Emerging strategies for nerve repair and regeneration in ischemic stroke: neural stem cell therapy
2024-03-05SijiWangQianyanHeYangQuWenjingYinRuoyuZhaoXuyutianWangYiYangZhenNiGuo
Siji Wang ,Qianyan He ,Yang Qu ,Wenjing Yin ,Ruoyu Zhao ,Xuyutian Wang ,Yi Yang,2,*,Zhen-Ni Guo,2,*
Abstract Ischemic stroke is a major cause of mortality and disability worldwide,with limited treatment options available in clinical practice.The emergence of stem cell therapy has provided new hope to the field of stroke treatment via the restoration of brain neuron function.Exogenous neural stem cells are beneficial not only in cell replacement but also through the bystander effect.Neural stem cells regulate multiple physiological responses,including nerve repair,endogenous regeneration,immune function,and blood-brain barrier permeability,through the secretion of bioactive substances,including extracellular vesicles/exosomes.However,due to the complex microenvironment of ischemic cerebrovascular events and the low survival rate of neural stem cells following transplantation,limitations in the treatment effect remain unresolved.In this paper,we provide a detailed summary of the potential mechanisms of neural stem cell therapy for the treatment of ischemic stroke,review current neural stem cell therapeutic strategies and clinical trial results,and summarize the latest advancements in neural stem cell engineering to improve the survival rate of neural stem cells.We hope that this review could help provide insight into the therapeutic potential of neural stem cells and guide future scientific endeavors on neural stem cells.
Key Words: bystander effect;cell replacement;extracellular vesicles;ischemic stroke;neural stem cells;neural stem cell engineering
Introduction
Ischemic stroke occurs due to a decrease in blood flow to certain areas of the brain caused by the formation of blood clots in the cerebral arteries,embolus detachment,changes in blood flow dynamics,and a lack of glucose and oxygen supply to local brain tissue,which together lead to a series of cascade reactions (Ferrari et al.,2022;Hernández and Pérez-Álvarez,2022).The pathological mechanisms of ischemic stroke include calcium overload,excitotoxicity,oxygen radical accumulation,inflammatory response,and an imbalance in apoptotic autophagy signaling (Magnoni et al.,1987;Tuo et al.,2022;Wang et al.,2023).In untreated patients with acute ischemic attacks involving large blood vessels,approximately 120 million neurons and 830 billion synapses are lost per hour,with an average stroke duration of 10 hours (Saver,2006).
Currently,early vascular recanalization therapy is the preferred and most effective treatment for ischemic stroke(Cougo-Pinto et al.,2015;Demaerschalk et al.,2016;Αnfray et al.,2021).Intravenous thrombolysis and endovascular therapy have developed rapidly in recent years;however,owing to the narrow time window,bleeding risk,and ineffective recanalization,a multitude of ischemic stroke patients are still unable to receive effective treatment.Even after rehabilitation treatment,most patients who survive stroke have functional impairments or permanently lose their independent function,which reduces their life quality and causes a heavy economic burden on society (Langhorne et al.,2011;Howard and Goff,2012).Therefore,alternative or supplementary treatment methods have been widely used.Stem cell transplantation,as a promising new therapy,has been applied in research on various central nervous system (CNS) diseases,aiming to reduce neural damage and promote neural function recovery through the stimulation and regulation if endogenous stem cells or supplementary cells (De Gioia et al.,2020).
Stem cell sources are divided into three categories:hematopoietic,mesenchymal/stromal,and neural (He et al.,2020).It is difficult to determine which type of stem cell is the most effective treatment for ischemic stroke in stem cell therapy (Kawabori et al.,2020).Hematopoietic lineage (such as bone marrow-derived mononuclear cells and peripheral blood-derived CD34+cells) and mesenchymal/stromal lineage(such as bone marrow-derived mesenchymal stem cells and adipose tissue-derived mesenchymal stem cells) can be extracted from abundant bone marrow or adipose tissue,umbilical cord blood,and peripheral blood;therefore,they are relatively easy to obtain and less likely to constitute a logical or ethical issue.Compared to the other two common types of stem cells,neural stem cells (NSCs) are specifically responsible for repairing the CNS.NSCs have the ability to selfrenew and form neurons and neuroglia.Furthermore,NSCs can better cooperate with the endogenous microenvironment and integrate into the endogenous host network,allowing them to play a distinctive role in neurological illness treatment(Hamblin and Lee,2021;Nie et al.,2023).
The aim of this article is to clarify the mechanism and clinical procedures of NSCs,as well as to provide a theoretical foundation and prospective therapeutic targets for future research and clinical application.Furthermore,we have compiled the most recent advances in NSC engineering and presented viable techniques for enhancing NSC survival and treatment efficiency.
Retrieval Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published up to July 31,2023.Α combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity:“ischemic stroke”;“neural stem cells”;“cell replacement”;“extracellular vesicles”;“exosome”;“immunomodulation”;“blood-brain barrier”;“angiogenesis”;“neuroplasticity”;and“NSC engineering”.The results were further screened by title and abstract,and those studies exploring the potential mechanisms or clinical strategies of NSC therapy in ischemic stroke,as well as studies involving NSC engineering,were included.No language or study type restrictions were applied.
