Wetting alternating with partial drying during grain filling increases lysine biosynthesis in inferior rice grain
2024-03-07YiJiangWenliTaoWeiyangZhangZhiqinWangJianchangYang
Yi Jiang, Wenli Tao, Weiyang Zhang, Zhiqin Wang, Jianchang Yang*
Jiangsu Key Laboratory of Crop Genetics and Physiology/Co-Innovation Center for Modern Production Technology of Grain Crops, Yangzhou University, Yangzhou 225009,Jiangsu, China
Keywords:Brassinosteroids Inferior grain Lysine biosynthesis Rice Wetting alternating with partial drying
ABSTRACT Lysine content is a criterion of the nutritional quality of rice.Understanding the process of lysine biosynthesis in early-flowering superior grain (SG) and late-flowering inferior grain (IG) of rice would advance breeding and cultivation to improve nutritional quality.However,little information is available on differences in lysine anabolism between SG and IG and the underlying mechanism,and whether and how irrigation regimes affect lysine anabolism in these grains.A japonica rice cultivar was grown in the field and two irrigation regimes, continuous flooding (CF) and wetting alternating with partial drying (WAPD),were imposed from heading to the mature stage.Lysine content and activities of key enzymes of lysine biosynthesis, and levels of brassinosteroids (BRs) were lower in the IG than in the SG at the early grainfilling stage but higher at middle and late grain-filling stages.WAPD increased activities of these key enzymes, BR levels, and contents of lysine and total amino acids in IG, but not SG relative to CF.Application of 2,4-epibrassinolide to rice panicles in CF during early grain filling reproduced the effects of WAPD, but neither treatment altered the activities of enzymes responsible for lysine catabolism in either SG or IG.WAPD and elevated BR levels during grain filling increased lysine biosynthesis in IG.Improvement in lysine biosynthesis in rice should focus on IG.
1.Introduction
In rice,whose grain feeds over half of the world’s population[1],content of the amino acid lysine is a criterion of nutritional quality[2,3].Insufficient intake of lysine not only leads to metabolic,physiological, and functional diseases in humans, but hinders the uptake and use of other amino acids[3–5].Accordingly,increasing grain lysine content is a target of rice breeding and cultivation[3–6].
Grain on a rice panicle can be divided into two types: superior grain (SG) and inferior grain (IG) [7–9].SG, located in the apical part of the panicle, usually flowers earlier, fills faster, and weighs more than the late-flowering IG, located in the basal part of the panicle [7–9].Although grain filling of SG and IG and its mechanisms have been extensively investigated [7–9], it is not known whether there is a difference in lysine content between the two types of kernel and what mechanism accounts for it.
Lysine is synthesized in the aspartate pathway in rice kernels,and there are three key enzymes involved in its biosynthesis:aspartate kinase (AK, EC 2.7.2.4), homoserine dehydrogenase(HSDH; EC 1.1.1.3), and dihydropicolinate synthase (DHDPS, EC 4.2.1.52).[10–12].In contrast, lysine α-ketoglutarate reductase(LKR; EC 1.5.1.8) and saccharopine dehydrogenase (SDH, EC 1.5.1.9) are considered bi-functional lysine-degrading enzymes[11–13].Either increasing activities of AK, HSDH, and DHDPS or inhibiting LKR and SDH activities increased lysine content in cereal seeds [10–13].However, little information is available about dymamics of lysine biosynthesis and catabolism in SG and IG during grain filling, and whether such dymamics are associated with lysine content.
It is believed [5,14,15] that plant hormones, especially brassinosteroids(BRs), function in amino acid biosynthesis in rice grain.A rice cultivar with a higher lysine content showed higher levels of grain 2,4-epibrassinolide (2,4-epiBL) and 2,8-homobrassinolide(2,8-homoBL)than one with a lower lysine content,whereas other phytohormones did not differ between the two cultivars [5,14].Applying 2,4-epiBL, a synthetic BR, increased grain lysine content,whereas applying other plant growth regulators, such as zeatin riboside or gibberellin 3, showed no effects [5,14].It is desirable to know how BRs function in SG and IG during grain filling, and how to regulate their levels to increase lysine biosynthesis there.
