Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma
2024-02-29YangSongHarryCHLauXiangZhangJunYu
Yang Song, Harry CH Lau, Xiang Zhang, Jun Yu
1Institute of Digestive Disease and Department of Medicine and Therapeutics, State Key Laboratory of Digestive Disease, Li Ka Shing Institute of Health Sciences, CUHK Shenzhen Research Institute, The Chinese University of Hong Kong, Hong Kong,China; 2Department of Gastroenterology, Zhongshan Hospital Xiamen University, Xiamen 361004, China
ABSTRACT Hepatocellular carcinoma (HCC) is a prevalent and aggressive liver malignancy.The interplay between bile acids (BAs) and the gut microbiota has emerged as a critical factor in HCC development and progression.Under normal conditions, BA metabolism is tightly regulated through a bidirectional interplay between gut microorganisms and BAs.The gut microbiota plays a critical role in BA metabolism, and BAs are endogenous signaling molecules that help maintain liver and intestinal homeostasis.Of note,dysbiotic changes in the gut microbiota during pathogenesis and cancer development can disrupt BA homeostasis, thereby leading to liver inflammation and fibrosis, and ultimately contributing to HCC development.Therefore, understanding the intricate interplay between BAs and the gut microbiota is crucial for elucidating the mechanisms underlying hepatocarcinogenesis.In this review, we comprehensively explore the roles and functions of BA metabolism, with a focus on the interactions between BAs and gut microorganisms in HCC.Additionally, therapeutic strategies targeting BA metabolism and the gut microbiota are discussed,including the use of BA agonists/antagonists, probiotic/prebiotic and dietary interventions, fecal microbiota transplantation, and engineered bacteria.In summary, understanding the complex BA-microbiota crosstalk can provide valuable insights into HCC development and facilitate the development of innovative therapeutic approaches for liver malignancy.
KEYWORDS Bile acid; gut microbiota; hepatocellular carcinoma; therapeutics; microbiota modulation
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy worldwide.This disease is typically diagnosed in advanced stages, because of its aggressive nature and rapid progression, thus resulting in high mortality and limited treatment options1,2.HCC arises primarily from chronic liver diseases with various etiologies, including viral hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease (NAFLD)3,4.The intricate interplay among genetic and environmental factors contributes to the complex tumorigenesis mechanism of HCC.Understanding the risk factors associated with HCC is essential for the development of diagnostic biomarkers, therapeutic targets, and preventive strategies against this lethal disease.
Recent studies have revealed the emerging role of the gut microbiota in HCC development and progression.The human gastrointestinal tract harbors a diverse and dynamic community of microorganisms forming the gut microbiota, which consists primarily of bacteria but also includes viruses and fungi5-7.These gut microorganisms play critical roles in dietary digestion, such as breaking down carbohydrates, producing vitamins,and metabolizing dietary components that human hosts cannot digest8-10.The gut microbiota also interacts with the host, and influences metabolism, immunity, and even brain function11,12.However, the physiological crosstalk between hosts and microorganisms is greatly impaired in disease conditions, because of pathological alterations in gut microbial composition and function, which are commonly known as dysbiosis.Gut dysbiosis has been implicated in promoting chronic liver inflammation,fibrosis, and HCC, through mechanisms such as immunomodulation, toxin release, and altered metabolite regulation, thereby influencing hepatocarcinogenesis.
Bile acids (BAs) are steroid acids that are synthesized in the liver and promote absorption of dietary lipids.The regulation of BA metabolism involves interactions between host cells and gut microbiota.In the context of HCC patients,there is a notable alteration in BA metabolism and the reciprocal iinteraction between host cells and gut microbiota.In general, a dysbiotic microbiota can disrupt BA homeostasis,and contribute to hepatocellular damage and carcinogenesis.In contrast, dysregulated BA metabolism reshapes the composition and function of the gut microbiota, and further promotes the occurrence and extent of microbial dysbiosis.Notably, because the intricate interaction between BAs and the gut microbiota is fundamental to HCC development,many studies have investigated the therapeutic potential of approaches targeting BAs and/or the gut microbiota.In this review, we summarize recent research on the interaction between BAs and the gut microbiota in HCC.Therapeutic strategies aimed at modulating BA metabolism and microbial profiles for HCC prevention and treatment are also discussed.
BA metabolism and interaction with the gut microbiota
BA synthesis
The liver plays a crucial role in transforming cholesterol into primary BAs, specifically chenodeoxycholic acid (CDCA)and cholic acid (CA) in humans, and α- and β-muricholic acid (MCA) in rodents.BA synthesis, orchestrated by multiple cytochrome P450 (CYP) enzymes, begins with cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme converting cholesterol to 7α-hydrocholesterol13.Subsequently, microsomal sterol 12α-hydroxylase (CYP8B1) balances CDCA and CA synthesis14.Concurrently, in various organs, an alternative pathway is initiated by sterol 27α-hydroxylase (CYP27A1),which converts cholesterol to 27-hydrocholesterol, thereby diversifying the pool of BA precursors15.
