Antioxidant and Hypoglycemic Ability of Ardisia gigantifolia Stapf Parts
2024-03-07ChaohaoGUShihaoLIANGYuexuanWUZhirenYAOJiaojiaoLIKaimeiZHU
Chaohao GU, Shihao LIANG, Yuexuan WU, Zhiren YAO, Jiaojiao LI, Kaimei ZHU*
1. Guilin Medical University, Guilin 541000, China; 2. Second Affiliated Hospital of Guilin Medical University, Guilin 541000, China
Abstract [Objectives] To study the antioxidant and hypoglycemic effects of different parts of Ardisia gigantifolia Stapf. [Methods] The hydroxyl radical scavenging activity, DPPH radical scavenging activity and total antioxidant capacity of ABTS of 75% ethanol extract of A. gigantifolia Stapf and the petroleum ether, ethyl acetate, n-butanol, chloroform and aqueous extract were measured with Vc as positive control. At the same time, acarbose was used as reference substance to determine the inhibitory effect of each polar site on α-glucosidase. [Results] All parts of A. gigantifolia Stapf had antioxidant activity, among which ethyl acetate had the strongest antioxidant activity, and the scavenging rate of hydroxyl radical and DPPH radical was higher than that of positive control. The results showed that petroleum ether, ethyl acetate and chloroform had a good inhibitory effect on α-glucosidase (better than acarbose). [Conclusions] The ethyl acetate part of A. gigantifolia Stapf had the best antioxidant activity and inhibitory effect on α-glucosidase. It provides a basis for further research and development of A. gigantifolia Stapf.
Key words Ardisia gigantifolia Stapf, Antioxidants, Free radicals, Hypoglycemic effect
1 Introduction
ArdisiagigantifoliaStapf is a medicinal plant in the Myrsinaceae family, and can be harvested all year round. After washing, removing fibrous roots, and drying in the sun, it can be used for medical purposes.A.gigantifoliaStapf is pungent and warm in nature, and has the effects of dispelling wind and dampness, strengthening bones and muscles, promoting blood circulation and removing blood stasis. It is used for the treatment of rheumatic pain, traumatic injury, postpartum blood stasis, carbuncle and ulcer[1-2]. In modern clinic, it is used to treat gouty arthritis, rheumatoid arthritis, hyperosteogeny, bone injury and fracture. Studies on chemical constituents show thatA.gigantifoliaStapf contains phenols, quinones, sterols, triterpenes and volatile oils, etc., and has good pharmacological effects in antithrombotic, antioxidant, anti-tumor and anti-rheumatoid aspects[3]. However, there is no report on the antioxidant capacity and hypoglycemic effect of some specific parts. In this study, five different polar solvents were used to extract 75% ethanol fromA.gigantifoliaStapf, and three different antioxidant activity methods were used to evaluate its antioxidant activity, and the inhibitory effect on α-glucosidase was used to evaluate whether it had hypoglycemic effect, which provided a scientific basis for further research and development ofA.gigantifoliaStapf.
2 Materials and methods
2.1 Medicinal materials and reagentsThe sample was provided by Tang Hui, a researcher from Guangxi Institute of Botany, Chinese Academy of Sciences, and was identified as the genuineA.gigantifoliaStapf. Anhydrous ethanol for analysis, analytically pure petroleum ether, analytically pure ethyl ether, analytically pure ethyl acetate, analytically pure n-butanol, ABTS kit (Beyotime Biotechnology Co., Ltd.), 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) (TCI Shanghai Co., Ltd.); ascorbic acid (Vc) (Tianjin Fuchen Chemical Reagent Co., Ltd.), α-glucosidase, 4-nitrobenzene-α-D-glucopyranoside (PNPG) (Sigma).
2.2 Systematic solvent extractionThe dry product ofA.gigantifoliaStapf (800 g) was crushed, soaked in 75% ethanol for one week according to the ratio of solid to liquid 1:10, and extracted for three times. 66 g of dry extract was obtained by rotating evaporation and concentration, dispersed with distilled water at 60 ℃, and extracted with petroleum ether, chloroform, ethyl acetate and water-saturated n-butanol for three times in turn. The combined extracts were concentrated under reduced pressure at 50 ℃ and dried.
2.3 Determination of total antioxidant capacity of each extracted part by ABTS method
2.3.1Preparation of standard curve. 200 μL of ABTS working solution and 10 mL (0.15, 0.3, 0.6, 0.9, 1.2 and 1.5 mM) of Trolox solution were added into the 96-well plate. After evenly mixing and incubating at room temperature for 2-6 min, the absorbance was measured at 405 nm wavelength.