Irrigation influences both the quantity and quality of rice yield[16–18].Alternate wetting and drying(AWD)has been extensively adopted in world rice production[17–19].Although it saves water,its influence on quantity and quality of rice yield and grain lysine content is disputed[19–21].In our earlier work[22–24]a wetting alternating with partial soil drying (WAPD) regime during grain filling,in which leaf photosynthesis was not impaired, accelerated grain filling, thereby increasing grain yield.It remains unclear whether a WAPD regime could increase grain lysine content,especially in IG, by increasing lysine biosynthesis.
This study aimed to test the hypothesis that a WAPD regime not only promotes rice grain filling but increases lysine biosynthesis by elevating grain BR levels.The experimental plan was to compare SG and IG with respect to grain filling, amino acid contents, activities of lysine biosynthetic enzymes, BR levels, and responses of these characters,leaf photosynthesis, and root activity,to application of exogenous BRs and a BR inhibitor.
2.Materials and methods
2.1.Plant materials and cultivation
A high-yielding rice (Oryza satica L.) cultivar Jinxiangyu 1 (a japonica inbred),was used.Seeds were sown on May 20 and seedlings were transplanted on June 15 in 2020 and 2021.The hill space was 0.25 m × 0.15 m with two seedlings per hill.The full amount of N was applied at 210 kg ha-1as urea on the day of rice transplanting,10 d after transplanting(DAT),38 DAT(young panicle differentiation), and 54 DAT(pistil and stamen differentiation)in the ratio 4:2:2:2.The respective rates of P(P2O5)and K(K2O)were 105 and 90 kg ha-1, applied on the transplanting day.Except for midseason drainage at 32–36 DAT, a water level of 4–5 cm was maintained in the field until heading (July 28 in 2020 and July 29 in 2021), when irrigation treatments were begun.
The experimental field was located on a farm at Yangzhou University, Yangzhou, China.The soil texture, contents of N, P,and K, and field moisture capacity were described previously[25].The main weather factors during the rice-growing season in both years are presented in Fig.S1.
Field experiments were also performed in 2018 and 2019 using the indica rice cultivar Yangdao 6.Except for the total N rate of 180 kg ha-1for this cultivar, other growth conditions were the same as Jinxiangyu 1.
2.2.Irrigation treatment
Irrigation treatments were applied from heading to the mature stage (74–129 DAT) of rice.They consisted of continuous flooding(CF)and wetting alternating with partial drying(WAPD).Under the CF regime, a 4–5 cm water level was maintained in the field until seven days before the plants were finally harvested,a practice consistent with farmer water management[26].In the WAPD regime,the water potential threshold was-10 kPa at 15–20 cm soil depth.The soil was naturally dried from the 4–5 cm water level to the threshold, then re-watered and naturally dried again (Fig.S2).The soil water potential was monitored with tensiometers as described previously [25].The experiment followed a randomized complete block design with three replications.Plot size was 6.25×5.10 m and plots were separated by a cement ridge(60 cm wide,40 cm tall, and 50 cm deep in the soil) built between plots to prevent water and fertilizer runoff.A removable rain shelter was built over the experimental field.
2.3.Sampling and measurement of grain contents
Approximately 600 panicles heading on the same day were tagged in each treatment.The SG and IG on tagged panicles were sampled separately following Yang et al.[8].Eight to ten tagged panicles from each plot were sampled every 3 d from anthesis to the final harvest.Of the sampled kernels (both SG and IG), 80%were used for measurement of amino acids, enzymatic activities,and BR levels, and 0.2 were used for measuring grain filling rate.The contents of lysine,aspartate,and total amino acids were measured following Zhang et al.[27] using a high-speed amino acid analyzer(Biochrom 30,Cambridge,UK).Activities of lysine biosynthetic and catabolic enzymes were determined as described previously:AK and HSDH,Azevedo et al.[28];DHDPS,Varisi et al.[29];LKR and SDH,Gaziola et al.[30].Both 2,4-epiBL and 2,8-homoBL in kernels were extracted and purified following Ding et al.[31], and quantified following Chen et al.[32].The grain-filling processes and increases of lysine and total amino acid contents in grain were fitted with Richards’ equation [33]:
where W is grain weight,C is content of lysine or total amino acids,A is maximum grain weight,or maximum content of lysine or total amino acids,t is time after anthesis(days),and B,k,and N are coefficients of the exponential expression.The active grain-filling period and active period of lysine or total amino acid increase (D), the mean increase rate (Gmean) and maximum increase rate (Gmax) of lysine or total amino acids, and days to reach the maximum increase rate of lysine or total amino acids(Dmax.g) were calculated using the equation following Zhu et al.[34].