After synthesis, primary BAs undergo conjugation with taurine or glycine, thus increasing their water solubility, and are then transported to the bile ductviabile salt export pump(BSEP) and multidrug resistance-associated protein 2 (MRP2).These conjugated BAs are secreted into the duodenum, where they participate in the digestion of dietary fats and fat-soluble vitamins.As much as 95% of conjugated BAs is reabsorbed by enterocytes through the apical sodium-dependent bile acid transporter (ASBT) in the distal ileum, then released into the portal circulation through basolateral BA transporters, including organic solute transporter α/β (OSTα/β) and multidrug resistance protein 3 (MRP3).These reabsorbed BAs are then transferred into hepatocytes through transporters such as Na+-taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptide 1 (OATP1)16, metabolized,and eventually released into bile juiceviaBSEP and MRP217.In general, BAs undergo approximately 10 enterohepatic cycles per day, and this cycle is substantially influenced by a variety of BA receptors and the gut microbiota.BA synthesis and transformation by gut microbiota are summarized in Figure 1.
BA receptors
BA receptors are necessary for regulating BA metabolism and homeostasis.The main BA receptors include farnesoid X receptor (FXR), Takeda G-protein-coupled receptor 5 (TGR5),pregnane X receptor (PXR), constitutive androstane receptor (CAR), and vitamin D receptor (VDR).These receptors together closely monitor a diverse set of signaling pathways that control BA synthesis, transport, and metabolism.
FXR is a nuclear receptor that acts as a transcription factor and initiates the expression of a diverse set of target genes.In the small intestine, FXR activation leads to the upregulation of fibroblast growth factor (FGF)-15/19, which is subsequently translocated into the liver and interacts with the FGF receptor 4 (FGFR4)/β-klotho complex on hepatocytes.This complex then inactivates CYP7A1, thus decreasing BA production.Hepatocytic FXR also upregulates BSEP, thereby enhancing BA efflux into the biliary canaliculi while suppressing the BA importer NTCP, and decreasing BA reabsorption from the blood into hepatocytes18,19.
TGR5, also known as G-protein-coupled bile acid receptor(GPBAR1), is expressed in various tissues and serves as a metabolic regulator of BA homeostasis20.TGR5 is the receptor of primary BAs (e.g., CDCA and CA) and microbiota- derived secondary BAs [e.g., lithocholic acid (LCA), deoxycholic acid (DCA), and their conjugated forms]; the later are more potent activators of TGR521.In contrast, TGR5 is critical in BA metabolism.In mice withTgr5deficiency, the total BA amount is significantly lower, by 21%–25%, than that in wildtype mice22and is accompanied by alterations in BA composition including decreased taurine-conjugated muricholic acid and increased taurocholic acid (TCA) and taurodeoxycholic acid (TDCA)23.In general, TGR5 activated by BAs stimulates various signaling pathways, such as the NF-κB, AKT, and ERK pathways, and has potential implications in liver inflammation, hepatocyte proliferation, and other pathological process.
