Organo-mineral complexes in soil colloids:Implications for carbon storage in saline-alkaline paddy soils from an eight-year field experiment
2024-03-07MengmengCHENShirongZHANGLuLIUBaojianCHANGYuyiLIandXiaodongDING
Mengmeng CHEN ,Shirong ZHANG ,Lu LIU ,Baojian CHANG ,Yuyi LI and Xiaodong DING,*
1College of Resources and Environment,Qingdao Agricultural University,Qingdao 266109(China)
2Jingke High-tech Institute of Environmental Sciences Co.,Ltd,Beijing 100094(China)
3Institute of Agricultural Resources and Regional Planning,Chinese Academy of Agricultural Sciences,Beijing 100081(China)
ABSTRACT The combination of organic carbon(OC)and reactive minerals is a crucial mechanism of soil carbon(C)storage,which is regulated by the formation of organo-mineral complexes on the surface of soil colloids.The effect of organic fertilizer on the storage mechanism of OC in soil colloids was studied through an 8-year field experiment,which included four treatments:i)no fertilization(control,CK),ii)only mineral N,P,and K fertilization(NPK),iii)NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and iv)NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2).The main results indicated that organic fertilizer addition significantly increased the content of aromatic-C,which was 158.7%and 140.0%higher in soil colloids than in bulk soil in the NPKC1 and NPKC2 treatments,respectively.X-ray photoelectron spectroscopy further demonstrated that the relative proportion of C=C group on the surface of soil colloids was increased by 20.1%and 19.1%in the NPKC1 and NPKC2 treatments,respectively,compared with the CK.In addition,compared with the NPK treatment,the content of reactive minerals(such as Fe and Al oxides)significantly increased with organic fertilization,which was positively correlated with C=C group in soil colloids.This indicates that aromatic-C may be retained by the formation of aromatic-mineral complexes with reactive minerals in soil colloids.Organic fertilization also significantly increased OC storage efficiency(OCSE),which was significantly higher in the NPKC1 treatment than in the NPKC2 treatment.Therefore,a moderate amount of organic fertilizer application is a better agronomic practice to increase OCSE and OC storage in saline-alkaline paddy soils.
Key Words:aromatic-C,aromatic-mineral complex,organic fertilization,reactive mineral,soil organic carbon,water-dispersible colloid
INTRODUCTION
Soil organic carbon(SOC)pool is the most important carbon(C)pool in terrestrial ecosystems(Shahriariet al.,2011;Karcheganiet al.,2012;Falahatkaret al.,2014;Havaeeet al.,2014;Ajamiet al.,2016;Ayoubiet al.,2020;Niet al.,2021),and largely determines agricultural productivity,soil fertility,and the sustainability of cultivated land(Chenet al.,2022).The sequestration capacity of organic carbon(OC)in agricultural soils ranges from 140 to 170 Pg C(Lal,2004),accounting for over 10%of the world’s terrestrial OC storage and playing a crucial role in global C neutralization(Qinet al.,2013).As the largest artificial wetland in terrestrial ecosystems,paddy soil exhibits enormous potential for C sequestration(Kalbitzet al.,2013;Tianet al.,2015;Zeraatpishehet al.,2021).The formation of Fe-OC association by adsorption or co-precipitation of organic compounds with Fe oxides in paddy soil is beneficial for C storage(Wanget al.,2017).A recent continental-scale study in China showed that OC content in paddy soil in the non-growing season increased by 39% to 127% when compared with that in adjacent upland soil(Chenet al.,2021a).Liuet al.(2014)also demonstrated that OC storage improvement in paddy soil could enhance soil quality and mitigate global climate change.Thus,maintaining or increasing OC storage is of great significance for improving soil quality and mitigating global climate change in saline-alkaline paddy soil(Chenet al.,2021a).
The most direct and effective way to increase the storage of OC in soil is through the input of exogenous organic matter(such as organic fertilizer)(Liet al.,2018).Organic fertilization contributes to improve the interaction between OC and soil mineral surface(such as ligand exchange),which prevents the decomposition of OC(Vogelet al.,2015).Soil minerals,especially highly reactive minerals,act as a key factor in the storage of OC in soil(Barret al.,2014;Huanget al.,2019).For instance,Yuet al.(2017)found that poorly crystalline minerals more readily formed organic mineral complexes with organic matter than crystalline minerals,which played a crucial role in maintaining soil C storage.Huanget al.(2020) found that more than 47.1% OC was bound to Fe/Al in paddy soil.Organic fertilizer addition promotes the formation of reactive mineralsviaelectron transport in paddy soil (Ginnet al.,2017;Huanget al.,2018;Wenet al.,2019).Fertilization,especially organic fertilization,can affect the biochemical properties of soil minerals(Huanget al.,2020).Therefore,the effect of organic fertilizer on mineral protection mechanism of OC needs to be further studied in paddy soil.