Neural Stem Cells and Neural Stem Cell Therapy
NSCs are primitive precursor cells with the ability to differentiate in the CNS.NSCs exist in the developing CNS and persist in certain areas of the brain in newborns and adults,appearing in the form of neuroepithelial cells,radial glial (RG) cells,subventricular zone (SVZ) astrocytes (type B cells),and the subgranular zone (SGZ) radial astrocytes,functioning as stem cells (Doetsch et al.,1999;Garcia et al.,2004).The symmetrical cell division process of neuroepithelial cells and RG cells,known as interkinetic nuclear migration,can maintain stem cell activity,whereas limited potential progenitor cells,such as intermediate progenitor cells (IPCs),no longer undergo interkinetic nuclear migration;therefore,interkinetic nuclear migration is a characteristic of NSCs in the CNS (Del Bene et al.,2008;Elkabetz et al.,2008).During early embryonic development,neural tube epithelial cells act as NSCs and line the ventricles,undergoing self-renewal and symmetric division to generate more neuroepithelial cells,potentially producing some early neurons (Kriegstein and Αlvarez-Buylla,2009;Bond et al.,2015).Αs the brain epithelium thickens,neural tube epithelial cells elongate and transform into RG cells.During corticogenesis,RG cells remain in the ventricular zone and maintain clear apical-basal polarity,differentiating into neurons and neuroglia,forming the basis of neural circuits (Kriegstein and Alvarez-Buylla,2009;Yoon et al.,2014).RG cells increase in number through asymmetric division and give rise to neurogenic IPCs (nIPCs) and oligodendrocyte IPCs (oIPCs),indirectly generating neurons and oligodendrocytes,respectively (Haubensak et al.,2004;Noctor et al.,2004).In newborns,a subgroup of RG cells act as NSCs,remaining mostly quiescent until activation after birth(Fuentealba et al.,2015;Furutachi et al.,2015).Most RG cells migrate to the cortical plate and continue to produce neurons and oligodendrocytes by differentiating into nIPCs and oIPCs,while some RG cells transform into ependymal cells and type B cells (Kriegstein and Alvarez-Buylla,2009).In the SVZ region of the lateral ventricle in adults,type B cells extend their basal processes towards blood vessels and their apical processes to contact the cerebrospinal fluid in the lateral ventricle.Type B cells undergo division to generate neural transiently amplifying progenitor cells (type C cells),which repeatedly divide to produce neuroblasts (type A cells) (Doetsch et al.,1999;Imayoshi et al.,2008).The newly generated neuroblasts migrate tangentially along chains to the olfactory bulb and differentiate into different subtypes of interneurons (Doetsch et al.,1999;Lim and Alvarez-Buylla,2014).Another region in the adult CNS that generates new neurons is the SGZ of the hippocampal dentate gyrus.Radial astrocytes in the SGZ,also known as Type I progenitor cells,generate IPCs within the granule cell layer,also known as Type II progenitor cells or D cells,which undergo limited proliferation and give rise to neuroblasts (Kempermann et al.,1997;Fukuda et al.,2003).The newly formed neuroblasts migrate tangentially along the SGZ and develop into immature neurons,which then radially migrate into the granule cell layer and differentiate into granule cells (Kempermann et al.,1997;Su et al.,2023).