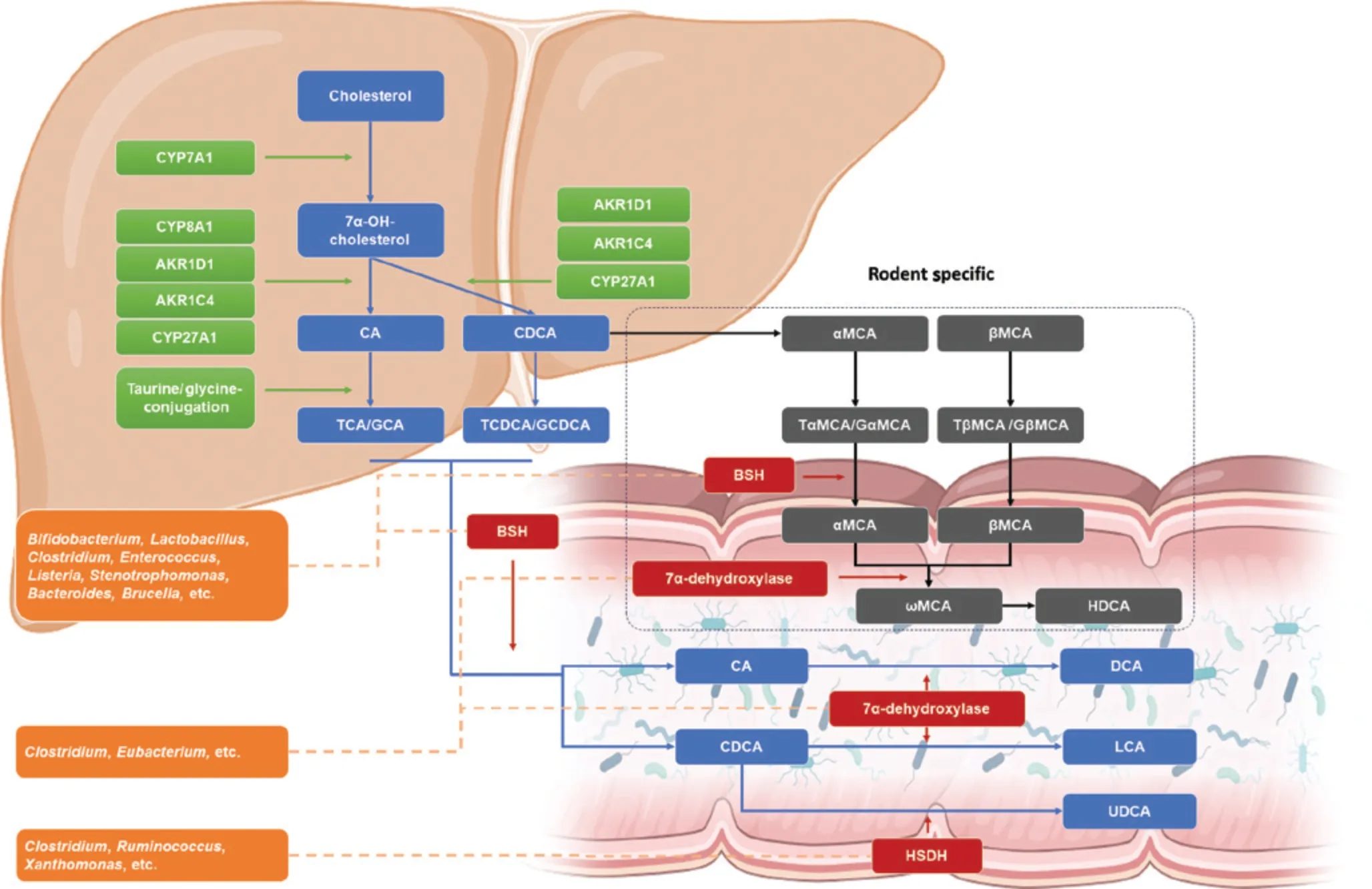
Figure 1 Bile acid synthesis and microbial transformation.In hepatocytes, cholesterol undergoes conversion to 7α-OH-cholesterol by CYP7A1 or alternatively CYP27A1 (not depicted in this figure), thus catalyzing further synthesis.In the presence or absence of CYP8A1,primary bile acids (BAs), cholic acid (CA), or chenodeoxycholic acid (CDCA) is synthesized.In rodents, CDCA is further transformed into αMCA and βMCA.Primary BAs are conjugated with taurine/glycine, thus enhancing hydrophilicity, before being secreted with bile.In the intestine, secondary BAs are converted from primary BA by gut microorganisms.Bacteria with bile salt hydrolase (BSH) activity, including Bifidobacterium, Lactobacillus, Clostridium, and Enterococcus, deconjugate taurine/glycine-conjugated primary BAs.Bacteria including Clostridium and Eubacterium, expressing 7α-dehydroxylase, convert CA to DCA and CDCA to LCA.Another enzyme, HSDH, expressed by bacteria including Clostridium, Ruminococcus, and Xanthomonas, transforms CDCA to UDCA.In rodents, αMCA and βMCA are converted to HDCA.BA, bile acids; BSH, bile salt hydrolase; CA, cholic acid; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7α-hydroxylase; CYP8A1,cholesterol 12α-hydroxylase; CYP27A1, cholesterol 27α-hydroxylase; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GβMCA, glycobetamuricholic acid; GαMCA, glycoalphamuricholic acid; HSDH, hydroxysteroid dehydrogenase; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TβMCA, taurobetamuricholic acid; TαMCA,tauroalphamuricholic acid; UDCA, ursodeoxycholic acid; αMCA, alphamuricholic acid; βMCA, betamuricholic acid; ωMCA, omegamuricholic acid.Figure generated with BioRender.com.
PXR and CAR are crucial nuclear receptors with major roles in regulating drug-metabolizing enzymes and transporters responsible for the metabolism and elimination of xenobiotics24.These receptors are expressed predominantly in the liver but also have high intestinal expression.PXR is activated by various BAs, whereas CAR is considered an indirect sensor of BAs25,26.Activation of PXR and CAR stimulates BA detoxification, thus potentially counteracting cholestasis27.Indeed,numerous studies have indicated the protective effects of PXR and CAR against BA toxicity.For example, a preclinical study has reported that PXR inhibits the pro-inflammatory response in hepatocytesin vitro, as evidenced by inactivation of the NF-κB signaling pathway28.In mice administered LCA or subjected to bile duct ligation, PXR protects hepatocytes from injury by promoting the expression of enzymes involved in BA metabolism and transport29.PXR also exhibits antiinflammatory and anti-fibrosis effects in the intestines30.Meanwhile, CAR, through a different protective mechanism,facilitates BA detoxification in the hepatocytes of CA-treated mice withFxrandPxrdeficiencies31,32.CAR also functions locally in intestinal CD4+effector T cells, by contributing to BA detoxification and inflammation resolution by upregulating the anti-inflammatory cytokine interleukin (IL)-10 and BA-detoxifying enzymes and transporters25.