Soil water-dispersible colloidal particles are among the most active soil particles and have large specific surface areas(Wenet al.,2014;Bagheriet al.,2021).As the main component of soil colloids,the conversion and accumulation of Al silicates and Fe/Al hydroxide mixtures are sensitive to fertilization practices(Regelinket al.,2014;Huanget al.,2020).Long-term organic fertilization can significantly increase the accumulation of poorly crystalline mineral colloids through mineral transformation(Wenet al.,2019).The dispersion and reaggregation of soil colloids may be greatly aggravated by the inherent wetting and drying alternation during rice cultivation(Hendersonet al.,2012).Through spectral analysis,after 22 years of organic fertilizer application in upland red soil,more metal ions were found at the interface of soil colloids than at soil interface (Wenet al.,2014).Huanget al.(2020)further proposed that organic fertilization could selectively enrich aromatic organic ligands on the surface of colloids and form organic-Fe complexes with poorly crystalline Fe,which can promote OC storage.However,the effect of the interaction between poorly crystalline Fe minerals and OC functional groups on OC storage mechanism in saline-alkaline paddy soil is still unclear,especially at soil colloid interface.Therefore,studying the interactive relationship between OC functional groups and minerals in soil colloids with organic fertilization will help clarifying the mechanism of C sequestration in saline-alkaline paddy soil.
It is estimated that,due to high salt content,the irrigated soil has declined approximately 25%crop productivity worldwide(Maoet al.,2016;Chenet al.,2023).Soils with excessive salt content account for about 7% of farmland in China,including the saline-alkaline soil in the Yellow River Delta area(Maoet al.,2016;Luoet al.,2017).High content of Na+will cause the dispersion of soil colloids,thus increasing the loss of C through microbial decomposition(Wuet al.,2021a).High soil pH affects the surface charge of Fe and Al oxides,which is not conducive to the stability and storage of SOC (Tavakkoliet al.,2015;Zhaoet al.,2018).Setiaet al.(2013)reported that soil C sequestration was inhibited by soil salinization and that the global loss of SOC was expected to reach 6.8 Pg by 2100.In our previous study,we found that organic fertilization could improve SOC storage by inhibiting soil C mineralization and improving the stability of soil structure in saline-alkaline paddy soil(Chenet al.,2022).However,the effect of organic fertilization on the mechanism of OC storage needs to be further explored at the soil colloid interface.Therefore,in the present study,we aimed to i)clarify the impact of 8-year organic fertilizer application on SOC stability,ii)elucidate the relationships between reactive minerals and the contents of SOC and soil colloid OC(SCOC),and iii)reveal the mineral protection and storage mechanisms of SCOC in saline-alkaline paddy soil.
MATERIALS AND METHODS
Experimental site and design
A field experiment was conducted in Dongying,Shandong Province,China(37°31′16.17′′N,118°32′28.96′′E),starting in May 2014.The study site has a warm temperate continental monsoon climate,with a mean annual temperature of 13°C and mean annual precipitation of 560 mm.The soil is classified as a salic Fluvisol according to World Reference Base for Soil Resources.The texture of topsoil(0-20 cm depth)was silt loam soil,containing 172.0 g kg-1clay,617.0 g kg-1silt,and 211.0 g kg-1sand,which is slightly salinized in this area.Before the field experiment(May 2014),soil properties were determined as follows:SOC 4.9 g kg-1,total N(TN)1.1 g kg-1,total P(TP)0.34 g kg-1,total K(TK)1.1 g kg-1,pH 8.1(1:5 soil/deionized water,weight:volume),and electrical conductivity(EC)0.41 mS cm-1.
In the present study,four treatments were included:no fertilization (control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1year-1)of organic fertilization(NPKC2).The experiment was in a completely randomized design with three replicates and each plot was in the size of 3.0 m×5.0 m(width×length).In the NPK,NPKC1,and NPKC2 treatments,the total inputs of N,P2O5,and K2O were 255,128,and 229 kg ha-1year-1,respectively.In addition,450 and 900 kg C ha-1year-1were applied to the NPKC1 and NPKC2 treatments,respectively.The mineral N,P,and K fertilizers were urea(N 46%),superphosphate(P2O516%),and potassium sulfate(K 50%),respectively.Organic fertilizer (pH 6.6) was produced from soybean litter and soybean,containing 261.0,24.0,16.0,14.0,and 1.8 g kg-1C,N,P,K,and FeO,respectively.Both organic and P fertilizers were applied once as base fertilizer.N fertilizer was applied four times as base fertilizer (20%),tiller fertilizer (40%),panicle fertilizer(30%),and kernel fertilizer(10%)and K fertilizer was used as tillering fertilizer(50%)and panicle fertilizer(50%).All fertilizers were applied artificially with the detailed information provided in a previous study(Chenet al.,2021b).Rice was the sole crop,and the seedlings were transplanted in June and harvested in October every year.Intermittent irrigation was provided using Yellow River water before rice planting.
Rice root sampling and estimation of rice root C input
At harvest,a 0.6-cm3quadrat (1 m in length,1 m in width,and 0.6 m in depth)was randomly selected in each plot to collect rice root samples.The root samples were carefully washed with distilled water.After drying at 60°C to a constant weight,root dry weight was determined.The C content in rice roots was determined using the potassium dichromate volumetric analysis method(Shaw,1959).There was no significant difference in root C content among the four treatments;therefore,we used the average C value to calculate C input from the roots,which was 395 g kg-1.Root biomass is not significantly different from year to year within the same treatment(Lianget al.,2016;Huanget al.,2020,2022;Haoet al.,2022).Thus,we used the root C input obtained in 2021 as a representative value for the entire experimental period.