Whether NSCs and endogenous adult neurogenesis occur in the hippocampal region of the adult human brain remains controversial.Earlier studies concluded that the SVZ and SGZ retain the ability to produce neurons throughout life(Eriksson et al.,1998;Spalding et al.,2013).Recently,doublepositive cells of doublecortin and polysialic acid-neural cell adhesion molecule (Table 1) have been detected using immunohistochemical staining and electron microscopy to assess newborn neurons.There are considerable amounts of neurons in the hippocampus of newborns,and neurogenesis processes still occur in the dentate gyrus (DG) in middle-aged children;however,these are highly depleted and are almost non-existent in adults (Sorrells et al.,2018).Meanwhile,single-cell RNA sequencing can be used to reveal neurogenesis trajectories in the DG of macaques,pigs,and mice,but clear granule cell trajectories or cells expressing nIPC or neural progenitor cell (NPC) markers (Table 1) have not been detected in adult humans (Hochgerner et al.,2018).Although there is increasing evidence pointing to a lack of neurogenesis in adults (Nano and Bhaduri,2022;Wiseman,2022),this has also redirected the focus of research on NSCs to promising new fields.Simultaneously,with the development of technologies,such as single-cell RNA sequencing,and bioinformatics tools,such as Monocle (Trapnell et al.,2014),our understanding of the continuous dynamic behavior of adult brain NSCs will deepen.
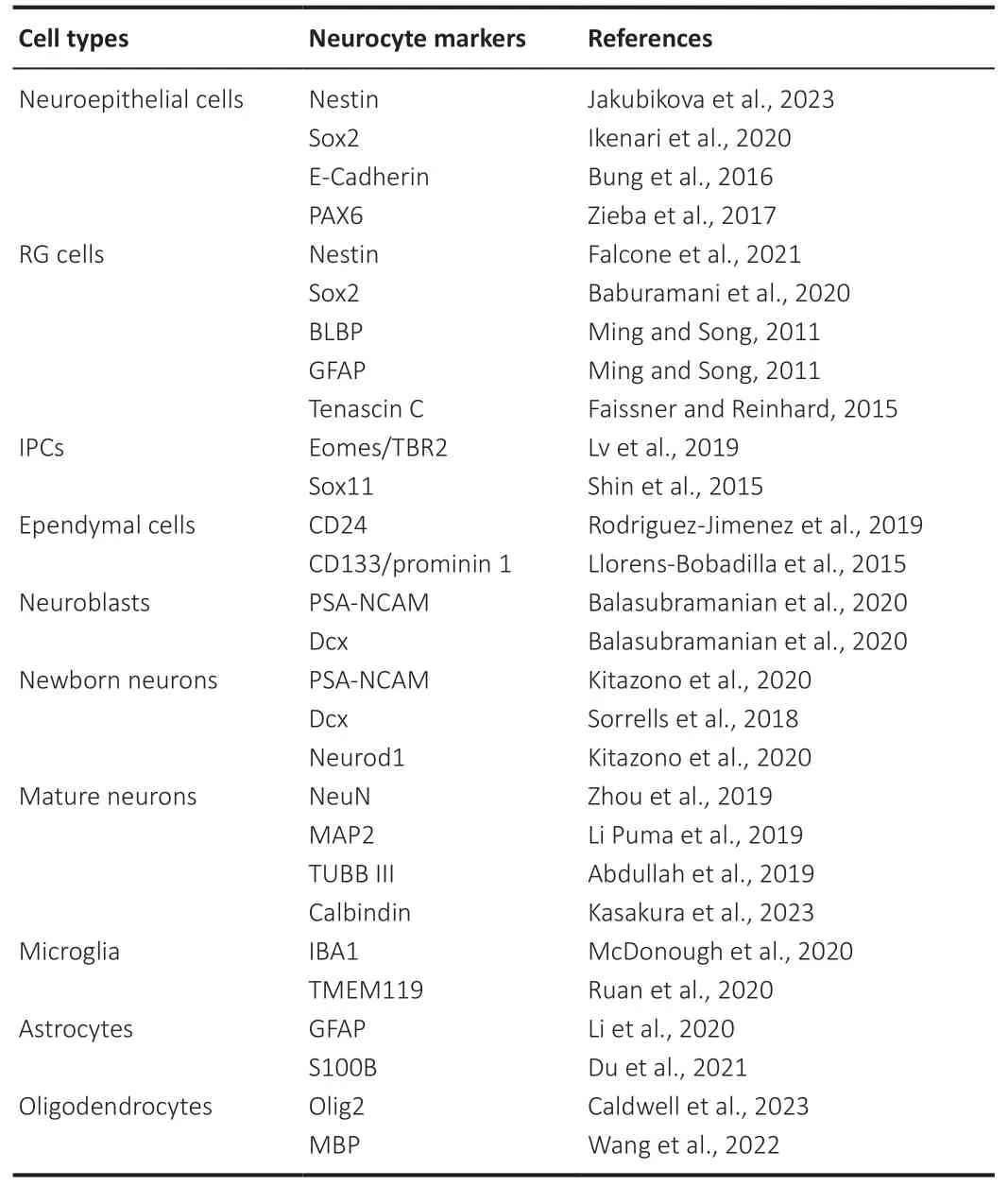
Table 1|List of markers for NPSCs,neurons,and neuroglia
In the adult CNS,under normal physiological conditions,neurogenesis is in a resting or inhibited state,possibly maintaining the relative stability of the internal neural network system (Kempermann et al.,1997).Numerous experimental studies have suggested that cerebral ischemia can stimulate cell proliferation and neurogenesis in adult rodents and monkeys and that ischemia-induced neurogenesis may persist for several months (Arvidsson et al.,2002;Kernie and Parent,2010).After cerebral ischemia,neurogenesis is stimulated through the upregulation of growth factors and nutritional factors,such as stem cell factor,epidermal growth factor,and vascular endothelial growth factor (VEGF) (Jin et al.,2002;Sun et al.,2003;Lei et al.,2023).New neurons originate from neural progenitor/stem cells (NPSCs) in the SVZ and SGZ,and some may derive from astrocytes in the parenchyma,which have potential neurogenic programs and can be reprogrammed and transformed into neurons via Notch signaling (Magnusson et al.,2014;Santopolo et al.,2020).The Notch signaling pathway plays an important role in maintaining the survival and stability of NSCs (Carlén et al.,2009).However,the limited repair capacity of endogenous NSCs may not be sufficient to compensate for neurodegeneration during disease processes.
Potential Mechanisms of Neural Stem Cell Therapy
In previous preclinical and clinical trials,NSCs were reported to be an effective treatment for ischemic stroke.Specifically,NSCs can affect key targets in neurological treatments and play a neuroprotective role.Research on these targets has been continuously explored (Figure 1),which lists potential therapeutic targets for NSC transplantation in the treatment of ischemic stroke.The role of these targets and their relationship with neural stem cell therapy for ischemic stroke requires further research and validation to better explore the therapeutic potential of NSCs.
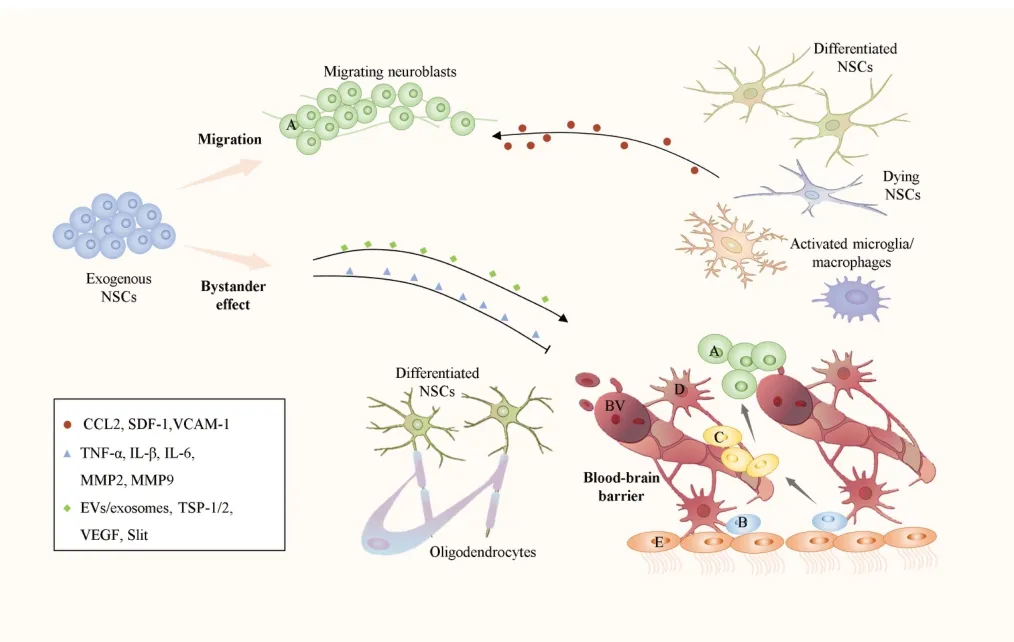
Figure 1|Mechanisms of NSCs in ischemic stroke.
Homing and cell replacement
The continuous self-renewal,differentiation,and chemotaxis of NSCs provides a theoretical basis for cell replacement(Kelly et al.,2004).Cell replacement is currently considered the primary pathway for NSC transplantation to improve neurological damage.