VDR is a nuclear receptor originally known for its binding to calcitriol, the active form of vitamin D.Recent studies have revealed that VDR is also activated by BAs as well as several LCA-associated synthetic compounds, including LCA-acetate and LCA-propionate33.In the liver, VDR downregulates the small heterodimer partner (SHP), and consequently increases CYP7A1 and BA synthesis34.In a preclinical study, significantly diminished levels of total BAs and CDCA have been observed in mice withVdrdeficiency, even after CDCA supplementation,thus indicating that VDR is essential for CDCA metabolism35.VDR also influences the expression of transporters involved in BA uptake and efflux, such as OSTα/β, MRP3, MRP2, and BSEP36,37.Notably, VDR interacts with other nuclear receptors including FXR and PXR, and consequently mediates BA metabolism.This crosstalk enables coordinated regulation of BA homeostasis.
BA metabolism is affected by the gut microbiota
In addition to host receptors, the gut microbiota plays a crucial role in BA metabolism, particularly in the synthesis of secondary BAs.These gut microorganisms inhabiting the gut express diverse enzymes that facilitate the conversion of primary BAs into secondary BAs.This transformation encompasses a series of intricate enzymatic reactions, including deconjugation,dehydroxylation, oxidation, epimerization, desulfurization,and reconjugation38.
Deconjugation is the initial step in BA modification, wherein the enzyme bacterial bile salt hydrolase (BSH) hydrolyzes the taurine and glycine conjugates of primary BAs.BSH activity has been observed in a wide range of bacterial genera, includingBifidobacterium,Lactobacillus,Clostridium,Enterococcus,Listeria,Stenotrophomonas,Bacteroides, andBrucella39-45.Once deconjugated, BAs become available for other microorganism-mediated biotransformation.Dehydroxylation, a crucial microbial transformation, involves the conversion of primary BAs to secondary BAs through the removal of a hydroxyl group.This process is performed primarily byLachnospiraceaeandPeptostreptococcaceaebacteria, which carry the enzyme 7α-dehydroxylase46-49.Of note, the product of dehydroxylation depends on the primary BA.For instance, 7α-dehydroxylation of CDCA results in LCA formation, whereas 7α-dehydroxylation of CA produces DCA48,50.Moreover, the oxidation and epimerization of BAs are essential for decreasing the toxicity and increasing the hydrophilicity of BAs.Bacterial hydroxysteroid dehydrogenase (HSDH) is an enzyme catalyzing the oxidation of primary BAs to ketonic BAs.These ketonic BAs then undergo epimerization and further generate of β-hydroxy BAs, such as isodeoxycholic acid (IDCA) and isolithocholic acid (ILCA)51.HSDH is expressed predominantly in the bacterial phyla Actinobacteria, Proteobacteria, and Firmicutes52,53.Several studies have suggested that this microorganismmediated process helps the gut microbiota maintain resistance against the competitive and hostile intestinal environment54.Some bacteria leveraging this characteristic modulate the composition of the BA pool, thus potentially enabling clinical applications.For instance, CDCA can be transformed into ursodeoxycholic acid (UDCA) through isomerization of its C-7 hydroxyl group55.This conversion is facilitated by 2 crucial enzymes: 7α-HSDH and 7β-HSDH.These enzymes have been identified in various organisms such asClostridium,Ruminococcus, andXanthomonas.56,57UDCA has been extensively studied for its health benefits and clinically applied in the treatment of cholestatic liver diseases58-60.Additionally,several bacteria express BA sulfatase enzymes and are involved in BA desulfurization.These enzymes remove sulfate groups from sulfated BAs, thus forming desulfated BAs, which are more easily reabsorbed by the intestine than their sulfated counterparts61,62.Finally, gut microorganisms can reconjugate unconjugated BAs with phenylalanine, tyrosine, and leucine, in a process that appears to be common in the human gut microbiota63.Microbial modification of BA was listed in Table 1.
