumcomosumthat can inhibit the proliferation of human nasopharyngeal carcinoma cells. It has the potential to be used in the research and development of anti-nasopharyngeal carcinoma drugs.
杂志排行
Medicinal Plant的其它文章
- Research Progress in the Treatment of New Bone Formation of Ankylosing Spondylitis
- Current Status of Mongolian Medicine Treatment for Breast Hyperplasia
- Anti-Tumor and Anti-Diabetic Effects of Sarsasapogenin
- Research Overview of Zhuang Medicine Fumigation Lotions
- Antioxidant and Hypoglycemic Ability of Ardisia gigantifolia Stapf Parts
- Incidence and Risk Factors of Sub-syndromal Delirium in Patients after Cardiac Surgery