Leaf water potential and photosynthetic rate of the flag leaf,root biomass, and root oxidation activity (ROA) were measured 10 d after heading (DAH) (D1) and 30 DAH (D2) in 2020 and 10 DAH (D1) and 29 DAH (D2) in 2021, respectively, when soil water potential was approximately-10 kPa in the WAPD.They were also measured 12 DAH (W1) and 32 DAH (W2) in 2020 and 12 DAH(W1) and 31 DAH (W2) in 2021, during the wetting period.Measurement details of these parameters were described previously[25].
At the mature stage (October 22 in both 2020 and 2021, 129 DAT),plants were harvested for measurement of rice yield components, quantity, and quality.Yield components were determined from the 20 plants and grain yield was measured for plants harvested in an area of 5.4 m2in each plot following Wang et al.[25].Approximately 800 g of grain from each plot was used for quality measurement following Zhang et al.[35].
2.4.Chemical treatment
At 4,8,and 12 DAH,10 μmol L-12,4-epiBL,10 μmol L-1brassinazole (BRZ, an inhibitor of BR biosynthesis), or 10 μmol L-12,4-epiBL + 10 μmol L-1BRZ were sprayed on panicles at the rate of 50 mL m-2for each application.Control plants were treated with the same volume of deionized water.Leaves were wrapped with plastic film during spraying to reduce the effect of the chemicals on leaf photosynthesis.Each chemical treatment was applied to three plots as replications with 6.3 m2(3.0 m × 2.1 m) for each plot.Activities of AK, HSDH, DHDPS, SDH, and LKR were determined 6,10,and 14 d after anthesis and contents of lysine and total amino acids in milled rice were measured as described above after final harvest.
2.5.Statistical analysis
SPSS 20.0(IBM,Armonk,NY,USA)was used to perform analysis of variance.Treatment means were compared by least significant difference (LSD).Pearson’s correlations among BR levels, enzymatic activities, and grain lysine content were calculated.
3.Results
3.1.Grain yield, grain filling rate, and milling, appearance,and eating qualities
In comparison with the CF, WAPD increased grain yield by 11.6%–12.3%, owing mainly to increases in seed-setting rate and 1000-kernel weight (Table 1), leaf photosynthesis and ROA(Figs.S3,S4).Because irrigation treatments were initiated at heading time, they did not affect numbers of panicles and spikelets(Table 1).Similar influences of irrigation regime on yield were observed in Yangdao 6 (Table S1).
As shown in Fig.S5,WAPD markedly increased grain filling and grain weight of the IG in comparison with the CF.No differences between the two irrigation regimes in the filling and weight of SG were observed (Fig.S5A–D; Table S2).
Similarly, WAPD markedly improved milling, appearance, and taste qualities of the IG (Tables S3, S4, S5).The SG did not differ in these quality traits between CF and WAPD.
3.2.Contents of lysine and total amino acids and their increasing rates
Contents of lysine and total amino acids in both SG and IG,based on mg g-1dry weight(DW),first increased and reached their peak values at 9–12 d after anthesis(DAA)for the SG and at 18–21 DAA for the IG,and then sharply decreased(Figs.1A,B,2A,B).If the contents were expressed as μg or mg kernel-1, their changes or increase rates (μg kernel-1d-1) showed trends similar to those of grain-filling rates (Figs.1C–F, 2C–F).During the rapid increase period (3–21 DAA for the SG and 9–33 DAA for the IG), the rates of increase of the mean(Gmean)and maximum(Gmax),and the contents of lysine and total amino acids for the IG were greater in the WAPD than in the CF(Figs.1A–F,2A–F;Tables S6,S7).However,in the SG they did not differ between the CF and the WAPD.Similarly,contents of lysine and total amino acids,either mg g-1DW or μg or mg kernel-1, in the milled IG were markedly increased in the WAPD in comparison with those in the CF, but did not differ in the milled SG between the CF and the WAPD (Table S8).
