Unraveling the potential of acute intermittent hypoxia as a strategy for inducing robust repair in multiple sclerosis
2024-03-05ValerieVergeNataliyaTokarskaJustinNaniong
Valerie M.K.Verge,Nataliya Tokarska,Justin M.Naniong
Multiple sclerosis (MS) is a debilitating inflammatory disease of the central nervous system characterized by immune-mediated segmental demyelination and variable degrees of axonal and neuronal degeneration that contribute to disability.Inducing efficient and effective repair programs following demyelination is a major goal and challenge in MS.Conventional MS therapies focus largely on modulating the immune aspects of the disease contributing to lesions.While this alleviates some symptoms and mitigates damage,it does not tackle the fundamental challenge of effective remyelination,which few MS patients experience,especially in the progressive phase of the disease.
The capacity for effective remyelination in the early relapsing-remitting phase of MS reveals that a repair pathway exists.Harnessing this pathway can improve repair outcomes and benefit patients when this pathway becomes less effective at mediating remyelination and preventing neurodegeneration as the disease becomes progressive.
The current paucity of treatments available for those with progressive forms of MS and the deficits created by ongoing neurodegeneration underlie the need for enhanced repair strategies.This need aligns well with our research focus,which is on devising therapies that increase the intrinsic repair capacity of the peripheral and central nervous systems following injury,including focal demyelination.In this regard,increasing neural activity via brief electrical nerve stimulation(ES) promoted enhanced remyelination and axon protection,drove a pro-repair immune response,and attenuated gliosis and inflammation in lysophosphatidyl choline-focally demyelinated rat peripheral nerves and dorsal columns (Ayanwuyi et al.,2022).However,nerve ES is invasive,requiring the placement of electrodes.A further complication is the fact that demyelinating lesions in MS can occur anywhere in the brain and spinal cord,making ES not a realistic therapeutic approach against the systemic nature of MS.Thus,the search for a non-invasive means of increasing neural activity and enhancing the intrinsic capacity for repair led us to examine acute intermittent hypoxia (AIH).AIH,which involves repeatedly breathing in air with alternating normal and low oxygen levels for short cycles,is an emerging noninvasive therapy that improves function in spared respiratory and nonrespiratory muscle-controlling neurons in rats and humans with partial spinal cord injury (Vose et al.,2022).AIH has strong translational potential,eliciting sustained increases in voluntary limb strength and walking distance in persons with chronic spinal cord injury.Importantly,ΑIH does not negatively affect memory,cognition,heart rate,or blood pressure(Vose et al.,2022).
AIH induces long-term facilitation,a form of respiratory plasticity/increased neural activity resulting from a repetitive exposure to brief (5 minutes) episodes of mild-moderate hypoxia (11%O2) alternating with normoxic (21% O2) intervals that can be evoked in awake animals.Αfter 3–10 episodes of ΑIH,long-term facilitation is sustained for at least 2 hours,closely mimicking the optimal ES nerve therapy promoting nerve regeneration,immune modulation,axon-protective phenotype,and remyelination (Nadeau et al.,2021;Αyanwuyi et al.,2022;Vose et al.,2022).AIH also results in increased brain-derived neurotrophic factor(BDNF) expression (Nadeau et al.,2021),which is linked to induction of repair programs and myelination (Geremia et al.,2010;Nadeau et al.,2021).The duration,dose,and number of intervals that elicit optimal AIH neuroplasticity without contraindication have been investigated and it is found that the responses to AIH occur within an optimal beneficial range of hypoxemia coupled with a low number of episodes/day– i.e.modest hypoxia (9–16% inspired air) coupled with low cycle #s (3–15 episodes/day) (Navarrete-Opazo and Mitchell,2014).It should be noted that all our nervous system repair studies adhere to the dose,duration,and number of intervals in line with prior and ongoing preclinical and clinical studies showing positive responses (Vose et al.,2022).
In our initial research,the capacity for AIH to promote repair/regeneration was assessed in a complete peripheral nerve transection with surgical repair model,with AIH therapy being directly compared to ES.In the study,once daily AIH treatment,where normal levels of oxygen (O2)at 21% were alternated over ten 5-minute cycles with O2at 11% was performed in each of the two days after nerve repair and directly compared to either Normoxia controls (21% O2for the identical number of 5-minute cycles) or the 1-hour ES treatment delivered at the time of repair that has been shown to result in optimal regeneration outcomes (Nadeau et al.,2021).This preclinical study revealed AIH &ES to have similar molecular signatures with respect to regeneration-associated gene expression and nerve regeneration outcomes,including accelerated remyelination.This is marked by a significant elevation in the expression of the pro-regenerative molecules BDNF and hypoxia-inducible factor 1α (Nadeau et al.,2021).With respect to efficient remyelination of the nerve,AIH therapy resulted in decreased numbers of degenerating myelin profiles and an improved G-ratio,a measure of myelin thickness relative to axon diameter,which correlated with improved functional outcomes 10 weeks postnerve repair (Nadeau et al.,2021).