Recent advances in microbial profiling, such as high-throughput sequencing (e.g., 16S rRNA gene sequencing and metagenomics), and enhanced bioinformatic tools, have spurred an increase in high-quality studies revealing the influence of distinct gut microorganisms on BA metabolism.In a cohort of patients with NAFLD, numerous microorganism-derived BAs, particularly DCA, have been found to increase in tandem with disease activity and fibrosis progression.This increase is concomitant with the enrichment ofBacteroidetesandLachnospiraceae, which bear genes responsible for DCA generation.In contrast, microbial populations susceptible to DCA are depleted, includingRuminococcus,Prevotella,Lactobacillus,andTuricibacter64.Another pilot study has shown that disulfiram ameliorates NASH by decreasingClostridium-mediated 7α-dehydroxylation activity, thus suppressing secondary BA biosynthesis and consequently activating hepatic FXR signaling65.Animal studies have provided further detail regarding the effects of gut microorganisms on BA metabolism.For instance, microorganism-derived BAs, such as HDCA, alleviate NAFLD in mice and concurrently enrich probiotic species such asParabacteroides distasonis66.In a separate study,P.distasonishas been found to produce various BAs, including LCA, DCA, ILCA, and 3-oxolithocholic acid, and to alleviate inflammatory responses by inhibiting Th17 cell differentiation and promoting M2 polarization of macrophages39.Additionally, in mice with liver injury, gut microbial dysbiosis,characterized by the depletion of bacteria with BSH activity,including Bacteroidetes, has been found to inhibit intestinal FXR/FGF-15 signaling, thus ultimately facilitating liver injury development67.These findings collectively highlight the intricate interplay between the gut microbial composition and BA metabolism, and have elucidated their major roles in various hepatic conditions.
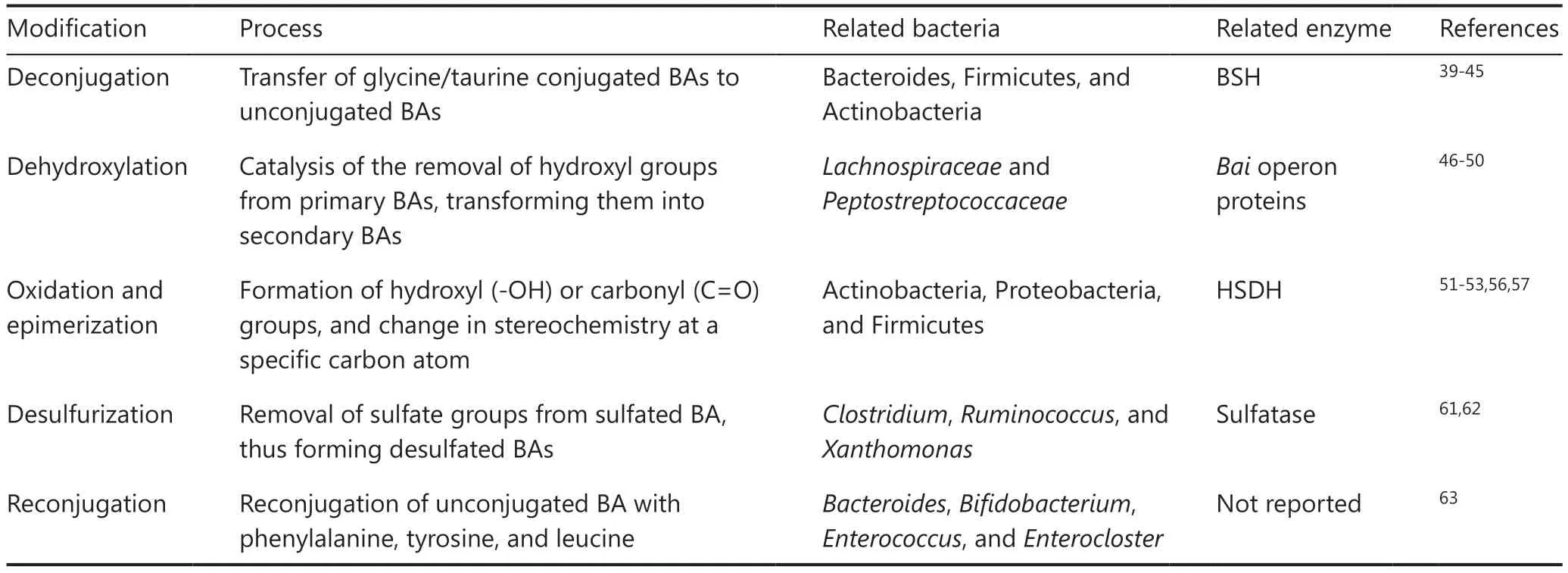
Table 1 Microbial modification of bile acids
BA metabolism and its influence on the composition of gut microbiota
Although BAs have been reported to have both positive and negative effects on the gut microbiota, BAs generally decrease the integrity of bacterial cell membranes, and increase permeability and cell death68.The extent of membrane damage depends largely on BA hydrophobicity: higher hydrophobicity is associated with greater detrimental effects on the bacterial membrane69.This finding explains why unconjugated BAs often have stronger antibacterial properties than their conjugated counterparts: conjugation markedly decreases lipophilicity68.Notably, BAs also exhibit antimicrobial activity through inducing DNA damage, oxidative and pH stress, and chelation of cellular ions such as calcium.BAs also indirectly exert antimicrobial activity by activating the inhibitory FXR pathway, and subsequently inducing inducible nitric oxide synthase (iNOS) and IL-18, thereby facilitating the maintenance of the integrity of the intestinal barrier70,71.