Soil sampling and determining
Soil samples from the four treatments were collected in October 2021.Five soil samples(0-20 cm depth)were randomly collected from each plot with a 5-cm diameter auger and mixed as a composite sample.The gravel and plant fragments were removed,and the samples were brought to the laboratory in sealed bags.After crushing,air drying,and sieving (5-mm mesh),the samples were divided into two parts:one was used for the extraction of soil colloids and the other was used to determine soil physicochemical properties.
Soil water-dispersible colloids were extracted as previously described(Schumacheret al.,2005;Huanget al.,2020).Briefly,soil samples were dispersed in deionized water at a water:soil ratio of 5:1(volume:weight),shaken at 170 r min-1for 8 h,and then centrifuged at 2500×gfor 6 min(H1850,Cence,China).The upper solution was lyophilized to obtain soil colloids for further analysis of the physicochemical properties.
Soil pH was determined at a water:soil ratio of 5:1(volume:weight)using a pH meter(PHS-2F,Shanghai INESA Scientific Instrument Co,Ltd.,China).Soil EC was measured using a conductivity meter at a water:soil ratio of 5:1 (DDS-11A,Shanghai INESA Scientific Instrument Co,Ltd.,China).Cation exchange capacity (CEC)was determined using the sodium acetate exchange method(Jaremko and Kalembasa,2014).The TN content was measured using a Kjeldahl N analyzer(KDN-19C,Shanghai Xianjian Instrument Co,Ltd.,China).The TP content was measured using the colorimetric method after digestion with H2SO4-HClO4.The cutting ring method was used to measure soil bulk density(BD)(Botulaet al.,2015).
The K2Cr2O7oxidation method was used to determine the contents of SOC and SCOC(Kuwatsukaet al.,1992).Furthermore,Fe/Al-bound OC(Fe/Al-OC)and calcium(Ca)-bound OC(Ca-OC)were determined as previously described(Xu and Yuan,1993).In brief,the bulk soil samples were dispersed for 5 min in 1.8 g cm-3NaI solution.After dispersion,the suspension was separated for 5 min at 4 000 r min-1(H1850)and the supernatant was decanted to separate soil free fraction.The above process was continued until there was no suspended matter.The residue of soil was used to extract Fe/Al-OC with 0.1 mol L-1NaOH and Na4P2O7,and Ca-OC was extracted using 0.5 mol L-1Na2SO4.A TOC analyzer(TOC-Vario,Elementar,Germany)was used to measure the extracted OC content.
Fe and Al oxides were measured by plasma emission spectroscopy (Optima 8000DV,PerkinElmer,USA) after extraction with(NH4)2C2O4(Krameret al.,2012).The specific surface area,total pore volume,and mean pore diameter of soil colloids were evaluated with the Brunauer-Emmett-Teller theory based on N desorption isotherms(NOVA2200e,Quantachrome,USA).
Fourier transform infrared(FTIR)spectroscopy
The functional groups of OC were characterized by transmission FTIR spectroscopy(Thermo Fisher Scientific,USA).Briefly,both soil and soil colloid samples were thoroughly mixed with KBr,ground in an agate mortar,dried at 105°C for 24 h,and tableted into wafers for spectral analysis.The spectra were recorded as the average of 32 scans with a wavelength resolution of 4 cm-1and a range of 400-4 000 cm-1.The absorption peaks at 1 030,1 410,1 630,3 420,and 3 619 cm-1indicated polysaccharide-C(Dovbeshkoet al.,1997),aliphatic-C(Parikhet al.,2014),aromatic-C(Xueet al.,2019;Chenet al.,2022),carboxyl or amidogen-C(Movasaghiet al.,2008),and phenol-C(Artzet al.,2008),respectively.Semi-quantitative analysis of OC functional groups was carried out according to the methods described by Zhuet al.(2016)and Chenet al.(2022).The absorption peak area of FTIR spectra was integrated by the wavenumber from 940-1 062,1 309-1 573,1 590-1 733,3 240-3 501,and 3 590-3 983 cm-1using OMNIC Specta software;the relative content was obtained as the proportion of each functional group absorption peak area in the five absorption peak areas.
X-rayphotoelectron spectroscopy(XPS)
The functional groups of OC and chemical morphology of Al and Si on the surface of soil colloids were determined using XPS(K-Alpha,Thermo Fisher Scientific,USA)(Xiaoet al.,2016;Huanget al.,2020).The spectra of C 1s,Al 2p3/2,and Si 2p3/2in soil colloids were acquired using a monochromatic AlKαX-ray source(1 486.6 eV).The binding energy scale was adjusted using C 1s spectrum resulting from the adventitious hydrocarbon at 284.8 eV.XPSPEAK 4.1 software was used to analyze and deconvolve the highresolution XPS spectra with Shirley background correction.The spectra were optimized using Gaussian-Lorentzian value(Zamaet al.,2018).