To determine whether the increase in lysine content was due to the increase in aspartate content in the IG in the WAPD,changes in aspartate content were measured in both SG and IG.As shown in Fig.S6, aspartate content in either SG or IG, expressed as either mg g-1DW or μg kernel-1, did not differ between CF and WAPD(Fig.S6A–D).Thus aspartate, the substrate for lysine synthesis,did not account for the difference in lysine content in the IG between the CF and the WAPD.As shown in Fig.S7, the mean increase rate (Gmean) and maximum increase rate (Gmax) of lysine or total amino acids during the rapid increase period were positively correlated(r=0.92**to 0.99**,Fig.S7A–D)with the contents(μg or mg kernel-1) of lysine and total amino acids in milled rice,suggesting that increased lysine biosynthesis during grain filling contributed to the increases in lysine and total amino acids in the milled IG under the WAPD regime.
3.3.Activities of key enzymes in lysine anabolism
Activities of the lysine biosynthetic enzymes AK, HSDH, and DHDPS showed similar changing trends during grain filling, first increasing to maximums at 9 DAA for SG and 18 DAA for IG and then decreasing(Fig.3A–F).Relative to IG,SG showed higher activities of these enzymes at early grain filling (3–12 DAA), but lower activities from 15 DAA.During 3–33 DAA, WAPD increased enzymatic activities in IG but not in SG, relative to the CF (Fig.3A–F).The activities of the three enzymes were closely correlated with lysine and total amino acid contents in the grain during the active grain-filling period (3–33 DAA) (Fig.S8A–F).
Activities of the lysine catabolic enzymes SDH and LKR and the ratio of SDH to LKR(SDH/LKR),reached their peak value at 18 DAA for the SG and at 33 DAA for the IG (Fig.S9A–F),in contrast to the changing trend of lysine content.Activities of SDH and LKR and SDH/LKR did not differ in either SG or IG between the CF and the WAPD (Fig.S9A–F), indicating that irrigation regime did not influence lysine catabolism.
3.4.BR levels and their relationship with lysine biosynthesis
In agreement with the activities of AK, HSDH, and DHDPS, the levels of 2,4-epiBL and 2,8-homoBL reached their maximum at 9 DAA in the SG and at 18 DAA in the IG(Fig.4A–D).WAPD increased 2,4-epiBL and 2,8-homoBL levels in the IG but did not increase them in the SG, relative to the CF (Fig.4A–D).In the active grainfilling period, levels of 2,4-epiBL or 2,8-homoBL were correlated(r = 0.64**to 0.93**, P < 0.01) with the activities of AK, HSDH, and DHDP and the contents of lysine and total amino acids(Fig.S10A–J).Application of 2,4-epiBL to panicles in the CF regime at early grain filling increased the activities of AK, HSDH, and DHDPS, grain weight of IG, and the contents of lysine and total amino acids in IG, whereas applying BRZ produced the opposite effects (Tables 2, 3).The negative effect of applying BRZ on lysine biosynthesis was eliminated by simultaneous application of 2,4-epiBL and BRZ.Although BRZ reduced the activities of AK, HSDH,and DHDPS, grain weight of the SG, and the contents of lysine as well as total amino acids in the SG, application of 2,4-epiBL or 2,4-epiBL + BRZ did not markedly affect lysine biosynthesis and amino acid content in SG (Tables 2, 3).Applying either 2,4-epiBLor BRZ did not affect the activities of SDH and LKR in either SG or IG(Table 2).