Interestingly, different bacteria exhibit distinct sensitivity to BAs.For example, bacteria expressing BA-metabolizing enzymes tend to have high resistance to the antimicrobial activity of BAs.Gram-positive bacteria appear to be more sensitive to BAs than gram-negative bacteria.However, specific gram-positive bacteria, includingBifidobacterium,Sporolactobacillus,Lactobacillus, andBacillus, are susceptible to BA-induced toxicity72,73.These findings highlight the intricate interactions between BAs and the gut microbiota, and may have promise in clinical applications.
BA-gut microbiota interactions in HCC
In the landscape of HCC, emerging research has examined the complex network of dysregulated gut microbiota and BAs, and revealed their effects on HCC development.These insights may illuminate potential avenues for innovative diagnostic,therapeutic, and preventive strategies for HCC.
Dysregulated BAs contribute to HCC development
As early as 1940, DCA, a critical secondary BA generated through microorganism-mediated 7α-dehydroxylation, was found to exhibit oncogenic potential74, particularly in HCC associated with obesity75, thus providing initial evidence linking BAs to HCC.Further support has been provided by another study showing that DCA administration stimulates hepatic stellate cell (HSC) senescence, and causes these cells to exhibit biological behaviors and phenotypes of liver malignancy76.On the basis of these insights, DCA is being explored as a potential therapeutic target for HCC.For instance, microbial products such as butyrate might reverse dysregulated BA profiles, including decreased DCA levels in hepatitis and HCC77.Another study has further elucidated this association by revealing a positive correlation between elevated DCA levels and HCC, alongside an abundance of gut bacteria with high BSH activity.Subsequent mechanistic investigations have highlighted the anti-tumor potential of a conjugated DCA form (glycodeoxycholic acid, GDCA), whereby gut bacteria rich in BSH activity deconjugate GDCA to DCA, thus promoting HCC development78.
Beyond DCA, other BAs including CDCA derivatives,such as LCA and UDCA, have regulatory roles in HCC79.LCA contributes to HCC and cholangiocarcinoma development by dysregulating MAFG in hepatocytes, disrupting lipid homeostasis, and subsequently promoting cholestasis injury80,81.In contrast, UDCA has emerged as a promising therapeutic agent for various chronic liver diseases, and its efficacy in the context of HCC is increasingly being recognized.Clinical observations suggest a potential inverse correlation between UDCA use and the incidence of HCC in HCV-associated liver cirrhosis82.Pre-clinical experiments have investigated several mechanisms underlying UDCA’s anti-HCC effects, including promotion of apoptosis83, facilitation of autophagy82, and inhibition of angiogenesis84.Glycoursodeoxycholic acid (GUDCA), a microbial derivative of UDCA, has potent anti-tumor properties.Diminished levels ofBacteroides fragilishave been found to lead to increased GUDCA levels, thereby alleviating HCC by activating the FXR/RXR pathway85.
Aberrant BA metabolism profoundly influences the tumor immune microenvironment (TIME).BAs involved in inflammatory regulation are associated with carcinogenesis through essential signaling pathways, including the NFκB, COX-2,and STAT3 pathways, and inflammatory factors, such as IL-6,IL-1β, and TNF-α86.This altered metabolism hampers the function of CD8+ T cells, avoids the recruitment of natural killer T (NKT) cells, and amplifies the polarization of M2-like tumor-associated macrophages, thereby fostering tumor immune escape and contributing to HCC development.Studies have indicated that gut microorganism-mediated conversion of primary-to-secondary BA regulates CXCL16 expression in liver sinusoidal endothelial cells, thus controlling NKT cell accumulation and mediating liver-selective tumor inhibition87.BAs also function as signaling mediators by stimulating nuclear receptors and promoting the polarization of M2-like macrophages, thereby creating an immunosuppressive TIME favoring the growth of tumor-initiating cells88.Moreover, the overproduction of DCA by specificClostridiumspecies has been found to facilitate the proliferation of regulatory T cells while inhibiting the accumulation of CD103+ dendritic cells.This mechanism may potentially compromise the anti-tumor function of CD8+ T cells and ultimately accelerate the progression of liver cancer89.
Regulation of HCC by BA receptors
Key BA receptors such as FXR and TGR5 govern the interplay between BAs and the gut microbiota.Mice lackingFxrexpression exhibit elevated serum and hepatic BAs, and therefore are susceptible to spontaneous hepatocarcinogenesis90.LiverspecificFxrknockout mice show changes in the expression of the tumor suppressor p53 and cell cycle regulator cyclin D1;however, these effects are reversed by CA supplementation,which disrupts signaling pathways involved in HCC progression91.Aberrant BA metabolism has been implicated in modulating the TIME, potentially through the elevation of TGR5 methylation, thereby facilitating tumor immune evasion and fostering HCC development92.