The main OC functional groups in soil colloids were C(O)O,C-O-C,C-C/C-H,and C=C and their binding energies were 289,286.7,285,and 284.6 eV,respectively(Xiaoet al.,2016).The Al 2p3/2XPS binding energies of allophane,boehmite AlO(OH),and AlOxwere 73.8,74.5,and 75.4 eV,respectively(Huanget al.,2020).The Si 2p3/2XPS was divided into C-Si-O(101.7 eV,short-range order Si structure),Si-Al (102.6 eV,layered Si structure),and Si-O (103.4 eV,SiO2) (Huanget al.,2020).The relative contents of functional groups and chemical speciation were obtained by peak integration.
SOC storage and OC storage efficiency(OCSE)
The SOC storage(Mg C ha-1)was calculated according to Chenet al.(2022),as shown in Eq.1:
where 0.2 is the soil depth(m)and 10 is the coefficient of adjustment unit.
The proportion of C input(rice roots and organic fertilizer)transformed into SOC was defined as OCSE.Carbon input was calculated using Eq.2 and OCSE(%)was calculated using Eq.3(Lianget al.,2016):
where Crootis the C input from rice roots per year,COFis the C input from organic fertilizer per year,SOC2021and SOC2014are the SOC storages in October 2021 and May 2014,respectively,and 8 is the experimental duration(years).
Statistical analyses
One-way analysis of variance(ANOVA)was used for testing the differences between the fertilization treatments,followed by Duncan’s multirange tests atP <0.05 by SPSS 22.0 software(SPSS Inc.,USA).Before Pearson’s correlation coefficient(r)calculation,the normality and distribution of the data were first tested using SPSS 22.0.Pearson’srvalue was used to evaluate the linear correlations among SOC storage,OCSE,and soil chemical properties using SPSS 22.0.Linear regression analysis was used to analyze the correlations among soil chemistry properties,OC composition,SOC storage,and OCSE,which was conducted using Origin 2021b software(Origin Lab,USA).All tables and graphs were prepared using Microsoft Excel 2019(Microsoft,USA)and Origin 2021b software,respectively.
RESULTS
Soil physicochemical properties
Compared with CK,soil pH and EC were significantly reduced(P <0.05)under organic fertilization;EC decreased by 18.2%-21.2%(Table I).Soil BD decreased(P <0.05)by 8.8%in the NPKC2 treatment compared with that in the CK.Organic fertilization significantly increased(P <0.05)soil CEC compared with the NPK treatment.Compared with the CK,the contents of soil TN and TP were significantly increased in the NPKC1 and NPKC2 treatments,and there were no significant differences(P >0.05)between the two treatments.Soil colloidal specific surface area was significantly increased(P <0.05)in the two organic fertilization treatments,and the total pore volume and mean pore diameter of soil colloids showed a similar trend to soil colloidal specific surface area.
Content and composition of OC
After 8 years of organic fertilization,the SOC content was significantly increased(P <0.05)by 51.9%and 63.5%in the NPKC1 and NPKC2 treatments,respectively,compared with CK(Fig.1).The SCOC content was significantly increased (P <0.05) by more than 26.6% under organic fertilization,in comparison with the NPK treatment(Fig.1).The Fe/Al-OC content was significantly increased(P <0.05)in the NPKC1 and NPKC2 treatments,with no significant differences between the two organic fertilization treatments(Fig.1).The same trend was observed for the Ca-OC content(Fig.1).
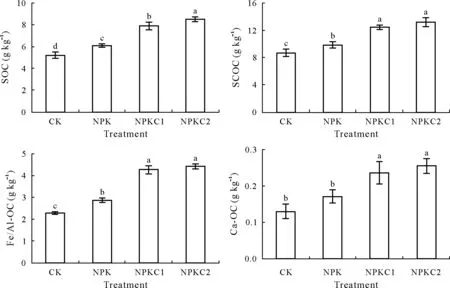
Fig.1 Effects of organic fertilization on soil organic C(SOC),Fe/Al-bound organic C(Fe/Al-OC),and Ca-bound organic C(Ca-OC),and soil colloid organic C(SCOC)contents.Vertical bars indicate standard deviations of the means(n=3).Bars with the same letter are not significantly different at P <0.05 according to the least significant difference test.CK=control(no fertilization);NPK=only mineral N,P,and K fertilization;NPKC1=NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization;NPKC2=NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization.