2.3.2Preparation of ABTS working solution. According to the instructions of the kit, ABTS solution and oxidant solution with the same volume were taken, stored at room temperature and kept away from light for 12-16 h, and diluted to 1:35-1:55 with 80% ethanol.
2.3.3Determination of total antioxidant capacity. 200 μL of ABTS working solution was added into 96-well plate, 10 μL of PBS was added to blank control group, 10 μL of samples with different concentrations were added to sample group, and 10 μL of Trolox solution was added to standard curve group. The solution was shaken gently and mixed evenly. The absorbance was measured at 405 nm after incubation at room temperature for 2-6 min. According to the standard curve, the total antioxidant capacity of the sample was calculated.
2.4 Determination of hydroxyl radical scavenging activity
According to the method of reference[4], each part ofA.gigantifoliaStapf was diluted to 1.60, 0.80, 0.40, 0.20, 0.10, 0.05 mg/mL solution, respectively, and the same concentration of Vc solution was used as control. 1 mL of samples were mixed with 2 mL of 1.8 mmol/L FeSO4solution and 1 mL of 1.8 mmol/L salicylic acid-ethanol, respectively, and 0.1 mL of 0.03% H2O2solution was added for reaction for 30 min at 37 ℃. The absorbance at 510 nm was determined by centrifugation at 8 000 r/min for 10 min. The scavenging rate of hydroxyl radicals was calculated according to the formula:
Hydroxyl radical scavenging rate (%)=[1-(A1-A2)/A0]×100%
(1)
whereA1is 1.0 mL of extracting solution +1.0 mL of FeSO4solution +1 mL of salicylic acid-ethanol+H2O2solution;A2is 1.0 mL of extracting solution+1.0 mL of FeSO4solution+1 mL of salicylic acid-ethanol+distilled water;A0is 1.0 mL of distilled water+1.0 mL of FeSO4solution+1 mL of salicylic acid-ethanol+H2O2solution.
2.5 Determination of DPPH free radical scavenging activity
According to the method of reference[4]: 2 mL of ethanol extract ofA.gigantifoliaStapf was added into 1 mL of DPPH-ethanol solution with a concentration of 500 μmol/L. After mixing evenly, the absorbance was measured at 517 nm after 30 min reaction at room temperature. The positive control was Vc, and the scavenging rate of DPPH free radicals was calculated according to the following formula.
DPPH radical scavenging rate (%) =[1-(A1-A2)/A0]×100%
(2)
whereA1is absorbance for 2.0 mL of extracting solution +2.0 mL of DPPH working solution;A2is absorbance for 2.0 mL of extract+2.0 mL of absolute ethanol;A0is absorbance for 2.0 mL of pure water+2.0 mL of DPPH working solution.
2.6 Inhibitory effect ofArdisiagigantifoliaStapf extracts in different solvents on α-glucosidaseAccording to the method of reference[5]: 10 μL ofA.gigantifoliaStapf extracts with different concentrations (1.60, 0.80, 0.40, 0.20, 0.50, 0.25 mg/mL) were added into a 96-well plate, 10 μL of PBS and 10 μL of 0.5 U/mL α-glucosidase solution were added respectively, then incubated at 37 ℃ for 15 min, 20 μL of 3 mmol/L PNPG solution was added, incubated for 10 min, and 150 μL of 0.1 mol/L Na2CO3solution was added to terminate the reaction. The absorbance was measured at 405 nm by microplate reader, and blank group, control group and sample control group were set up separately. The experiment was carried out according to Table 1, with acarbose as the positive control.

Table 1 Inhibitory effect of α-glucosidase
The inhibitory effect of the extract on α-glucosidase is calculated according to the formula:
Inhibition rate=[1-(A1-A2)/A3]×100%
(3)
whereA1is the absorbance value of sample group;A2is the absorbance value of blank group;A3is the absorbance value of control group.
2.7 Statistical processingSPSS 20.0 software was used for statistical analysis. The measurement data were expressed by mean±standard deviation, and the difference was statistically significant whenP<0.05.
3 Results and analysis
3.1 Determination of total antioxidant capacity by ABTS methodThe total antioxidant capacity test kit (ABTS method) was used to detect the antioxidant activity of each extract ofA.gigantifoliaStapf, and the results showed that each extract had certain antioxidant activity (Table 2).

Table 2 Antioxidant activity of the extracts of Ardisia gigantifolia Stapf
3.2 Scavenging ability of hydroxyl radicalsThe mechanism of scavenging hydroxyl radical is that hydroxyl radical can react with benzene ring on salicylic acid molecule to produce 2, 3-dihydroxybenzoic acid. The absorbance value was measured at 510 nm. If there are antioxidants in the reaction system, the absorbance value will decrease. The scavenging ability of hydroxyl radicals is shown in Fig.1A.