Through dual labeling with specific antibodies against human cell nuclei and glial fibrillar acidic protein or neuronal nuclei antigen,transplanted NSCs exhibit directional migration and differentiation during ischemic stroke (Ryu et al.,2016).Furthermore,non-invasive imaging techniques,such as magnetic particle imaging and near-infrared fluorescence imaging,are also potential tools for tracking the behavior of NSCsin vivo(Yang et al.,2021;Chen et al.,2023).The monocyte chemoattractant protein-1,also known as CC chemokine ligand 2,and the chemokine stromal cellderived factor-1 alpha,also known as CXC chemokine ligand 12,are upregulated in ischemic regions and recruit NSCs that express CC chemokine receptor 2 and CXC chemokine receptor 4 to migrate across the blood-brain barrier (BBB)to the ischemic injury area.The ligand-receptor systems of CXC chemokine ligand 12/CXC chemokine receptor 4 and CC chemokine ligand 2/CC chemokine receptor 2 are crucial pathways for homing (Kokovay et al.,2010;Andres et al.,2011a).Furthermore,NSCs achieve therapeutic homing by migrating to the ischemic peripheral area via the interaction between integrin CD49d expressed by NSCs and vascular cell adhesion molecule-1 (Guzman et al.,2008).NSCs can survive and differentiate into neuroglia and neurons with different neurotransmitter subtypes,exhibit mature functional neuronal electrophysiological characteristics,and integrate into host neural circuits (Grønning Hansen et al.,2020;Palma-Tortosa et al.,2020).Transplanted NSCs compensate for damaged neural networks by replacing cells lost following CNS injury,contributing to the improvement of neural function.
However,continuous research has shown that this type of cell replacement is inefficient,and perhaps only 1–10% of transplanted NSCs can survive (Sortwell et al.,2000;Bakshi et al.,2005),the reason for which is closely related to the harsh conditions of the ischemic microenvironment in the stroke infarction core area and penumbra.The challenges of low survival and nerve regeneration rates require an immediate solution.
Bystander effect
The bystander effect is widely accepted as a neuroprotective mechanism in stem cell transplantation (Eckert et al.,2015).Some studies have indicated that transplanted NSCs exert therapeutic effects not only through limited cell replacement but also through paracrine mechanisms by secreting biologically active compounds,such as chemokines,cytokines,growth factors,nutrients,and extracellular vesicles (EVs),which promote endogenous regeneration and mediate neural network reconstruction (Palma-Tortosa et al.,2021;Bonetto and Grilli,2023;Ni et al.,2023).Recent research has proposed that apoptotic NSCs provide neuroprotection through the secretion of substances,such as peroxidedoxin-1 and galectin-1 (Han et al.,2023),providing an alternative explanation for the neuroprotective mechanism when the survival rate of NSCs is low.The bystander effect refers to the mechanisms that regulate immune responses,increase BBB stability,and promote vascular and nerve regeneration.EVs/exosomes and other vesicles containing biologically active factors have developed rapidly in recent years and are discussed separately in the following text.
EVs and exosomes
EVs and exosomes play an important role in paracrine secretion.EVs are secreted by various cell types including stem cells and other mature cells.Exosomes are EVs with a diameter of 30–200 nm that carry specific molecules,such as various types of nucleic acids,lipids,and proteins(Jeske et al.,2020;Li et al.,2021b),in addition to microRNΑs(miRNΑs),which can target multiple genes by inhibiting mRNΑ transcription,thus regulating the proliferation,differentiation,and apoptosis of target cells (Li et al.,2021b;Xu et al.,2021b).NPSCs promote the repair of neural functions,enhance the integrity of the BBB,and improve the inflammatory microenvironment by releasing exosomes as paracrine factors(Rong et al.,2019;Zhang et al.,2021;Hur et al.,2022).Αnimal experiments have confirmed that EVs from NPCs have a strong inhibitory effect on the inflammatory response in ischemic areas (Tian et al.,2021),and their mechanism is related to the action of miRNA-rich EVs on the inhibition of the MAPK pathway (Tian et al.,2021).Furthermore,in the pig middle cerebral artery occlusion (MCAO) model,NSC EVs can effectively reduce infarction volume,cerebral edema,and hemorrhagic transformation,resulting in a more favorable neuroprotective effect (Webb et al.,2018a).Recently,a study using NSCs and NSC-derived exosomes for combined treatment found that exosomes promoted the survival and differentiation of transplanted NSCs,regulated the behavior of NSCs by carrying targeted genes,and improved the microenvironment.However,no significant therapeutic effect was observed when exosomes were used alone,possibly because of their dosage (Zhang et al.,2023).
Numerous preclinical studies have suggested that EVs could be used for cell-free therapies and appear to be a safer option(Vogel et al.,2018;Wiklander et al.,2019;Sil et al.,2020).Transplanted NSCs have potential problems,especially the risk of immunogenicity and instability of differentiation (Αttwood and Edel,2019).Additionally,the low survival rate of NSCs may be another issue,as the high-level immune inflammatory biological signals and cell debris produced by dead NSCs can also damage the microenvironment to a certain extent(Zhang et al.,2019b).Some studies have proposed using EVs extracted from stem cells as a replacement for stem cells in neuroprotective and restorative stroke therapies (Zhang et al.,2019d;Upadhya et al.,2020).
The induction of EV-mediated formation from human neural stem cells (hNSCs) significantly increased the survival rate of cellsin vitroand showed better therapeutic effects in a rat MCAO model (Zhang et al.,2020).Combining NPC exosomes overexpressing miR-126 with endothelial progenitor cellexosomes could enhance the protective effects of exosomes against nerve damage through the potential regulation of pathways such as reactive oxygen species/NADPH oxidase 2 and brain-derived neurotrophic factor (BDNF)/BDNF receptor tyrosine kinase receptor B (Xu et al.,2023).
EVs/exosomes possess biological properties that can be used for drug delivery and loading of bioactive cargo (Kim et al.,2020;Wu et al.,2021;Xu et al.,2021a;Ulpiano et al.,2023).The construction of engineered exosomes derived from NSCs effectively resolves certain issues,such as poor drug half-life and low BBB permeability (Naqvi et al.,2020;Hering and Shetty,2023).BDNF-hNSC exosomes reduced stress-induced damage in NSCs and inhibited inflammation,significantly improving the survival rates of NSCs and poststroke neurological functions (Zhu et al.,2023).