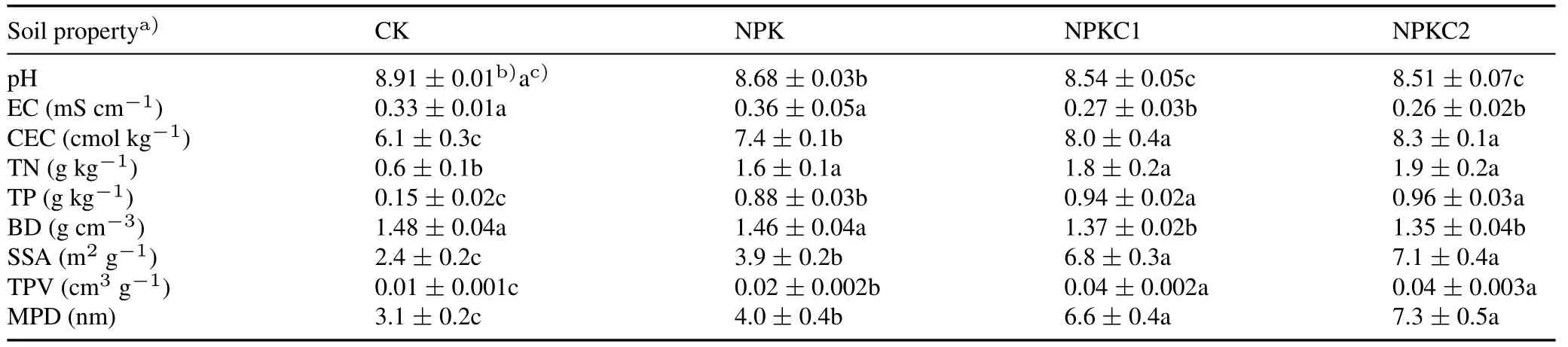
TABLE ISoil physicochemical properties in four treatments:no fertilization(control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2)
Organic C functional groups quantified byFTIR
The FTIR analysis showed that the main OC functional groups on the surface of soil and soil colloids were aromatic-C,polysaccharide-C,aliphatic-C,carboxyl-or amidogen-C,and phenol-C (Fig.2).The relative contents of aromatic-C,polysaccharide-C,and aliphatic-C showed an increasing trend,while carboxyl or amidogen-C and phenol-C decreased with organic fertilization in both soil and soil colloids(Table II).On the surface of soil colloids,the relative content of aromatic-C was significantly increased(P <0.05)by 42.8%in the NPKC1 treatment.The relative content of carboxyl-or amidogen-C was significantly lower(P <0.05)in the two organic fertilization treatments than in the NPK treatment.

Fig.2 Fourier transform infrared spectra of soil(a)and soil colloids(b)in four treatments:no fertilization(control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2).
Chemical speciation of C,Al,and Si
The functional groups of OC obtained by fitting the C 1s peak in soil colloids were C=C,C-C/C-H,C-O,and C(O)O(Figs.3a and S1-S3,see Supplementary Material for Figs.S1-S3).The relative content of C=C was significantly increased(P <0.05)in the two organic fertilization treatments compared to the NPK treatment,while C-O and C(O)O were decreased(Table III).The chemical speciation of Al 2p3/2was allophane,AlO(OH),and AlOx(Fig.3b).The relative content of allophane significantly increased in the two organic fertilization treatments,whereas no significant effect(P >0.05)was found for AlO(OH)and AlOx.The main chemical speciation of Si 2p3/2was C-Si-O,Si-Al,and Si-O (Fig.3c),in which the relative content of C-Si-O was significantly increased (P <0.05) under organic fertilization.

Fig.3 X-ray photoelectron spectroscopy peak-fitting spectra of C 1s(a),Al 2p3/2 (b),and Si 2p3/2 (c)on soil colloid surface in the NPKC1 treatment.NPKC1=NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization.a.u.=absorbance unit.
Reactive minerals in soil and soil colloids
Compared with the NPK treatment,the content of FeO in soil increased(P <0.05)by 20.0%-26.7%with organic fertilization(Fig.4a).The content of FeO in soil colloids was also significantly higher(P <0.05)in the two organic fertilization treatments than in the untreated soil(Fig.4b).The AlO content was significantly increased(P <0.05)in both soil and soil colloids with organic fertilization compared to the other treatments(Fig.4c,d).The contents of AlO and FeO in soil colloids showed no significant difference(P >0.05)between the two organic fertilization treatments.

Fig.4 Effects of organic fertilization on FeO and AlO contents in soil(a and c)and soil colloids(b and d).Vertical bars indicate standard deviations of the means(n=3).Bars with the same letter are not significantly different at P <0.05 according to the least significant difference test.CK=control(no fertilization);NPK=only mineral N,P,and K fertilization;NPKC1=NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization;NPKC2=NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization.

TABLE IIOrganic C functional groups in soil and soil colloids in four treatments:no fertilization(control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2)
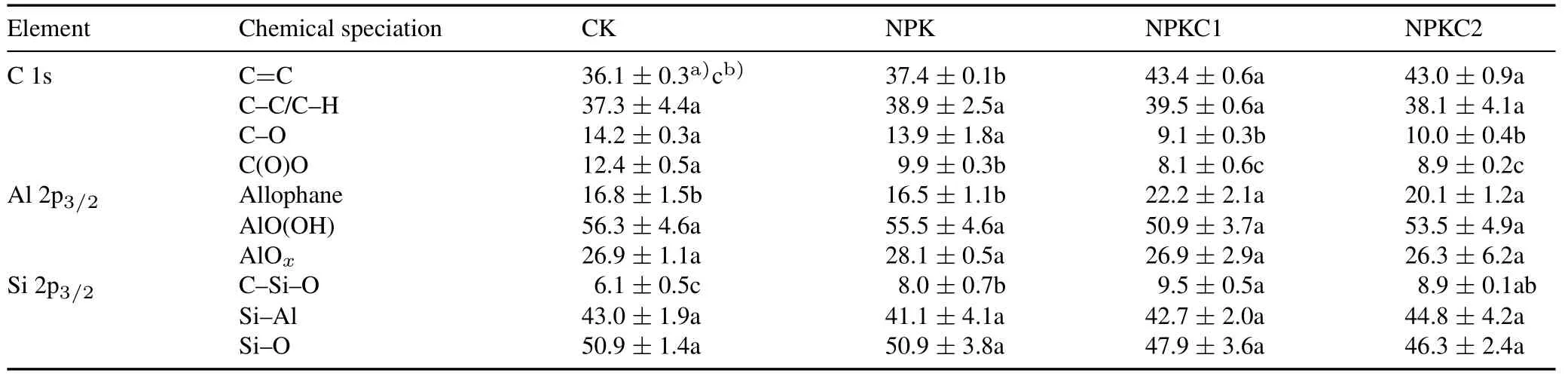
TABLE IIIChemical speciation of C 1s,Al 2p3/2,and Si 2p3/2 on soil colloid surface in four treatments:no fertilization(control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2)
SOC storage and OCSE
The C input from rice roots was 1 190 and 1 290 kg C ha-1year-1in the NPKC1 and NPKC2 treatments,respectively(Table IV).The SOC storage significantly increased(P <0.05)with organic fertilization compared to the NPK treatment.A similar trend was found for OCSE,which increased (P <0.05) by 41.0% in the NPKC1 treatment compared to the NPK treatment.