3.3 Scavenging effect on DPPH radicalThe mechanism of DPPH scavenging ability is that under the action of antioxidants, purple DPPH radical can be reduced to yellow non-free radical DPPH-H. The absorbance value was measured at 517 nm. There is a linear relationship between the change degree of absorbance value and the scavenging degree of free radicals in a certain range. The study on antioxidant activity of the extracts ofA.gigantifoliaStapf showed that the extracts had strong antioxidant activity. The scavenging rate (EC50) of DPPH radical and hydroxyl radical by VCwas (0.14±0.005) and (0.182±0.015) mg/mL, respectively, and the scavenging rate of extracts ofA.gigantifoliaStapf was higher than that of positive control. Ethyl acetate and n-butanol had the highest scavenging rate for the two free radicals, indicating that the active antioxidant components ofA.gigantifoliaStapf were mainly dissolved in ethyl acetate and n-butanol (Fig.1B).
3.4 Inhibitory effect ofA.gigantifoliaStapf extracts in different solvents on α-glucosidaseThe inhibitory effect of each extract ofA.gigantifoliaStapf on α-glucosidase showed that all the effective parts had certain inhibitory effect on α-glucosidase (Fig.2A), and the inhibitory effect of all the effective parts was higher than that of the positive control of acarbose (IC50=3.2±0.002 mg/mL). The inhibitory effect of ethyl acetate site was the most obvious, and it was much higher than that of the positive control of acarbose in the same concentration (Fig.2B). This indicated that the active components ofA.gigantifoliaStapf which exerted α-glucosidase inhibition were mainly dissolved in ethyl acetate.
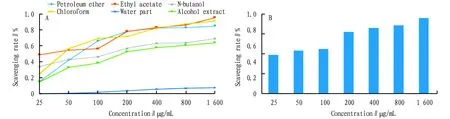
Fig.2 Inhibitory effect on α-glucosidase by different parts (A) and ethyl acetate (B) of Ardisia gigantifolia Stapf
4 Discussion
The antioxidant activity of extracts fromA.gigantifoliaStapf in different solvents was compared, and the results showed that ethyl acetate was superior to other solvents. In this study, the antioxidant capacity of each part was evaluated comprehensively by three methods: ATPS total antioxidant capacity, DPPH free radical and hydroxyl free radical. The antioxidant sequences obtained by the three evaluation systems were consistent, which showed that the ethyl acetate extract had the strongest antioxidant activity and the water extract had the worst antioxidant activity. Studies have shown that phenols are the main components of antioxidant activity in plants[6]. Phenols are mainly represented by flavonoids, which play an antioxidant role by complexing metal ions and inhibiting the generation of free radicals[7-8]. There are many compounds inA.gigantifoliaStapf, such as phenols, quinones, sterols, coumarins, triterpenes and volatile oils. At present, nine phenolic components such as phenolic acids and phenolic glycosides were isolated fromA.gigantifoliaStapf, and flavonoids such as quercetin, kaempferol and catechin were detected fromA.gigantifoliaStapf by Liang Weietal, which indicated that catechin and quercetin were the main material basis of antioxidant activity[9].
By comparing the inhibitory effects of different parts ofA.gigantifoliaStapf on α-glucosidase, the results showed that petroleum ether, ethyl acetate and chloroform parts were superior to other parts, indicating that the effective components may be mainly dissolved in these three parts. Diabetes mellitus is a disease that seriously affects human health. The occurrence of diabetes mellitus is closely related to oxidation, and glucose in starch is linked in the form of α-1,4 glycosidic bonds and α-1,6 glycosidic bonds. α-glucosidase inhibitors generally have the chemical structure of monosaccharides or oligosaccharides, and can effectively inhibit the activity of glycohydrolase and effectively control the postprandial blood sugar level of diabetic patients. α-glucosidase inhibitors also have anti-cancer, anti-HIV and anti-atherosclerosis effects[5].
To sum up,A.gigantifoliaStapf has good antioxidant and hypoglycemic effects. Nowadays, people pay more and more attention to health.A.gigantifoliaStapf, as a kind of Chinese herbal medicine which can be used as medicine and food, has high development value. This study provides a theoretical basis for the further development and utilization ofA.gigantifoliaStapf.
杂志排行
Medicinal Plant的其它文章
- Research Progress in the Treatment of New Bone Formation of Ankylosing Spondylitis
- Current Status of Mongolian Medicine Treatment for Breast Hyperplasia
- Anti-Tumor and Anti-Diabetic Effects of Sarsasapogenin
- Research Overview of Zhuang Medicine Fumigation Lotions
- Incidence and Risk Factors of Sub-syndromal Delirium in Patients after Cardiac Surgery
- Effect of Elephantopus scaber L. Extract on Acute Pleurisy in Rats
