The wheat sucrose synthase gene TaSus1 is a determinant of grain number per spike
2024-03-07LipingShenLiliZhangChangbinYinXiaowanXuYangyangLiuKuochengShenHeWuZhiwenSunKeWangZhonghuHeXueyongZhangChenyangHaoJianHouAoyueBiXueboZhaoDaxingXuBotaoYeXuchangYuZiyingWangDanniLiuYuanfengHaoFeiLuZifengGuo
Liping Shen, Lili Zhang, Changbin Yin, Xiaowan Xu, Yangyang Liu, Kuocheng Shen,He Wu, Zhiwen Sun, Ke Wang, Zhonghu He,f, Xueyong Zhang, Chenyang Hao, Jian Hou,Aoyue Bi, Xuebo Zhao, Daxing Xu, Botao Ye, Xuchang Yu, Ziying Wang, Danni Liu,Yuanfeng Hao,*, Fei Lu,g,*, Zifeng Guo*
a Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
b China National Botanical Garden, Beijing 100093, China
c State Key Laboratory of Plant Cell and Chromosome Engineering,Institute of Genetics and Developmental Biology,Innovative Academy of Seed Design,Chinese Academy of Sciences,Beijing 100101, China
d Institute of Crop Sciences, Chinese Academy of Agricultural Sciences (CAAS), Beijing 100081, China
e University of Chinese Academy of Sciences, Beijing 100049, China
f International Maize and Wheat Improvement Center (CIMMYT) China Office, c/o CAAS, Beijing 100081, China
g CAS-JIC Centre of Excellence for Plant and Microbial Science (CEPAMS), Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China
Keywords:Domestication selection Fertile spikelet number per spike (FSN)Geographical differentiation Grain number per spike (GNS)TaSus1
ABSTRACT Some haplotypes of the sucrose synthase gene TaSus1 are associated with thousand-grain weight(TGW)in wheat (Triticum aestivum L.).However, no mutations have been identified within the gene to test this association.The effects of TaSus1 on grain number per spike (GNS) also are largely unknown.Our previous genome-wide association study identified TaSus-A1 as a candidate gene controlling fertile spikelet number per spike (FSN).In the present study, we generated two independent mutants for the three TaSus1 homoeologs by CRISPR/Cas9-mediated genome editing.The triple mutants displayed lower FSN,GNS, grain number per spikelet (GNST), and TGW than wild-type plants.In 306 hexaploid wheat accessions,two single-nucleotide polymorphisms in TaSus-A1 contributed differently to GNS.Introgression of the two alleles into a wheat genetic background confirmed their effects.The alleles differed in geographical distribution among the accessions.
1.Introduction
Wheat (Triticum aestivum L.) is a staple food crop worldwide.Wheat grain yield is influenced by three components: spike number per unit area, grain number per spike (GNS), and thousandgrain weight (TGW) [1].GNS is closely associated with fertile spikelet number per spike (FSN) and grain number per spikelet(GNST)[2].TGW is determined mainly during the grain filling period [3].
Sucrose synthase (SUS) is involved in sucrose biosynthesis and functions in carbon partitioning.Sucrose transport influences the allocation of carbon resources during grain filling in crops [4–6].Increasing the photosynthetic and sucrose export capacity of source tissue can increase crop yields[7–9].In Arabidopsis thaliana,SUS is encoded by six genes.SUS1 and SUS4 are expressed specifically in phloem companion cells,SUS2 and SUS3 are highly induced during seed development, and expression of SUS5 and SUS6 is restricted to sieve elements [10].The maize (Zea mays L.) genome contains three SUS genes:Shrunken1(Sh1),Sus1,and Sus2.Sh1 and SUS1 function redundantly in starch biosynthesis [11].However,there has been limited research conducted on wheat sucrose synthase.Different haplotypes of TaSus1 are associated with differing TGW [12], but the effects of TaSus1 on other grain yield-related traits are largely unknown.Moreover, understanding the genomic basis of phenotypic variation in germplasm is necessary for making accurate selection decisions and for combining desired allelic combinations to improve wheat grain yield.