Immunomodulation
Following the onset of an ischemic event,non-infectious neural inflammation pervades the pathophysiological process(Boltze and Perez-Pinzon,2022) and plays a dual positive and negative role (Tsygan et al.,2019).The precise control of neural inflammation is beneficial for neural functional recovery.As resident immune cells of the CNS,microglia are activated first after ischemic injury,and monocyte-derived macrophages are recruited to infiltrate the brain (Zhang et al.,2019c;Blank-Stein and Mass,2023).Transplanted NSCs can effectively play an immune-regulating role for up to 30 days,including reducing the expression of pro-inflammatory cytokines,such as tumor necrosis factor-α,interleukin (IL)-1β,IL-6,and adhesion molecules,downregulating microglia/macrophage activity,improving BBB damage,reducing the cascade of inflammatory signals,and relieving inflammatory reactions (Chang et al.,2013a;Lockard et al.,2022).
Microglia/macrophages can modify the phenotype activated or polarized by various factors such as inflammation and ischemia.Activation by lipopolysaccharide can lead to M1 phenotype modification and the release proinflammatory factors.In contrast,activation by IL-4 leads to an M2 phenotype modification with an anti-inflammatory effect,which helps clear dead cell fragments and release neuroprotective factors.Microglia/macrophages also regulate local brain immune inflammation homeostasis (Liu et al.,2020;Lyu et al.,2021).To some extent,promoting M2 polarization and inhibiting M1 polarization can benefit neurological function recovery after stroke (Mesquida-Veny et al.,2021;Blank-Stein and Mass,2023).IL-4-dependent NSCs can inhibit the activation of the nuclear factor-κB/ p65 pathway and induce macrophage M2 polarizationin vitro(Ji et al.,2020).Α study comparing the effects of EVs from different sources in a stroke model showed that using NSC EVs resulted in better functional outcomes in MCAO mice than the use of mesenchymal stem/stromal cells (MSC) EVs,which were related to macrophage M2 polarization,increased Treg cells,and decreased pro-inflammatory Th17 lymphocytes (Webb et al.,2018b).
Maintaining blood-brain barrier stability
The BBB is an important target of cerebral ischemiareperfusion injury,and can become disrupted by the upregulated expression of matrix metalloproteinase-2 and matrix metalloproteinase-9,which degrade the basement membrane after ischemia (Shen et al.,2018;Ji et al.,2023).Damage-associated molecular patterns released from necrotic cells activate pattern recognition receptors in immune cells,and immune cells and inflammatory factors pass through the damaged BBB,damaging normal or aggravating the function of damaged nerve cells (Sakai and Shichita,2023).
NPSCs have been shown to enhance BBB stability in animal models (Lin et al.,2018;Gao et al.,2020).Eckert et al.(2015) transplanted human induced pluripotent stem cellderived (hiPSC) NSCs into the mouse hippocampus 24 hours after MCAO and injected Texas Red dextran 70 kDa into the mouse inferior vena cava 48 hours after MCΑO (24 hous after transplantation).Owing to the complete BBB in the opposite area,the tracer remained in the capillary cavity and exhibited a clear vascular edge.In the untreated cortex on the stroke side,abundant tracer diffused from the leaking capillaries,whereas transplantation of hiPSC-NSCs significantly reduced tracer leakage (Eckert et al.,2015).The same study also evaluated the extravasation level of blood-derived IgG into the brain parenchyma through the BBB;Western blotting showed that the IgG level in the hiPSC-NSC-transplanted MCAO group was significantly lower than that in the control MCΑO group(Eckert et al.,2015).Further analysis showed that NSCs significantly downregulated matrix metalloproteinase-2 and matrix metalloproteinase-9 activity in the experimental group and played a neuroprotective role by maintaining BBB integrity(Eckert et al.,2015).
Endogenous regeneration: angiogenesis and neuroplasticity
Newly formed collateral blood vessels and regenerated capillaries maintain cerebral blood flow and support long-term functional recovery after stroke (Lin et al.,2008;Yang and Torbey,2020).NSC transplantation promotes angiogenesis and neuroplasticity.Compared to the control group,the area implanted with CTX0E03 in the brains of immature mice showed a significant increase in the formation of new blood vesselsin vivoand enhanced the formation of common tubules in the extracellular matrix (Hicks et al.,2013).Furthermore,NSC transplantation can increase post-ischemic angiogenesis,as assessed by double labeling rat endothelial cells with 5-bromo-2′-deoxyuridine and anti-von Willebrand factor (Ryu et al.,2016).NSC therapy induces an increase in the immune reactivity of VEGF and promotes vascular regeneration,which may be involved in the activation of the JΑK2/STΑT3 signaling pathway (Clayton et al.,2008;Ha et al.,2022).Simultaneously,angiogenesis and neurogenesis have a certain synergistic effect,sharing parts of their molecular mechanisms (stromal cell-derived factor-1 and angiopoietin-1;Ruan et al.,2015;Lin et al.,2019;Paro et al.,2023).
Plasticity of the brain after stroke involves the formation of new connecting wires by redundant neurons,forming neural circuits,and compensatory functions (Doeppner et al.,2014;Carmichael,2022).NSCs can reduce glial scar formation and enhance the plasticity of dendritic and axonal structures after stroke (Doeppner et al.,2014).Furthermore,transplanted NSCs can stimulate the proliferation and migration of endogenous NSCs in the SVZ and DG by secreting growth factors,such as fibroblast growth factor-2,insulinlike growth factor,BDNF,VEGF,and chemokines (matrix metalloproteinases and monocyte chemoattractant protein-1;Ruan et al.,2015;Ryu et al.,2016).Neurons differentiated from transplanted NSCs extend axons from the lesion site and form synapses,thereby supporting neural function repair.Studies have shown that NPSCs may enhance the plasticity of host neurons by secreting factors such as thrombospondins-1/2,VEGF,and Slit,including the restoration of axonal transport and sprouting of dendrites and axons(Andres et al.,2011b).