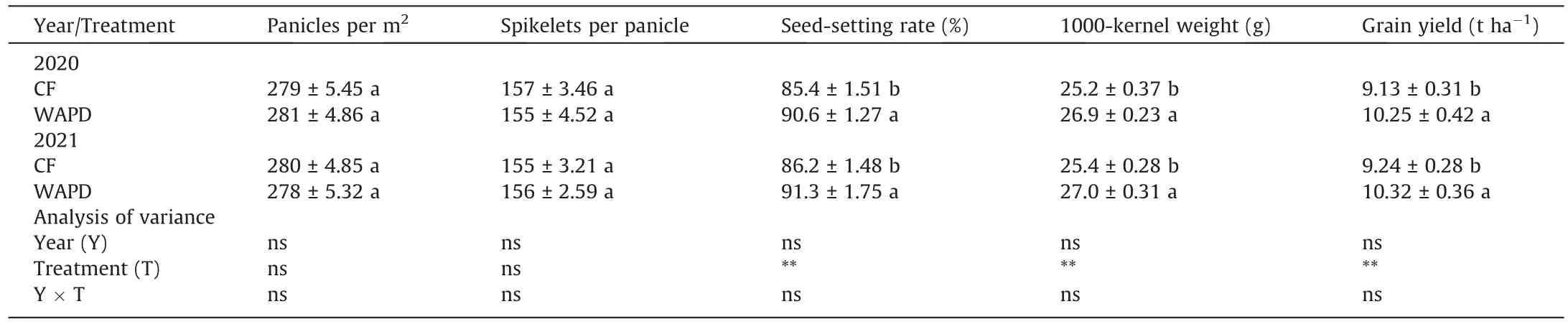
Table 1 Grain yield and its components in rice cv.Jinxiangyu 1.

Fig.1.Lysine content based on dry weight(A and B)and based on per kernel(C and D)and lysine increase rate(E and F)of rice grain(cv.Jinxiangyu 1).SG,superior grain;IG,inferior grain.CF and WAPD represent respectively continuous flooding and wetting alternating with partial drying,during grain filling.The lysine increase rate is fitted with Richards’ equation [33].Values in (A–D) are mean ± SE.
In Yangdao 6 the influence of irrigation regimes on lysine content in milled rice, changes in enzymatic activities and BR levels in filling grain,and effects of chemical applications on grain weight and lysine biosynthesis and catabolism in either SG or IG were similar (Tables S9–S13).
4.Discussion
4.1.Differences in lysine biosynthesis between SG and IG
In comparison with SG,IG showed a smaller mean increase rate(Gmean),a smaller maximum increase rate(Gmax),and a lower content of lysine based on either per unit dry weight or per grain at early grain filling and at maturity expressed as μg kernel-1(Figs.1,2; Table S8).Three conditions can result in a lower content of lysine in cereal seeds: insufficient substrate supply, lower ability of biosynthesis,and higher catabolic activity[4–6,12–14].The content (mg g-1DW) of aspartate, the substrate for lysine biosynthesis, did not differ between SG and IG at the early filling stage, and was higher in IG than in SG at later filling stages(Fig.S6).The activities of the two key enzymes in lysine catabolism, SDH and LKR,were lower in IG than in SG before 24 DAA (Fig.S9).Neither the adequate supply of substrate for lysine biosynthesis nor the reduced activities of lysine catabolic enzymes responsible for lysine catabolism explain the lower lysine content in IG during the active grain-filling period.The lower activities of the three lysine biosynthetic enzymes in IG than in SG at the early grainfilling stage and the correlation of these activities with grain lysine content suggests that the lower content of lysine in the IG was due mainly to lower biosynthetic ability at the early grain-filling stage.
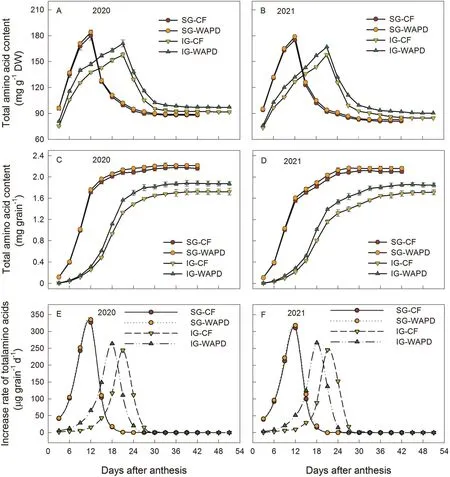
Fig.2.Contents of total amino acids based on dry weight(A and B)and based on per kernel(C and D)and total amino acids increase rate(E and F)of rice grain(cv.Jinxiangyu 1).SG, superior grain; IG, inferior grain.CF and WAPD represent respectively continuous flooding and wetting alternating with partial drying, during grain filling.The total amino acid increase rate was fitted with Richards’ equation [33].Values in (A–D) are presented as mean ± SE.