Our previous work identified TaSus-A1 as a candidate gene controlling FSN in a genome-wide association study (GWAS) of 210 European wheat accessions [13].In this study, we generated two independent TaSus1 mutant lines using CRISPR/Cas9-mediated gene editing and investigated the effects of homoeologous genes.The triple mutants had lower FSN, GNS and TGW than wild-type.Because sucrose synthase plays a crucial role in controlling the production and breakdown of sucrose in plants, we measured the levels of different types of sugars in both the wild type and the mutant.The mutant exhibited a considerable reduction in fructose content.We also identified the geographical distribution of the TaSus-A1 SNPs across global wheat accessions.Two singlenucleotide polymorphisms in TaSus-A1 was found to contribute differently to GNS.We introgressed the two alleles into a wheat genetic background to confirm their effects.
2.Materials and methods
2.1.Plant materials and growing conditions
A panel of 306 wheat accessions (Table S1) was grown in the field at Zhaoxian, Hebei, China in 2020 and 2021.Each accession was planted in a six-row plot.The row was 1.5 m in length with 15 plants, and the row spacing was 10 cm.Spikes from the main shoots of five plants were randomly selected for investigation of morphological traits at physiological maturity.Grain number per spikelet (GNST) and fertile spikelet number per spike (FSN) were determined using the middle part of a spike.A spikelet with at least one kernel was defined as a fertile spikelet.
A BC4F7 near-isogenic line(NIL)population was derived from a Jingshuang 16(G)/Lumai 21(C)//4*Jingshuang 16 cross.Successive generations of backcrossing resulted in a high degree of uniformity in the genetic background of the lines,with very high similarity to the parent Jingshuang 16.The NILs were grown in Zhengzhou,Henan,China.The mean GNS of 15 lines carrying the C allele were used to determine the effects of the allele on the trait.
Transgenic lines and their corresponding wild types were grown in a greenhouse under a 16-h light/8-h dark photoperiod and a temperature of ~20 °C day/~16 °C night.
Twelve developmental stages of wheat spikes were determined from morphological features of the inflorescence [14]: green anther stage (GA), yellow anther stage (YA), tipping stage (TP),heading stage (HD), anthesis (AN), and 5, 10, 15, 20, 25, 30, and 35 d after anthesis DFA).
2.2.Plasmid construction and wheat transformation
TaSus1 was knocked out using the CRISPR/Cas9 system.TaSus1 homoeologs in the three genomes, TraesCS7A02G158900 (TaSus-A1), TraesCS7B02G063400 (TaSus-B1), and TraesCS7D02G159800(TaSus-D1), were retrieved from the International Wheat Genome Sequencing Consortium database [15].Conserved exon sequences of the three homoeologs were used to design a single guide RNA(sgRNA) using WheatCrispr (https://crispr.bioinfo.nrc.ca/Wheat-Crispr/; Fig.S1).The sgRNA was cloned into the pWMB110-SpCas9-TaU3 vector following Liu et al.[16].The resulting vector was transformed into wheat cultivar Fielder (wild type) by Agrobacterium tumefaciens (strain EHA105)-mediated transformation.Seeds of 11 T0events were collected, and mutations were identified by Sanger sequencing.A 4-bp deletion and a 1-bp insertion were the most frequently identified mutations at the target sites.
2.3.Measurement of sugar content
Sugar content was measured in 2-week-old wild-type and tasus1-1 seedlings.Glucose, fructose, and sucrose were detected by MetWare (https://www.metware.cn/).Agilent 7890B gas chromatograph coupled to a 7000D mass spectrometer with a DB-5MS column(30 m length×0.25 mm i.d.×0.25 μm film thickness,J&W Scientific, Folsom, CA, USA) was used for GC–MS/MS analysis of sugars.