The notable impact of AIH on nerve and myelin repair in the peripheral nervous system led us to examine whether a similar potential exists for central nervous system repair following a focal demyelinating episode.When coupled with work by the Milner lab (Halder et al.,2018) showing that manipulation of the O2axis by chronically exposing mice to low O2(10%) as a form of hypoxic pre-conditioning was mitigating against the development of experimental autoimmune encephalomyelitis disease,it supports that a protective,adaptive and regenerative response to reduced oxygen may be possible.
In a first-of-its-kind preclinical study,we employed the MOG35–55progressive experimental autoimmune encephalomyelitis female mouse model of MS which exhibits many of the pathological features of MS including compromised blood-brain barrier,inflammation,demyelination,gliosis,and axonal and neuronal degeneration (Tokarska et al.,2023) to elucidate the repair potential of ΑIH.In these animals,once daily AIH treatment was performed for 1 week in female mice who had reached a near-peak clinical score of 2.5,akin to a patient seeking medical help while having an MS attack.As mentioned above,the AIH protocol selected was in line with that being delivered in preclinical research and spinal cord injury clinical trials and in the dose and frequency known to induce increased neural activity and promote neuroplasticity (Navarrete-Opazo and Mitchell,2014) and repair responses at a level comparative to optimal nerve ES (Nadeau et al.,2021).
What we observed (Tokarska et al.,2023) was a significant positive impact of AIH on every facet of repair and neuropathology examined one week after cessation of treatment that was maintained for at least two weeks after the end of treatment(latest timepoint examined;Figure 1).These included: (i) improved functional outcomes/clinical scores;(ii) inflammation that was reduced by~80% with a shift in the polarization state of the remaining inflammation toward a pro-repair state.The shift in polarization state was shown by increased microglial/macrophage expression of the pro-repair markers CD206 and Activin A and reduced expression of the pro-inflammatory markers inducible nitric oxide synthase and tumor necrosis factor alpha;(iii) a more rapid clearance of myelin debris,allowing for more efficient remyelination;(iv) the rephosphorylation of neurofilaments,placing the axons in a protected state from calpain-driven neurodegeneration;(v)a more robust recruitment of oligodendrocyte precursor cells (OPCs) to the inflamed regions/zones of demyelination as indicated by increased numbers of cells positive for Olig2,a marker of cells of oligodendroglial lineage,and plateletderived growth factor receptor alpha,a phenotypic marker of OPCs;(vi) increased differentiation of OPCs into mature myelinating oligodendrocytes(OLs) as assessed by the heightened expression of CC1,a marker of mature myelinating OLs;(vii) evidence of robust remyelination shown by increased linear myelin basic protein expression in zones of demyelination,re-established nodes of Ranvier positive for the paranodal marker Caspr,increased numbers of thinly myelinated axons with reduced degenerating myelin profiles and a higher G-ratio associated with active remyelination at this early repair timepoint.
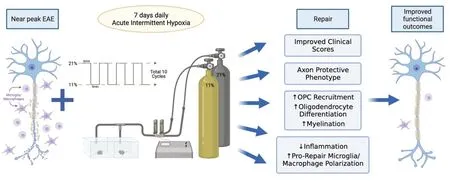
Figure 1|Schematic diagram summarizing the beneficial impacts of acute intermittent hypoxia (once daily treatment for 7 days;ten cycles of normal 21% O2 alternating with 11% O2) relative to normoxia controls on outcomes in female mice with EAE,an experimental model of multiple sclerosis.
The fact that these mice already showed significant and near-maximal myelin loss and inflammation at the near-peak clinical score of 2.5 just prior to commencement of ΑIH treatment and that Normoxia control treatment did not improve the outcomes while AIH did,supports the existence of a genuine repair response initiated by the treatment.