Beyond FXR and TGR5, other receptors, such as PXR, CAR,and VDR, participate in HCC regulation.High PXR levels in clinical specimens have been associated with poor prognosis in sorafenib-treated patients with HCC.In vitroPXR overexpression has been found to facilitate HCC cell persistence through sorafenib treatment93-95.The role of CAR in HCC is controversial, on the basis of human and rodent studies.In rodents, phenobarbital, a CAR activator, has been found to support tumor formation in the liver96,97.However, different studies have found inconsistent effects of CAR in human HCC cell lines98,99.VDR polymorphisms and methylation are associated with susceptibility to HCC99,100.VDR has a protective role in HCC development, possibly through the regulation of liver inflammation and fibrosis101.This intricate network of BA receptors regulates BA metabolism and homeostasis, and influences HCC progression.These receptors, known for their roles in normal BA physiology, are increasingly recognized for their effects on pathological processes in HCC, including liver inflammation, hepatocyte proliferation, and other factors contributing to HCC progression.Table 2 summarizes the roles of the natural ligands and the potential functions of these receptors in HCC.
Gut microbiota dysbiosis and HCC
The connection between gut microbiota and liver functions enables the microbial community to directly influence hepatic processes, thereby profoundly influencing the course of HCC.The underlying mechanisms linking the gut microbiota and HCC involve a complex interplay among a leaky gut barrier,microbial metabolites, host signaling pathways, and immune responses.
Imbalances between commensal and pro-inflammatory bacteria in dysbiotic microbiota may trigger an inflammatory response and lead to impaired gut barrier integrity114.Increased intestinal permeability can result in influx of increased bacterial ligands and enterotoxins into the portal vein, and subsequently affect the liver.For example, the microbial products lipopolysaccharides activate immune cellsviathe Toll-like receptor 4 (TLR4) and downstream NF-κB pathway, thus inducing pro-inflammatory cytokines115, and promoting intestinal inflammation and HCC progression116.
Notably, gut microbial dysbiosis alters metabolite profiles.Key gut metabolites associated with HCC include BAs and short-chain fatty acids, the latter of which have been extensively reviewed elsewhere.Although most BAs act locally and are reabsorbed into the liver, a fraction of BAs circulate systemically and function as signaling molecules by activating nuclear receptors, such as FXR and TGR5.The prominent aforementioned roles of FXR and TGR5 in HCC have been extensively documented91,92, thus underscoring the major role of gut microbiota-mediated BA metabolism in the context of HCC.
Gut microbiota dysbiosis, together with altered metabolite profiles and compromised gut barrier integrity, profoundly disrupts immune homeostasis in the liver and consequently fosters carcinogenesis.Clinical studies have revealed augmented numbers of regulatory T cells and diminished numbers of CD3+ and CD8+ T cells in the peripheral blood and tumor specimens of patients with HCC—findings correlated with poorer prognosis117-119.Modulation of the gut microbiotahas potential to reinvigorate anti-tumor responses.As previously described, gut microorganism-mediated conversion of primary-to-secondary BAs regulates NKT cell accumulation87,and activated NKT cells secrete interferon (IFN)-γ and TNF,both of which are essential for the anti-tumor response120.Moreover, individuals with HCC with enrichment in gut probiotics such asBifidobacterium longumandEnterococcus hirae,along with higher levels of reactive CD8+ T cells, have been found to experience prolonged disease-free periods121.These findings thus underscore the substantial effects of the gut microbiota in modulating anti-tumor effects against HCC.

Table 2 Bile acid receptors and their implications in HCC
Impaired interaction between BA metabolism and the gut microbiota in HCC
Given that almost all HCC occurs in the milieu of chronic liver diseases or cirrhosis, and that a dysbiotic gut microbiota is a prominent feature, BA homeostasis may be disrupted122.The roles of various specific primary and secondary BAs, as well as their derivatives shaped by the gut microbiota, may potentially exhibit substantial variability in the context of HCC.To provide a comprehensive overview, we have compiled evidence from human cohort studies and animal models regarding the profiles of BAs and gut microbiome signatures (Table 3).