2.4.RNA extraction and RT-qPCR assays
Fresh carpel tissues were removed from spikes and ground into powder in liquid nitrogen.Total RNA was isolated from the samples using a Plant RNA Extraction kit (TIANGEN, # DP441).Firststrand cDNA was synthesized using a qPCR RT Kit (TOYOBO,#FSQ-101).Quantitative PCR was performed using a Bio-Rad CFX machine and SYBR Premix Ex Taq Mix (TaKaRa #RR420A).The wheat Actin gene was used as an internal control.All primers used are listed in Table S2.We used previously published datasets to examine the expression patterns of TaSus-A1, TaSus-B1, and TaSus-D1 in other five tissues (grain, leaf, root, spike, and stem)[17].
2.5.Identifying SNPs in TaSus-A1
The latest version of VMap (VMap 2.0) [18] is based on 1,062 wheat accessions with multiple ploidy levels.Among these accessions, we selected 306 hexaploid wheat accessions collected from Europe, Asia (excluding the Middle East), the Middle East, South America, Africa, North America, and Oceania (Table S1) to characterize regional differences in the sequence of the TaSus1-A1 gene.A large-scale screening of gene-based SNPs was performed using 40,710,923 SNPs (with minor-allele frequency [MAF] > 0.05).VCFtools v0.1.13 (https://github.com/vcftools/vcftools/releases/tag/v0.1.13) was used to identify SNPs in genes (vcftools --vcf emd2_315merge_clean.VCF --chr $chr --from BP --to BP --recode--recode-info-all).The input file was in VCF file format,corresponding to the chromosome,starting position,and ending position of the gene.TASSEL v5.2.64 (https://www.maizegenetics.net/copy-of-tassel)was used to open the VCF file.Some SNPs were degenerate bases.Using the run_pipeline.pl in TASSEL, degenerate bases were replaced by ‘‘NA”.SNPs in TaSus1-A1 were identified and confirmed to be significantly associated with the corresponding traits.We used a previously published dataset[12]to examine the percentages of the two alleles for the TaSus-A1 SNP in cultivars from China, Europe, and North America collected over the past century.
3.Results
3.1.Phenotypes of TaSus1 CRISPR/Cas9 mutant lines
Two independent lines (tasus1-1 and tasus1-2) with all three homoeologous genes mutated were selected from the T1 progeny.Translation of TaSUS1 was expected to be terminated prematurely in the mutants owing to the introduction of premature stop codons(Fig.S1; Table S3).
Knocking out all three TaSus1 homoeologs significantly shortened flowering time and accelerated spike growth relative to the wild type (Fig.S2; Table S4).Spike morphology differed in GNS between the mutants and the wild type(Fig.1A–C;Table S5).There were negative effects on all aspects of spike morphology in the two TaSus1 mutants:FSN,spike length(SL),GNST,grain weight per spikelet (GWST),TGW, GNS, grain weight per spike (GWS), and spike weight (SW) (Fig.1A–D; Table S5).
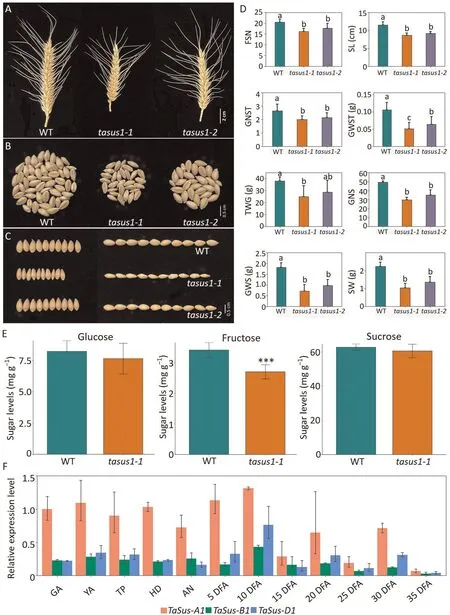