The mechanism explaining differences in lysine synthesis between SG and IG during grain filling is not fully understood.Many factors, such as source–sink relationship, carbon and nitrogen anabolism, and hormonal levels, could contribute to differences in lysine biosynthesis [12,27,36,37].Levels of 2,4-epiBL and 2,8-homoBL were much lower in IG than in SG during the active grain-filling period, in agreement with activities of AK,HSDH, and DHDPS and with grain lysine content (Figs.3, 4, S10).Application of 2,4-epiBL increased and that of BRZ reduced activities of these enzymes and lysine content in IG (Tables 2, 3, S12,S13).The lower levels of the BRs in IG than in SG during grain filling and the respective increases and reductions in lysine biosynthetic activity produced by application of the BR and inhibitor suggest that BRs regulate lysine synthesis and that a lower level of BRs leads to lower lysine synthesis in IG.Little is known, however,about how BRs regulate lysine biosynthesis.There are reports[5,14,37,38]that BRs mediate many biological processes including plant growth and development, organogenesis, plant configuration, fertility conversion, and stress resistance.It is possible that BRs could increase signal transduction in the aspartate-to-lysine pathway and expression of genes undertaking lysine synthesis,and accordingly, modulate amino acid biosynthesis [5,38].
The finding that the per-kernel lysine or total amino acid content was much greater in the milled SG than in the milled IG, but the per-dry-weight content was lower, may be explained by the lower grain dry weight and higher protein content of milled IG than milled SG (Fig.S5; Table S4).Because the content of amino acids including lysine is usually proportional to protein content in a cereal seed and is also influenced by grain weight [3–5], a lower grain dry weight can lead to a lower per-kernel content of amino acids, whereas a higher protein content and lower dry weight can lead to a higher dry-weight content of amino acids in milled IG.
4.2.The role of a WAPD regime in regulating lysine anabolism
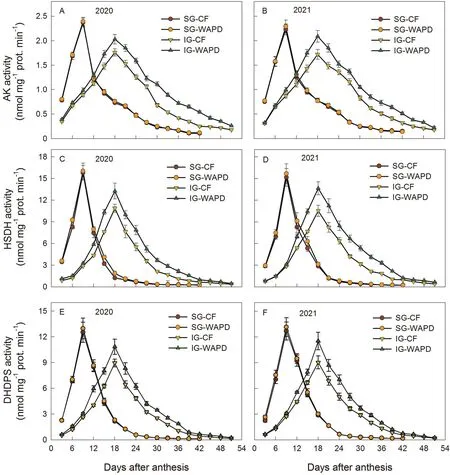
Fig.3.Activities of aspartate kinase(AK,A and B),homoserine dehydrogenase(HSDH,C and D),and dihydropicolinate synthase(DHDPS,E and F)in rice grain(cv.Jinxiangyu 1).SG,superior grain;IG,inferior grain.CF and WAPD represent respectively continuous flooding and wetting alternating with partial drying,during grain filling.Values are mean ± SE.
The finding that in comparison with the CF,the WAPD imposed during grain filling increased not only lysine but total amino acid content in IG (Figs.1, 2; Table S8) suggests that promoting lysine synthesis also increases other amino acid biosynthesis in grain.In the WAPD regime, increased grain filling rate, activities of enzymes responsible for lysine synthesis,and BRs levels in IG led to the increases in contents of amino acids including lysine(Figs.3,4,S5).Increases in leaf photosynthesis(Fig.S3C,D)and ROA(Fig.S4C,D)under the WAPD regime could also lead to an increase in amino acid biosynthesis in the grain by supplementing carbohydrates or providing more nutrients [5,21,39].The WAPD regime not only increased seed-setting rate and grain weight,leading to an increase in grain yield,but improved milling,appearance,cooking,and taste qualities of the IG (Tables 1, S1–S5).We conclude that a WAPD regime during grain filling can simultaneously improve rice grain yield and quality, in particular by increasing lysine content in the IG.