Neural Stem Cell Therapy Strategies
With the deepening of research on stem cell mechanisms,especially since the publication of the Stem Cell Therapies as an Emerging Paradigm in Stroke guidelines in 2007 (Stem Cell Therapies as an Emerging Paradigm in Stroke Participants,2009;Savitz et al.,2011),many stem cell products and related experimental studies on the treatment of ischemic stroke have emerged.Many preclinical and clinical studies have evaluated the efficacy and safety of exogenous NSCs,which have been confirmed (Mack,2011;Kalladka et al.,2016).
Sources and types of neural stem cells
To apply NSC therapy in stroke patients,the first step towards success is to prepare a perpetually growing human neural cell line with the ability to differentiate into mature neurons.Since the 1990s,various NSC cell lines have been mastered,including:
(i) The direct extraction of NSCs from the developing fetal brain,which are cultured and proliferatedin vitrousing growth media containing basic fibroblast growth factor or epidermal growth factor (Reubinoff et al.,2001;Lee et al.,2008a),such as the CTX0E03 cell line,ReNcell VM (Stroemer et al.,2009;Song et al.,2019).These cell lines are widely used in preclinical studies,and the preliminary results from preclinical and clinical trials are encouraging;however,they are currently only used in patients with chronic stroke (Stroemer et al.,2009;Kalladka et al.,2016).
(ii) Embryonic stem cells obtained from embryonic neural tissue,especially the outer layer of the embryo,can be efficiently differentiated into NSCs by inhibiting SMAD signaling transduction and via other methods (Chambers et al.,2009),such as the NSI-566 cell line (Zhang et al.,2019a).Similar to the previous type of stem cells,these cell lines are widely used in preclinical studies,and the safety has been demonstrated in clinical trials in amyotrophic lateral sclerosis and spinal cord injury (Curtis et al.,2018);however,fewer clinical trials have been conducted in the direction of stroke.(iii) Somatic cells,including fibroblasts,can be reprogrammed into human iPSCs,and induced to differentiate into NSCs using the same methods as ESCs (Liu,2013;De Paola et al.,2023).LDN193189,a bone morphogenetic protein inhibitor,can induce the differentiation of ischemia-induced multipotent stem cells by regulating bone morphogenetic protein signaling (Minato et al.,2022).Additionally,fibroblasts can be transiently induced into functional NPSCs or iPSCs through programmed rearrangement factors (Oct4,Sox2,klf4,and c-Myc;Gascón et al.,2017;Machado et al.,2020).
The use of iPSCs has emerged as a highly promising area.Recently,iPSC-derived NSCs has been applied in preclinical trials,such as for spinal cord injury,amyotrophic lateral sclerosis,and stroke.Transplantation has been demonstrated to be successful in spinal cord injury models and can considerably increase motor function recovery (Nagoshi and Okano,2018).iPSC-derived NSCs can effectively improve the function of neuromuscular and motor units in mice with amyotrophic lateral sclerosis,and prolong lifespan (Nizzardo et al.,2014).Chau et al.(2014) reported that the mice transplanted with iPSC-NPCs performed better in sensory motor function testing after stroke compared to the control group.It is highly anticipated that iPSC-derived NSCs will be extensively utilized in clinical trials in the future.These cell lines can be produced from the patient’s own cells,circumventing ethical and potential immune rejection issues.The disadvantage is that iPSC-derived NSCs still carry the risk of residual tumorigenicity and chromosomal abnormalities(Miura et al.,2009);therefore,further verification is required to ensure the safety.
(iv) Human teratoma cell lines (NTera2/clone D1 or NT2 cells) are considered a type of neural cell line and can be differentiated into neuron-like cells (NT2N) through vitamin A inductionin vitro(Hara et al.,2008;Eckert et al.,2015).Before the discovery of iPSCs,neurons derived from NT2/D1 cells were widely used.Kondziol et al.(2000) transplanted LBS neurons differentiated from NT2/D1 cells into stroke patients and showed that transplantation of NT2/D1 derived neurons was feasible.However,these cell lines carry a risk of tumorbuilding,and the current results suggest that their efficacy in stroke patients is limited (Stonesifer et al.,2017).
Delivery potential (route and dosage)
Endovascular transplantation is less damaging and more readily accepted.The main disadvantage of intravenous (IV)therapy is that most NSCs remain in peripheral organs,such as the spleen,lungs,and liver.However,research has shown that NSCs remain in the spleen and have neuroprotective effects,such as anti-inflammatory properties (Lee et al.,2008b).Compared to the IV route,the intra-arterial delivery of NSCs to the brain is more efficient (Pendharkar et al.,2010);however,this treatment has disadvantages,such as causing microemboli and lacunar infarction.The intracerebral(IC) (cortex or hippocampus) pathway utilizes stereotactic instruments to accurately inject NSCs into damaged braintissue but carries the risk of perioperative complications (Muir et al.,2020).A network meta-analysis suggested that IC is the best approach for transplantation intervention in animal models of ischemic stroke (Yang et al.,2022b).Transplanting NSCs through lumbar puncture is the most common method of intrathecal or intracerebroventricular delivery,which is less invasive than IC delivery and is widely distributed throughout the brain (Wang et al.,2021).Furthermore,intranasal administration is a non-invasive and repeatable method;however,related research is limited regarding with some testing of NSC and NSC EV transplantation in rodents(Αchón Buil et al.,2023).Research has shown that EVs can be efficiently delivered to neurons,microglia,and astrocytes in the brain (Kodali et al.,2019;Upadhya et al.,2020).A single-center,randomized,double-blind,parallel-controlled clinical trial of the intranasal administration of hNSCs will be conducted in China,and the results are expected soon (Xie et al.,2022).