Fig.1.Phenotypic characterization of spike traits in TaSus1 CRISPR/Cas9 mutant lines.Two triple-knockout lines for TraesCS7A02G158900, TraesCS7B02G063400, and TraesCS7D02G159800 were generated by CRISPR/Cas9-mediated genome editing.(A, B) Representative images of spikes (A) and kernels from individual spikes (B) of wildtype(WT)Fielder and knockout plants tasus1-1 and tasus1-2.(C)Representative images showing width and length of kernels from WT and mutant lines.(D)Fertile spikelet number per spike(FSN),spike length(SL),grain number per spikelet(GNST),grain weight per spikelet(GWST),thousand-grain weight(TGW),grain number per spike(GNS),grain weight per spike(GWS),and spike weight(SW)of WT and knockout lines.Values are means±SD(n=5).Significant differences were determined by ANOVA.Different lowercase letters indicate significant differences (P < 0.05).(E) Glucose, fructose, and sucrose contents in 2-week-old WT and tasus1-1 seedlings.**, significant difference at P < 0.01 by Student’s t-test.(F) RT-qPCR analysis showing the relative transcript levels of TaSus-A1, TaSus-B1, and TaSus-D1 in carpels at each of the 12 stages of development (normalized to Actin): green anther stage (GA), yellow anther stage (YA), tipping stage (TP), heading stage (HD), anthesis (AN), and 5, 10, 15, 20, 25, 30, and 35 days after anthesis (DFA).The experiment was conducted in three technical replicates.Values are means ± SD.
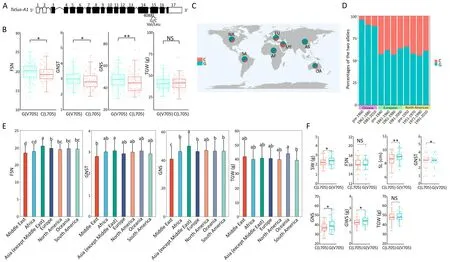
Fig.2.A SNP in TaSus-A1 (TraesCS7A02G158900) is associated with grain number per spike (GNS).(A) TaSus-A1 gene structure and associated SNP (chromosome 7A:115,204,602;position 4088 bp of the TaSus1 genomic region,or 2113 bp of the TaSus1 coding sequence,in the 15th exon).(B)Fertile spikelet number per spike(FSN),grain number per spikelet (GNST), grain number per spike (GNS), and thousand-grain weight (TGW) for accessions carrying each of the two alleles for the above SNP in 306 hexaploid wheat accessions distributed worldwide.(C)Geographical distribution of the TaSus-A1 alleles in seven continents or regions.(D)Proportions of the two alleles of the SNP in Chinese,European,and North American cultivars collected over the past century.Red and blue indicate the C and G alleles,respectively.(E)FSN,GNST,GNS,and TGW across the seven continents or regions.Significant differences in seven continents were identified by ANOVA.Different lowercase letters indicate significant differences(P<0.05).(F)Introgression of the G allele of TaSus-A1 improved grain yield-related traits.Spike weight(SW),FSN,spike length(SL),GNST,GNS,grain weight per spike(GWS),and TGW are shown for lines carrying the C allele (NILC) or the G allele (NILG).Values are means ± SD (n = 5) in (B) and (F).Significant differences in the two alleles were determined by Student’s t-test (two sided, *, P < 0.05; **, P < 0.01).NS, not significant.
TaSus1 mutant plants displayed consistent phenotypes for GNS in repeated experiments (Fig.S3).Thus, loss of TaSus1 function affected GNS.To investigate any alteration in carbohydrate contents in the mutants, we measured fructose, glucose, and sucrose levels in 2-week-old tasus1-1 and wild-type seedlings.Fructose content was lower in tasus1-1 than in Fielder, whereas there were no differences in glucose or sucrose content between tasus1-1 and wild-type seedlings (Fig.1E).