What are the potential molecules/mechanisms whereby AIH is leading to such beneficial repair responses? Some molecules/mechanisms may include: (i) increased axonal activity which has been linked to oligodendrogenesis and initiation of myelination by OLs and OPCs(Fekete and Nishiyama,2022);(ii) correcting the dysregulation of the axon/glial communication linked to demyelination and neuropathic pain in experimental autoimmune encephalomyelitis mice;(iii) the creation of a pro-myelinating environment by AIH through its ability to accelerate myelin debris clearance and increase recruitment and differentiation of OPCs into mature oligodendrocytes by molecules such as Activin A which is elevated in pro-repair microglia/macrophages in response to AIH;and(iv) upregulation of specific molecules such as BDNF which has multiple roles in myelin repair and whose levels are reduced levels in MS (Nocitiand Romozzi,2023).Elevated BDNF such as is seen in response to AIH (Nadeau et al.,2021) can increase mature OL numbers and remyelination in pathological states.BDNF can also impede infiltration of pro-inflammatory macroglia/macrophages and increase neurofilament phosphorylation which protects axons from degradation by calpains and increases axon caliber helping drive the onset of myelination (Fekete and Nishiyama,2022;Tokarska et al.,2023).
Ongoing investigations are aimed at identifying the molecular basis underlying the differential responses from AIH and normoxia treatments.We are targeting some of the key molecules potentially underlying these beneficial adaptive stress responses and assessing whether these AIH-driven repair responses are also observed for male counterparts.Whether other time points of intervention or duration of treatment or addition of task-specific training may also beneficially impact outcomes,mitigate chronic symptoms,or prevent progression remains to be explored (Vose et al.,2022).
While AIH is novel and promising as a repair strategy to decrease disease burden in MS,a complete understanding of the mechanisms underlying AIH’s effects on these outcomes has yet to be elucidated,including how it might affect comorbidities.What is encouraging however,are the increasing numbers of studies that report emerging beneficial adaptive impacts of AIH in a number of pathologies that include,but are not limited to,reduced cognitive decline such as in Alzheimer’s disease,improving stroke recovery,initial investigations evaluating safety and efficacy in amyotrophic lateral sclerosis,increased neuroplasticity in people with spinal cord injury,and improvements in metabolic disorders (Vose et al.,2022;Zhang et al.,2023).Further,a comprehensive examination of preclinical and clinical applications involving rodents and humans with spinal cord injury has concluded AIH to be a beneficial therapy with little indication of adverse effects (Vose et al.,2022).However,challenges in interpreting the clinical outcomes do exist,including the variability in the experimental protocols and design,small participant numbers,an incomplete understanding of mechanisms involved and detailed investigations to ensure that the beneficial adaptive responses to AIH do not transition into maladaptive ones.Αdditionally,some individuals do not respond as avidly to AIH.Thus,there is a need to better understand and perhaps screen individuals for biomarkers/genetic variations that may identify those who might be the best responders.An example of this is a recent focus on the 30–50% of individuals in the USΑ carrying the BDNF Val66Met polymorphism known to be associated with impaired neuroplasticity,as a potential marker of poor responders to ΑIH,a biomarker that has yet to be systematically investigated in ΑIH clinical trials (Vose et al.,2022).As the outcomes of ongoing clinical trials are reported,hopefully some of these queries will be answered and treatment protocols optimized to best treat the pathology at hand.
In conclusion,there are intrinsic repair programs that we can tap into and significantly enhance with the right therapeutic intervention.We are gaining a better understanding of how to beneficially manipulate stress pathways to advantage repair and lay the foundation for clinical translation.Most notably,our novel finding that AIH holds tremendous promise for myelin repair,axon protection,and immune modulation in a progressive preclinical model of MS makes us hopeful that with further investigation this can be translated,especially for those with progressive forms of MS for which there is a current paucity of therapies.
This work was supported by MS Canada research grants #2362 &# 920666,Canadian Institutes of Health Research(CIHR)grants #142328 𬵲,and University of Saskatchewan College of Medicine CoMRAD grant to VMKV.NT and JMN were supported by University of SaskatchewanCollege of Graduate and Postdoctoral Studies and College of Medicine Scholarships.
Valerie M.K.Verge*,Nataliya Tokarska,Justin M.Naniong
Department of Anatomy,Physiology and Pharmacology,University of Saskatchewan,Saskatoon,SK,Canada (Verge VMK,Tokarska N,Naniong JM)
Cameco MS Neuroscience Research Center,University of Saskatchewan,Saskatoon,SK,Canada(Verge VMK,Tokarska N,Naniong JM)
*Correspondence to:Valerie M.K.Verge,PhD,valerie.verge@usask.ca.
https://orcid.org/0000-0001-6648-3242(Valerie M.K.Verge)
Date of submission:October 6,2023
Date of decision:December 26,2023
Date of acceptance:January 8,2024
Date of web publication:January 31,2024
https://doi.org/10.4103/NRR.NRR-D-23-01663
How to cite this article:Verge VMK,Tokarska N,Naniong JM(2024)Unraveling the potential of acute intermittent hypoxia as a strategy for inducing robust repair in multiple sclerosis.Neural Regen Res 19(11):2339-2340.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