The WAPD regime or application of 2,4-epiBL did not markedly affect lysine biosynthesis and contents of lysine and total amino acids in the SG(Figs.1–3;Tables 2–3,S8-S13).One likely interpretation is that the levels of phytohormones such as BRs, and ability of amino acid biosynthesis are high in SG, and this grain could be insensitive or even unresponsive to the WAPD regime or 2,4-epiBL application.In another study [40], levels of polyamines,numbers of endosperm cells, and cell division rate differed among rice cultivars in IG but not in SG,and an effect of polyamine application on endosperm cell division and grain filling was observed only in IG.These findings suggest that IG has greater potential to increase grain quantity and quality than SG and that improvement in rice grain filling and amino acid biosynthesis should focus on IG.

Fig.4.Contents of 2,4-epibrassinolide (2,4-epiBL, A and B) and 2,8-homobrassinolide (2,8-homoBL, C and D) in the grain of rice (cv.Jinxiangyu 1).SG, superior grain; IG,inferior grain.CF and WAPD represent respectively continuous flooding and wetting alternating with partial drying, during grain filling.Values are mean ± SE.
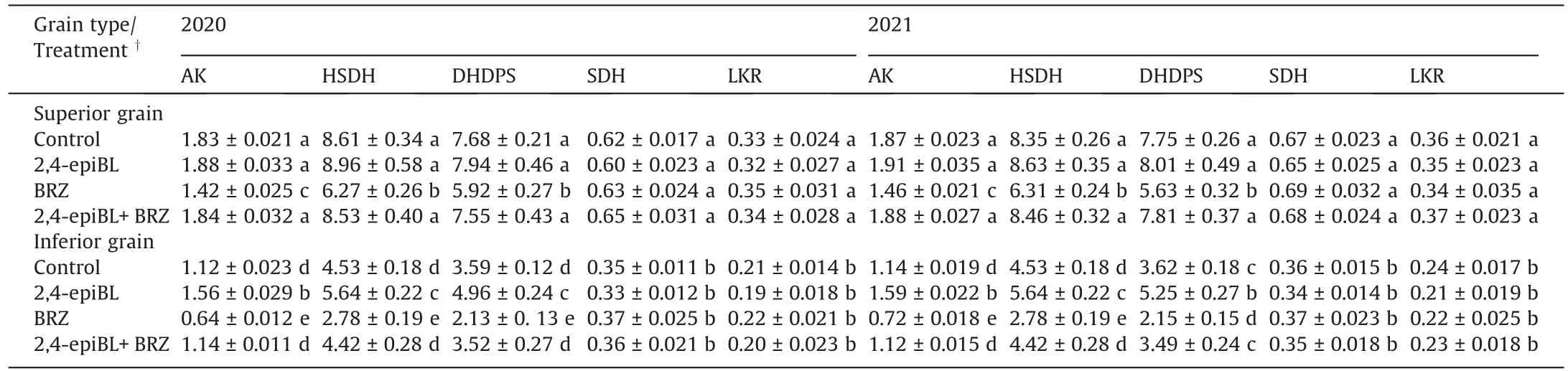
Table 2 Effects of chemical applications on activities of enzymes involved in lysine biosynthesis and catabolism in filling grain of rice (cv.Jinxiangyu 1).
Usually, higher activitees of AK, HSDH, and DHDPS are accompanied by higher activities of SDH and LKR to counterbalance increased lysine synthesis [11–13].But in the present study, the activities of AK, HSDH, and DHDPS were increased in the IG,whereas the activities of SDH and LKR were not greatly increased in either SG or IG under the WAPD regime (Figs.3, S9).Probably more lysine is incorporated into protein under such conditions[3–5].Thus, an increase in in lysine biosynthesis is not necessarily accompanied by an increase in lysine catabolism,such as under the WAPD regime.