Correlations among SOC storage,OCSE,and soil chemical properties
Pearson’s correlation analysis showed that SCOC was positively correlated with Fe/Al-OC(r=0.973,P <0.01)and Ca-OC (r=0.903,P <0.01) (Table V).Linear regression analysis indicated that Fe/Al-OC(R2=0.90)and Ca-OC(R2=0.71)were positively correlated with SOC storage(Fig.5).The SCOC content was also positively correlated with SOC storage(R2=0.82)(Fig.5).The C=C group was positively correlated with FeO(r=0.844,P <0.01)and AlO(r=0.878,P <0.01)in soil colloids(Table VI);however,carboxyl-or amidogen-C(r=-0.852,P <0.01)and phenol-C(r=-0.877,P <0.01)showed negative relationships with SOC storage(Table V).

Fig.5 Relationships between soil organic C(SOC)storage and Fe/Al-bound organic C(Fe/Al-OC),soil Ca-bound organic C(Ca-OC)and SOC storage,soil colloid organic C(SCOC)and SOC storage,and SCOC and organic C storage efficiency(OCSE).The shaded bands with grey color show 95%pointwise confidence intervals(n=12).

TABLE IVC input and soil organic C(SOC)storage and organic C storage efficiency(OCSE)in four treatments:no fertilization(control,CK),only mineral N,P,and K fertilization(NPK),NPK plus a low level(450 kg C ha-1 year-1)of organic fertilization(NPKC1),and NPK plus a high level(900 kg C ha-1 year-1)of organic fertilization(NPKC2)
DISCUSSION
Effect of organic fertilizer application on OC content and composition
Reactive mineral colloids containing Fe and Al provide an important substrate for the storage of C compounds(Kalbitzet al.,2005).Kramer and Chadwick(2018)pointed out that more than 50%of SOC was retained by reactive minerals at the global scale.In the present study,compared with mineral fertilization,organic fertilization significantly increased(P <0.05)the Fe/Al-OC content(Fig.1),which indicated that organic ligands played a crucial role in C storage by binding with hydroxides in paddy soil(Zhouet al.,2009).Interestingly,the content of SCOC was higher than that of SOC,especially in the two organic fertilization treatments(Fig.1).This might be due to i)the larger surface area of soil colloids(Table I),which can absorb organic materials(Jianget al.,2014);ii)the specific interactions of organic matter with minerals and metal ions at the colloidal interface(such as ligand exchange)(Filimonovaet al.,2016);and iii)the complex and stable macromolecular compounds(such as humic acid)in soil colloids(Schumacheret al.,2005).In addition,Ca can combine with OC to improve soil structural stability,thus improving SOC storage capacity(Rowleyet al.,2018).Sowerset al.(2018)found that Ca could form ternary organo-mineral complexes with Fe and OC,which had a synergistic effect on C storage.In the present study,Ca-OC increased by 38.6%-49.1%with organic fertilization,compared with the NPK treatment(Fig.1),and Ca-OC was positively correlated with SOC storage(Fig.5).These results further indicated that organic fertilization could improve SOC storage by increasing the Ca-OC content in saline-alkaline paddy soil.