Currently,there are registered clinical trials (e.g.,NCT03080571,NCT02605707,NCT02425670,and NCT01151124) focusing on the treatment of ischemic stroke using stem cells;however,only the IC pathway is currently being utilized for the delivery of NSCs.Mesenchymal stem/stromal cells,bone marrow mononuclear cells,and CD34+hematopoietic stem cells derived from mononuclear cells(CD34) have shown a preference for the IV and intra-arterial pathways over the IC pathway (Fang et al.,2019;Savitz et al.,2019;Jaillard et al.,2020;Kawabori et al.,2020).This indicates that the delivery mode varies significantly depending on the intended therapeutic outcome,and further research is required to determine the safety and efficacy of each delivery method in the context of ischemic stroke treatment.The application of NSCs in clinical trials ranges from 2 million to 80 million doses,and according to published literature,up to 20 million doses administered through the IC pathway have not resulted in adverse events and are deemed safe (Kalladka et al.,2016).
Time window
In clinical trials,stroke is classified into acute (within 1 week),subacute (within 6 months),and chronic (after 6 months)phases (Kawabori et al.,2020).The transplantation of NSCs during the acute phase of stroke is controversial.In the relevant clinical trials currently underway,it is assumed that the longer the treatment is administered from the onset of stroke,the lower the risk to the recipient’s safety posed by the therapy.The transplantation time window is important because changes in the ischemic microenvironment after stroke vary significantly over time.Transplanting stem cells at the early stage of ischemic events (within 2–3 weeks) is more advantageous for enhancing endogenous repair mechanisms,such as axonal plasticity,blood vessels,and nerves,whereas late-stage transplantation after inflammation subsides may be more conducive to stem cell survival (Carmichael,2006).Experiments on MCAO rats also showed that cell survival was better in the ultra-early stage (within 48 hours),before the establishment of the inflammatory reaction,compared to transplantation at 6 weeks after stroke (Darsalia et al.,2011).
Advances of NSCs in clinical trials
The initiation and advancement of clinical trials are more challenging than preclinical animal experiments.Since September 4,2023,59 stem cell clinical studies on ischemic stroke have been registered at ClinicalTrials.gov(e.g.,NCT03080571,NCT02605707,NCT02425670,and NCT01151124);unregistered studies have been omitted in this review.Two (NCT01151124 and NCT03296618) of the five studies (NCT04631406,NCT03296618,NCT03629275,NCT02117635,and NCT01 151124;Table 2) using NSCs have published their findings.The information provided in this article was sourced from data collected by ClinicalTrials.gov on September 4,2023.Table 2lists information on ongoing and completed clinical studies,including the sources,pathways,doses,and outcome measures of NSCs.By analyzing these data,researchers can identify potential trends and patterns in future research on NSC treatments.

Table 2|Clinical trials with neural stem cells (NSCs) for the treatment of ischemic stroke obtained from ClinicalTrials.gov
In an important study on the treatment of ischemic stroke with NSCs,the results of PISCES (NCT01151124) showed no immune-or cell-related adverse events,with a slight improvement in the National Institutes of Health Stroke Scale score.The safety results of this study fully support further research on ischemic stroke,and subsequent clinical trials,PISCES-II (NCT02117635) and PISCES III (NCT03629275),focused on exploring its efficacy (Olmsted et al.,2022).Α study using NSI-566 (NCT03296618) showed some improvements in the National Institutes of Health Stroke Scale score,Fugl-Meyer Motor Score,and modified Rankin Scale,and new tissue generation was observed in the infarcted area of some subjects (Zhang et al.,2019a).Furthermore,an ongoing NSC(NR1) trial (NCT04631406) is examining the safety and acceptability by delivering escalating dosages of NR1 at a single time point in participants post-injury,which is expected to be completed by the end of 2024,with promising results.
Engineering of Neural Stem Cells
The clinical conversion of NSCs is difficult,and the most common outcome of transplanting exogenous NSCs is cell death (Liang et al.,2013).Currently,NSC engineering techniques (Figure 2),such as preconditioning,embedding in biomaterial scaffolds,and genetic engineering,can be used to increase the survival rate of NSCs,making them more suitable as a treatment for stroke (Bernstock et al.,2017;Αbati et al.,2019).

Figure 2| Techniques for engineering neural stem cells (NSCs).
Preconditioning
Preconditioning of the microenvironment has proven to be effective in preclinical studies.Preconditioning of the microenvironment is beneficial for improving the survival rate of NSCs.Sublethal hypoxic preconditioning increases the expression of HIF target genes with cell-protective effects,such as erythropoietin;upregulates B-cell lymphoma 2,hypoxia-inducible factor-1α,and erythropoietin receptor expression;reduces the caspase-3 activation rate;and causes cell death (Theus et al.,2008;Li et al.,2022).Preconditioning NSCs with conductive polymer materials alters the VEGF-A pathway genes and genes involved in cell survival,effectively improving the functional results in animal models (George et al.,2017).Recently,it was demonstrated that nanoscale electrical stimulation promotes the differentiation of NSCs by balancing autophagy-related signals with mTOR signalsin vitro(He et al.,2021).
The exposure of NSCs to small-molecule drugs,cytokines/neurotrophic factors,and other positive factors before transplantation has beneficial effects.Pretreatment with the anti-inflammatory drug minocycline upregulates nuclear factor erythroid 2-related factor 2 and nuclear factor erythroid 2-related factor 2 regulated antioxidant genes,including NQO1 and HO-1,and induces the secretion of neurotrophic factors such as BDNF,glial cell-derived neurotrophic factor,and VEGF from NSCs (Sakata et al.,2012).A more direct pretreatment approach is the use of neurotrophic factor BDNF treatment to promote the expression of chemokine receptors and adhesion molecules,which is conducive to NSC migration(Rosenblum et al.,2015).Pretreatment with IL-6 upregulates STΑT3-mediated SOD2 expression,increases manganese superoxide dismutase levels,and renders NSCs more resistant to oxidative stress (Jung et al.,2011).In addition,proinflammatory cytokines simultaneously induce adverse effects by stimulating NSCs to differentiate into astrocytes(Suzuki et al.,2009).The combination of minocycline and proinflammatory cytokines,such as IL-6,TNF-α,and IL-1β,can restore the neural and oligodendrocyte-generating potential of NSCs (Vay et al.,2016).Non-hormonal male contraceptive adjudins can inhibit oxidative stress,activate the Αkt signaling pathway,and improve the survival rate of NSCs (Zhang et al.,2017).Recently,a study used Metal-Organic Framework Nanoparticles to mediate miRNA-124 to promote NSCs to accelerate their directional differentiation into neurons.Ca-MOF@miR-124 nanoparticles combined with NSCs to treat stroke enhanced the efficacy of stem cell therapy (Yang et al.,2022a).