3.2.Expression patterns of TaSus1 homeologs
All three genes (TaSus-A1, TaSus-B1, and TaSus-D1) were expressed at high levels in spikes, roots, stems, and grain but at low levels in leaves(Fig.S4).Because carpel development determines the final size and weight of grain,we measured the expression of all three TaSus1 homeologs in carpel tissues collected at 12 developmental stages.TaSus-A1 was expressed at high levels during early spike development and at lower levels at subsequent developmental stages (Fig.1F).Although the expression levels of the three homoeologous genes were similar in other tissues(Fig.S4), the transcript abundance of TaSus-A1 was much higher than those of TaSus-B1 and TaSus-D1 during early carpel development (GA to 10 DFA stages) (Fig.1F).
3.3.Effects of one SNP in TaSus-A1 on GNS
Because TaSus-A1 was identified as a candidate gene influencing grain yield-related traits in our previous GWAS,and its expression level during carpel development was higher than those of TaSus-B1 and TaSus-D1 (Fig.1F), we hypothesized that it functions in grain development and focused on examining the relationships between SNPs in TaSus-A1 and spike traits.
The TaSus-A1 gene consists of 17 exons and 16 introns(Fig.S5A).Five SNPs were found in TaSus-A1, with single SNPs in exons 2, 6, 10, and 15 and intron 6 (Fig.S5A).The SNP in exon 15 leads to an amino acid change.These five SNPs generated four haplotypes, which were named Hap-1, Hap-2, Hap-3, and Hap-4(Fig.S5A).GNS was significantly higher in accessions with Hap-1 than in those with Hap-2 or Hap-3 (Fig.S5B).Only the fifth SNP represented a nonsynonymous mutation, whereas the other SNPs were synonymous substitutions or were located in intron regions.It was accordingly chosen for further examination.
The fifth SNP (chromosome7A: 115,204,602; position 4,088 bp of the TaSus-A1 genomic region, or 2113 bp of the TaSus1 coding sequence,in the 15th exon;Figs.2A,S5A)changes a valine to a leucine(amino acid 705 of TaSus-A1,V705 or L705).Accessions carrying the G allele(V705)showed significantly higher FSN,GNST,and GNS than those carrying the C allele(L705)(Fig.2B;Table S6).However,the G allele(V705)showed no effect on TGW.
3.4.Geographic differentiation and breeding selection of the SNP in TaSus-A1
The G allele(V705)and the C allele(L705)of TaSus-A1 were present in respectively 208 and 92 accessions(Table S1),with varying geographical distributions.For each geographical area, we determined the distribution of the two alleles among local accessions.More accessions harbored the G allele (V705) than the C allele(L705, Fig.2C; Tables S1, S7).The G allele (V705) was present in a larger fraction of the accessions in Asia (except the Middle East)and Africa than of those in other areas (Fig.2C; Tables S1, S7).A higher proportion of landraces from the Middle East carried the C allele (L705) than those from other areas (Fig.2C; Tables S1, S7).More fertile spikelets and more kernels per individual spikelet and individual spike were found in accessions from Asia (except the Middle East) than in those from the Middle East (Fig.2E;Table S8).The frequency of the C allele(L705)increased in Chinese and North American cultivars over this period, with a slight decrease among North American cultivars between 1991 and 2010 (Fig.2D; Table.S9).
3.5.Use of TaSus-A1 alleles for improvement of grain yield-related traits
The C-to-G SNP in TaSus-A1 increased GNS without influencing TGW, indicating its potential to increase grain yield.To compare the contributions of the TaSus-A1 alleles to grain yield-related traits and evaluate their value in wheat breeding, we developed near-isogenic lines (NILs).
Compared with the line carrying the C allele(NILC),the line carrying the G allele (NILG) had longer spikes (8.65 cm for NILC,8.99 cm for NILG,P<0.01).More kernels per spikelet(2.95 for NILC,3.13 for NILG,P<0.05)with a similar number of fertile spikelets led to more kernels per spike (37.57 for NILC, 39.88 for NILG, P < 0.05)in NILG.The larger number of kernels per spike and similar TGW resulted in higher GWS (1.82 g for NILC, 1.96 g for NILG, P < 0.05)and greater SW (2.27 g for NILC, 2.42 g for NILG, P < 0.05) in NILG(Fig.2F; Table S10).These results suggest that the G allele of TaSus-A1 at this SNP has the potential to increase GNS without reducing grain size, thereby increasing grain yield.We developed a marker to distinguish the C and G alleles.Restriction endonuclease ApaL I can be used to identify the SNP in fragments of TaSus1-A1 (Fig.S6).