TABLE VPearson’s correlation coefficients(r)between soil organic C(SOC),soil colloid organic C(SCOC),Fe/Al-bound organic C(Fe/Al-OC),Ca-bound organic C(Ca-OC),polysaccharide-C,aliphatic-C,aromatic-C,carboxyl-or amidogen-C,and phenol-C,SOC storage,and organic C storage efficiency(OCSE)(n=12)

TABLE VIPearson’s correlation coefficients(r)between FeO,AlO,allophane,and C-Si-O and organic C(SCOC),C=C,C-C/C-H,C-O,and C(O)O in soil colloids(n=12)
Organic C contains various organic functional groups with different stabilities(Chenet al.,2022).For example,aromatic-C is more stable in soil because of its symmetrical structure and is not easily decomposed by microorganisms(Huanget al.,2018;Liet al.,2022).In the present study,the FTIR results revealed that the relative content of aromatic-C in both soil and soil colloids increased with organic fertilization(Table II).The XPS results also indicated that the relative content of aromatic-C (C=C) in the organic fertilization treatments was higher than that in the NPK treatments (Fig.3,Table III).This might be because that there were more aromatic components in the organic fertilizer produced from soybean litter and soybean(Sarkeret al.,2018;Feiet al.,2021)and that organic fertilizer addition increased the retention of aromatic-C in both soil and soil colloids(Huanget al.,2016;Xueet al.,2019).Notably,the relative content of aromatic-C in soil colloids was higher than that in bulk soil(Table II),suggesting that organic fertilization was more helpful in preserving stable aromatic-C in soil colloids in saline alkaline paddy fields.This was largely due to the formation of Fe minerals on the surface of soil waterdispersible colloids(Huanget al.,2020).Evidence has shown that Fe minerals selectively retain aromatic compounds on the surface of soil colloids (Wenet al.,2021).Aromatic compounds derived from lignin or low-molecular-weight components can resist biodegradation through interactions with metals(Krauset al.,2003;Wenet al.,2019).In addition,C(O)O compounds,which are relatively easy to degrade(Wuet al.,2021b),were found to be the least abundant in the two organic fertilization treatments,especially in the NPKC1 treatment(Table III).Therefore,organic fertilization could improve the stability of SOC,especially at the colloidal interface in saline-alkaline soil.
Effect of organic fertilization on mineral availability
Reactive minerals are tightly coupled with the biochemical cycle of soil C and their formation and transformation are greatly affected by fertilization(Huanget al.,2020).For instance,Ginnet al.(2017)reported that the formation of poorly crystalline minerals was promoted by the application of organic fertilizers.Huanget al.(2018)found that oxalateextractable FeO was significantly increased by microbial reduction with the application of organic fertilizer.In the present study,the FeO content in the organic fertilization treatments was higher than that in other treatments(Fig.4a).This might be because that the formation of FeO minerals was promoted by the alternation of drying and wetting after organic fertilizer application,leading to the dissolution and reprecipitation of Fe phase(Huanget al.,2016).
Soil colloids are usually composed of complexes or mixtures of metallic minerals(such as FeO and AlO)and OC,which carry variable charges on the surface (Zhanget al.,2021).Huanget al.(2020)found that there were more Fe/Al minerals in the water-dispersible colloids,because Fe/Al minerals could act as substitute electron acceptors to drive the biogeochemical cycle of Fe/Al during paddy field flooding (Ginnet al.,2017).In the present study,relative to bulk soil,FeO and AlO contents in soil colloids were higher in the four treatments,especially in the organic fertilization treatments(Fig.4).One possible reason was that organic fertilizer application decreased soil pH (Table I),which resulted in more positive charges on soil colloidal surface through FeO and AlO retention(Ariaset al.,1995;Huanget al.,2018).The increase in positive charges at the colloidal interface promoted the formation of organic mineral complexes between FeO/AlO and SCOC (Xiaoet al.,2015).Another reason was that soil EC decreased with organic fertilization(Table I),which reduced the dispersion of organic and mineral colloids and then increased the accumulation of FeO and AlO on soil colloids(Lianet al.,2019;Songet al.,2020).
Allophane is a poorly crystallized mineral widely present in the crust(Xiaoet al.,2016).Compared with traditional crystalline minerals such as goethite and boehmite,allophane has a larger specific surface area and provides more stable chemical bonds,which can bind with organic ligands to retain more OC(Sharmaet al.,2010).In the present study,compared with mineral fertilization,organic fertilization significantly increased (P <0.05) the allophane content,but the boehmite content showed no significant difference among all the treatments (Table III).These results are in line with those of previous studies(Xiaoet al.,2016;Huanget al.,2020),which suggested that organic fertilization could significantly increase Al-reactive minerals in salinealkaline paddy soil.Moreover,as an important composition of silicate minerals,the effect of biological geochemical cycles of Si was important on C storage in different terrestrial ecosystems(Nguyenet al.,2019).Huanget al.(2020)found that the formation of short-range order Si structure was promoted by the application of organic fertilizer using highresolution transmission electron microscopy,29Si nuclear magnetic resonance spectroscopy,and XPS.In the present study,compared to mineral fertilization,organic fertilization significantly increased (P <0.05) the content of shortrange order Si structure(C-Si-O)(Table III).C-Si-O was positively correlated with SCOC and C=C,which suggested that C could bind with Si and form organo-mineral complexes with Si.This is conducive to the long-term storage of OC in paddy soil(Songet al.,2018;Huanget al.,2020).
Mineral protection and SOC storage in soil colloids
A saline-alkaline environment is not conducive to OC sequestration(Tavakkoliet al.,2015).In the present study,organic fertilization significantly decreased soil pH(Table I),which was negatively correlated with SOC storage (Fig.S4a,see Supplementary Material for Fig.S4).This might be because that high soil pH promotes the dissociation of Fe and Al oxides and humus functional groups in soil colloids and then decreases the stability and storage of SOC(Zhaoet al.,2018)and that soil pH could also regulate soil organic matter turnover by affecting bacterial community composition(Kemmittet al.,2006).In addition,high salt concentration could cause soil organic and mineral colloid dispersion to reduce SOC storage(Wonget al.,2010).In the present study,EC was negatively correlated with SOC storage(Fig.S4b)and there was lower EC in the organic fertilization treatments(Table I),which indicated that organic fertilizer application could improve SOC storage by decreasing EC in saline-alkaline soil.Therefore,organic fertilizer application could increase SOC sequestration by improving soil salinealkaline environment in saline-alkaline paddy soil.