Applying BRZ reduced grain weight, contents of lysine and total amino acids,and activities of AK,HSDH,and DHDPS,whereas applying 2,4-epiBL or BRZ did not change the activities of SDH and LKR,in both SG and IG(Tables 2,3,S12,S13).A possible explanation is that a higher level of BRs is necessary to maintain a higher grain-filling rate and a higher activity of lysine biosynthesis in the two types of filling kernels.Decreases in BRs levels, such as by application of BRZ,can inhibit grain filling and reduce the activity of lysine biosynthesis,leading to a reduction in grain weight and contents of amino acids not only in IG but in SG.The observation that application of 2,4-epiBL or BRZ did not affect the activities of SDH and LKR may be explained by an assumption that BRs function mainly in mediating lysine biosynthesis and not in lysine catabolism.
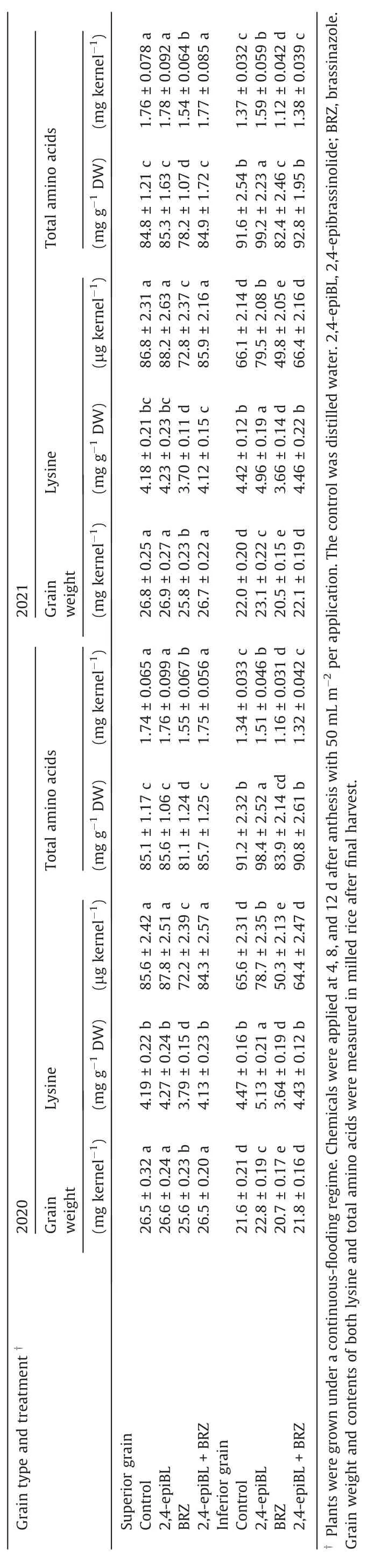
Table 3 Effects of chemical applications on grainweight andcontents of lysine andtotalaminoacidsin milled rice (cv.Jinxiangyu 1).
5.Conclusions
A WAPD regime imposed during grain filling elevated BR levels and increased lysine biosynthesis,increasing contents of lysine and total amino acids in IG.BRs function in mediating lysine biosynthesis.Neither WAPD or application of a lysine biosynthesis inhibitor altered lysine catabolism.Improvement of amino acid biosynthesis in rice should focus on IG.
CRediT authorship contribution statement
Yi Jiang:Investigation,Formal analysis,Visualization,Writing–original draft.Wenli Tao:Investigation,Formal analysis,Visualization.Weiyang Zhang:Investigation, Data curation, Formal analysis.Zhiqin Wang:Investigation, Formal analysis.Jianchang Yang:Funding acquisition, Project administration, Conceptualization, Supervision, Resources, Methodology, Writing – review &editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32071943, 32272198).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.11.008.
杂志排行
The Crop Journal的其它文章
- Corrigendum to ‘‘GmTOC1b negatively regulates resistance to Soybean mosaic virus”.[Crop J.11 (2023) 1762–1773]
- Decoding the inconsistency of six cropland maps in China
- OsDA1 positively regulates grain width in rice
- A polygalacturonase gene OsPG1 modulates water homeostasis in rice
- The ABA synthesis enzyme allele OsNCED2T promotes dryland adaptation in upland rice
- Trehalose: A sugar molecule involved in temperature stress management in plants