Biomaterial embedding
Recent studies have shown that owing to their unique physical and biological properties,biopolymer network hydrogels can be used as biomaterial scaffolds for NSCs,providing a more favorable microenvironment (Moshayedi et al.,2016;Li et al.,2021a).Hydrogels are highly versatile materials comprised of hydrophilic polymers that can form a three-dimensional network structure capable of absorbing large quantities of water to swell,but not dissolve (Totten et al.,2022).Hydrogels outperform many natural polymers in terms of physical and biological properties,providing physical and biological barriers for transplanted NSCs (Gopalakrishnan et al.,2019).On the one hand,hydrogels can provide physical scaffolds to avoid undesirable cell distribution,such as cell aggregation caused by transplanting exogenous stem cells into saline or culture medium (Orive et al.,2009).On the other hand,hydrogel has good biocompatibility and can effectively evade the immune and inflammatory response after implantation,providing support for the survival and proliferation of stem cells (Lin et al.,2009;Sindhu et al.,2020).
The use of hydrogels has developed rapidly in the field of stem cells.The hydrogel skeleton can be further modified by biological agents,such as proteins and antibiotics,to achieve“functionalization”,which provides additional support or signals for NSCs (Shendi et al.,2016;Totten et al.,2022).Αnti-Fas-conjugated hyaluronic acid microsphere gels (Shendi et al.,2017) and liposome sodium alginate microcapsules (Xu et al.,2022) have been developed in recent years.Liposomal sodium alginate microcapsules can also alter autophagy markers (such as P62 and LC3-II) to enhance autophagy flux and promote cell survival (Xu et al.,2022).The increased control and support provided by functionalized hydrogels have led to advanced applications in stem cell therapy,regenerative medicine,and tissue engineering research.Therefore,the continued development and refinement of functionalized hydrogels is a crucial area of focus in the field of stem cell research.
Pre-transplant genetic engineering
Extracorporeal genetic engineering can regulate the expression of related genes to make NSCs more adaptable to oxidative stress following ischemia and hypoxia (Gowing et al.,2017;Αchón Buil et al.,2023).Previous studies have focused on upregulating specific pathways,such as BDNF,glial cellderived neurotrophic factor,neurotrophin-3,and VEGF (Zhang et al.,2008;Chen et al.,2009;Chang et al.,2013b).With the concept of global protein conjugation with small ubiquitinlike modifier (SUMOylation) proposed in the stem cell field,future research may shift more towards the simultaneous regulation of multiple pathways (Lee and Hallenbeck,2013;Karandikar et al.,2023).Global protein small ubiquitin-like modifier modification is the post-translational modification of proteins by small ubiquitin-like modifier (Yang et al.,2014).Furthermore,global SUMOylation has been found to play a cytoprotective role,with its level positively correlating with the degree of protection (Yang et al.,2014;Lu et al.,2019;Karandikar et al.,2023).Thus,identifying targets to improve the pretreatment method for the global SUMOylation of NSCs is a highly valuable strategy (Bernstock et al.,2019).Mild hypothermia therapy can upregulate global SUMOylation of stem cells and increase their ability to tolerate hypoxic stress(Cai et al.,2022).
CRISPR-Cas9 technology can accurately modify NSCs by targeting specific gene sequences and synthesizing and modifying genes (Jinek et al.,2012;Cong et al.,2013).Gene editing in NSCs (GE-NSCs) not only preserves the functional characteristics of self-renewal,migration,and differentiation,but also can increase the therapeutic efficacy of NSCs,providing further exploration of gene loci as therapeutic cell products for CNS diseases (Bressan et al.,2017).Dever DP et al.confirmed that the enzyme galactosylceramidase GE-NSCs can overexpress galactosylceramidase lysosomal enzymes and cross-correct Krabbe disease fibroblastsin vitro,indicating that these GE-NSCs may become cellular and gene therapy methods for a range of neurodegenerative diseases and CNS damage,including lysosomal storage disorders (Dever et al.,2019).The selective modification of NSCs using CRISPR-Cas9 technology provides new ideas and possibilities for ischemic stroke treatment.
Limitations
This article has certain limitations that should be acknowledged.First,there are few clinical trials on the application of NSCs in ischemic stroke,and the content available for reference is limited.Therefore,in the NSC therapeutic strategies,we referred to the delivery route of other types of stem cells applied in clinical trials of ischemic stroke.Furthermore,owning to the complexity of the molecular mechanisms and space constraints,we did not include a thorough descriptions of the numerous signaling pathways implicated in NSC engineering.
Conclusions
The use of transplanted NSCs in cerebral ischemia models has shown favorable results in preclinical trials and is expected to have significant effects in further clinical trials.NSC therapy may enable the development of new therapies for a wide range of neurological conditions,including spinal cord injury,neuroimmune disorders,and degenerative disorders,such as Parkinson’s disease,Alzheimer’s disease,and multiple sclerosis.However,there are still some issues to be addressed before they can be widely used as a clinical treatment:(1) further validation of the safety and effectiveness of NSC clinical applications;(2) in-depth investigation of the therapeutic mechanisms of NSCs and exploring the feasibility of using NSC-derived EVs for treatment;(3) exploring the optimal cell source,transplantation route,time window,and dosage of exogenous NSC transplantation;and (4) promoting the development of NSC engineering.In this article,we provide an overview of potential NSC mechanisms and various processing techniques to boost the effectiveness of NSC therapy.Although important challenges remain in fully understanding the complex mechanisms of NSCs and optimizing their clinical applications,ongoing research in this area is significant.
Author contributions:SW,QH,YY and ZNG wrote the manuscript.SW,QH,YQ,WY,RZ,XW,YY and ZNG revised the manuscript.SW,YY and ZNG participated in the conceptualization of the manuscript.All authors approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