Organic C can be increased through root residue input and exogenous C addition (such as organic fertilization),which is the most direct and effective method(Chenet al.,2022).Heet al.(2018)reported that,compared with mineral fertilization,22-year organic fertilization increased SOC storage by 3.7-18.2 Mg C ha-1.In the present study,SOC storage significantly increased(P <0.05)by 40.1%-47.4%after 8-year organic fertilization (Table IV).This was because that i)root and stubble residues might be better than exogenous organic amendments for SOC storage (Huanget al.,2020);ii)there was more root C input in organic fertilization treatments(Table IV);and iii)Fe/Al-OC and Ca-OC were positively correlated with SOC storage(Fig.5),which was much higher in the NPNC1 and NPKC2 treatments(Fig.1).In addition,a previous study found that aromatic-C compounds could be retained by forming complexes with Fe(Chenet al.,2022).Interestingly,more aromatic-C(C=C)and FeO were found in soil colloids(Table II,Fig.4b).The C=C group was positively correlated with FeO(Table VI),which could promote the complex formation of aromatic-C with Fe(Wenet al.,2019;Huanget al.,2020).This may be an important mechanism for the long-term SOC storage in terrestrial ecosystems(Rumpelet al.,2015).Thus,compared with mineral fertilization,organic fertilization might be a better agronomic practice to improve SOC storage in saline-alkaline paddy soil.
Organic fertilization is a good agronomic practice for improving the sequestration coefficient of OC(Zhaoet al.,2016;Chenet al.,2021a;Liuet al.,2022).Previous studies have reported that organic fertilization significantly increases OCSE(Lianget al.,2016).The present results showed that,compared with the NPK treatment,organic fertilization significantly increased OCSE,which was as high as 53.1%in the NPKC1 treatment (Table IV).This was probably because i) organic fertilizer reduced enzymatic activities(such as polyphenol oxidase and invertase)in soil compared with mineral fertilizer(Mandalet al.,2008;Fanet al.,2014),which led to a reduction in OC mineralization rate(Yuet al.,2012);ii)the long-term addition of organic fertilizer reduced the leaching loss of SOC by soil aggregate physical protection(Chenet al.,2022),which resulted in the transformation of organic matter into SOC pool;and iii)organic fertilizer addition was beneficial to the formation and transformation of reactive minerals,which formed organic mineral complexes at the soil colloidal interface,and thus increased OCSE and SOC storage (Huanget al.,2020).In our previous study,more OC was lost through CO2emissions in the NPKC2 treatment than in the NPNC1 treatment(Chenet al.,2022).In addition,SOC storage was positively correlated with OCSE in the present study (Table V).These results are supported by previous research (Huaet al.,2014),which indicated that the increase in SOC storage might be due to the improvement of OCSE(Huanget al.,2020).Therefore,an appropriate amount of organic fertilizer application could maintain higher OCSE and SOC storage in saline-alkaline paddy soil.
CONCLUSIONS
This study provided direct evidence that eight years of organic fertilization increased the stable SOC in soil colloids.Compared with mineral fertilization alone,organic fertilization greatly increased the content of aromatic-C,especially in soil colloids.In addition,reactive Fe,Al,and Si minerals also significantly increased with organic fertilization,which were positively correlated with C=C in soil colloids.This indicated that aromatic C=C might be retained by the formation of aromatic-mineral complexes with reactive minerals in soil colloids.In addition,organic fertilization significantly increased OCSE,which in turn improved SOC storage.In contrast,the OCSE in the NPKC1 treatment was significantly higher than that in the NPKC2 treatment.Therefore,an appropriate amount of organic fertilizer application could increase the contents of stable OC and reactive minerals and improve the formation of organo-mineral complexes in soil colloids,which is a better agronomic practice to improve OCSE and SOC storage in saline-alkaline paddy soils.
ACKNOWLEDGEMENT
This study was funded by the National Key R&D Projects of China(No.2021YFD1900901-06),the Agricultural Science and Technology Innovation Projects,China(ASTIP No.CAAS-ZDRW202201),the Modern Agricultural Industrial Technology System of China(No.SDAIT-17-05),and the Provincial Natural Science Foundation of Shandong,China(No.ZR2020MC154).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Removal of atmospheric methane by soil ecosystems and its controlling variables from microbial to global scales
- Preface:Special issue on soil ecology and sustainability for celebrating the 70thanniversary of ISSCAS
- Responses of nitrogen cycling and related microorganisms to brackish wetlands formed by evapotranspiration
- Assessment of soil total phosphorus storage in a complex topography along China’s southeast coast based on multiple mapping scales
- Application of controlled-release urea increases maize N uptake,environmental benefits and economic returns via optimizing temporal and spatial distributions of soil mineral N
- Effects of herbicide butachlor application on the growth of periphytic biofilms and nitrogen loss in paddy systems