4.Discussion
A previous study[12]revealed a connection between the haplotypes of TaSus1 and TGW;however,no mutants of TaSus1 had been developed.We validated the effects of TaSus1 on TGW by phenotypic analysis of newly generated CRISPR/Cas9 mutant lines and measured the effects of TaSus1 on GNS in wheat.The measurement of sugar content in tasus1-1 was consistent with the effects of loss of sucrose synthases in other species [19,20], demonstrating that the sucrose metabolic pathway is indeed affected in the TaSus1 mutant.The greater severity of the phenotype of tasus1-1 than that of tasus1-2(Fig.1A–D)may be due to differences in the underlying editing mutations (Fig.S1).We found that the expression level of TaSus-A1 was higher than that of TaSus-B1 or TaSus-D1 during early carpel development (GA-5 DAF stages) (Fig.1F), suggesting that TaSus-A1 may play a more important role in carpel development.In a previous transcriptome study [17], expression of TaSus-A1 was higher in other organs than in the grain (Fig.S4), possibly because the kernels used for expression profiling were at a later development stage.
Kernels with faster growth rates have higher sucrose synthase activity than those with slower rates of dry matter accumulation,highlighting the importance of sucrose synthase activity in rapidly growing kernels [21].We suggest that the shorter times to reach the various stages of spike development in the TaSus1 knockout lines were responsible for the shorter spikes,fewer kernels per spikelet,and fewer fertile spikelets per spike,leading to fewer kernels per spike and lower GWS compared with the wild type.
Among the 306 wheat accessions, those from Asia (excluding the Middle East) had the greatest proportion with the G allele(V705) of the SNP (high GNS), while those from the Middle East had the lowest proportion.The frequency of the C allele (L705)fluctuated among European cultivars.The consistently high frequency of the G allele (V705) and the phenotypic differences between cultivars suggest a role for this TaSus-A1 SNP in determining GNS in wheat cultivars in these seven continents and regions.Overall,our work demonstrates that TaSus1 controls GNS in wheat and its potential application for wheat improvement.
CRediT authorship contribution statement
Liping Shen:Validation,Visualization,Writing–review&editing.Lili Zhang:Validation.Changbin Yin:.Xiaowan Xu:Resources.Yangyang Liu:Software,Formal analysis,Visualization.Kuocheng Shen:Software, Formal analysis, Visualization.He Wu:Validation.Zhiwen Sun:Validation.Ke Wang:Validation.Zhonghu He:Resources.Xueyong Zhang:Resources.Chenyang Hao:Resources, Data curation.Jian Hou:Resources.Aoyue Bi:Resources, Data curation.Xuebo Zhao:Resources, Data curation.Daxing Xu:Resources, Data curation.Botao Ye:Investigation.Xuchang Yu:Investigation.Ziying Wang:Investigation.Danni Liu:Investigation.Yuanfeng Hao:Conceptualization, Supervision,Writing–review&editing.Fei Lu:Conceptualization,Supervision,Writing – review & editing.Zifeng Guo:Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing –review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Jingdan Han,Institute of Botany,Chinese Academy of Sciences, for assistance with TaSus1 homoeologs analysis.This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDA24010104-2).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.11.007.
杂志排行
The Crop Journal的其它文章
- Flowering-time regulation by the circadian clock: From Arabidopsis to crops
- Global characterization of OsPIP aquaporins reveals that the H2O2 transporter OsPIP2;6 increases resistance to rice blast
- Drought-triggered repression of miR166 promotes drought tolerance in soybean
- The OsBSK1-2-MAPK module regulates blast resistance in rice
- Natural variation of an autophagy-family gene among rice subspecies affects grain size and weight
- Rice gene OsUGT75A regulates seedling emergence under deep-sowing conditions