A noteworthy aspect of BA dysregulation by gut microbiota in HCC is the imbalance between non-toxic hydrophilic BAs and toxic hydrophobic BAs130.Microbial deconjugation/reconjugation activities in the gut influence the hydrophobic or hydrophilic properties of BAs.HCC animal models have shown an intrahepatic increase in hydrophobic BAs and a decrease in hydrophilic BAs128,131.Typically, hydrophobic BAs,primarily unconjugated BAs (some conjugated BAs, such as glycocholic acid are also hydrophobic), are toxic to hepatocytes.These accumulated BAs activate pro- inflammatory signaling pathways, thus inducing liver damage and fibrosis132.Enhancing intestinal excretion of hydrophobic BAs through a diet with 2% cholestyramine has been shown to alleviate HCC progression128.Chronic inflammation and fibrosis caused by BA accumulation in turn favor the establishment of an immunosuppressive microenvironment in the liver.Additionally,dysregulated BAs promote HCC development by fostering immunosuppressive M2-like tumor- associated macrophage infiltration88and diminishing numbers of NKT cells87.These finding underscore the critical roles of gut microorganisms in shaping the hydrophobic or hydrophilic properties of BAs, and controlling HCC development.
HCC is also associated with cholestasis injury, characterized by the abnormal production or excretion of bile, thus leading to changes in intestinal BA composition and further exacerbation of the dysbiotic gut microbiota.People with cholestasis often exhibit gastrointestinal symptoms and intestinal bacterial overgrowth133.Primary biliary cholangitis(PBC), a classic cholestatic disease, is characterized by diminished microbial diversity.Specifically, certain genera involved in BA transformation, such asFaecalibacterium,Bacteroides,Clostridium,Lactobacillus, andStreptococcus, exhibit alterations in individuals with PBC.However, these alterations are reversed with UDCA therapy134.Other cholestatic diseases,including primary sclerosing cholangitis135, intrahepatic cholestasis of pregnancy136, and parenteral nutrition-associated cholestasis137, have been associated with gut microbiota dysbiosis.These results suggest that BA alterations may lead to increased colonization and survival of gut pathobionts, and that therapeutic approaches targeting the gut microbiota may consequently be effective against disorders associated with dysregulated BA metabolism in HCC.
Therapeutic strategies targeting BAs and the gut microbiota against HCC
The intricate relationship between BAs and the gut microbiota has opened new avenues for therapeutic interventions against HCC.Numerous studies have suggested that targeting BAs and microorganisms may feasibly prevent HCC development,inhibit tumor progression, and improve patient outcomes.
Therapeutic strategies targeting BAs
Given their complexity, the therapeutic potential of many agonists and antagonists in BA metabolism has been investigated,and some have shown great promise in treating various liver diseases, including NASH, PBC, and HCC.
The use of UDCA potentially correlates with diminished incidence of HCC in individuals with hepatitis C virus-associated liver cirrhosis.Notably, the 5-year HCC incidence in individuals receiving UDCA is 17.9%, a percentage significantly lower than the incidence of 39.1% in individuals not receiving UDCA138.Obeticholic acid (OCA), a synthetic BA analogue, potently activates FXR139.Experimental models of HCC have demonstrated the anti-tumor effects of OCA140,141.Currently, clinical data regarding OCA in HCC are lacking;however, multiple clinical trials have hinted at its therapeutic effectiveness in treating chronic liver diseases, such as NASH.For instance, in a phase 3 clinical trial, histological fibrosis was ameliorated in 12% of patients in the placebo group compared with 23% in the OCA 25 mg group (P= 0.0002)142.Furthermore, another study investigating the long-term effectiveness of OCA in patients with PBC has demonstrated favorable outcomes observed over a 3-year follow-up period.Notably a significant change was observed in ALP concentrations from baseline [105.2 U/L (SD 87.6)] to 3 years [95.6 U/L(SD121.1);P< 0.0001]143.

Table 3 Selected evidence of associations between the gut microbiota and bile acid profile signatures in patients with HCC and HCC mouse models
As previously discussed, FXR is a promising therapeutic target for various liver diseases and potentially HCC.Beyond OCA, several other FXR agonists have been developed for management of liver diseases.However, clinical data supporting the effectiveness of various FXR agonists specifically in the treatment of HCC are currently scarce, although these agonists have shown promising potential in managing diverse liver conditions.For instance, in studies focused on NASH, MET409, a synthetic FXR agonist, has shown considerable effects by decreasing liver fat content over a 12-week period in patients with NASH.That study reported mean relative decreases of 55% (80 mg) and 38% (50 mg), as compared with a 6% decrease in the placebo group (P< 0.001)144.Furthermore, other FXR agonists such as nonsteroidal agonists, vonafexor, and tropifexor have shown effects against liver diseases in phase 2 clinical trials145-147.These findings suggest that FXR agonists may have roles in maintaining liver homeostasis and may potentially serve as alternative therapeutic targets for treating HCC.
Analogs of FGF19, downstream of BA-induced FXR activation, are also being extensively investigated for their therapeutic potential against NASH148,149and cholestatic liver diseases150.In animal models of HCC, FGF19 analogs have shown anti- fibrosis and anti-tumor effects by suppressing hepatic BA synthesis151.Currently, several ongoing phase 1/2 trials are studying the efficacy of targeting FGF19 in patients with HCC.In particular, FGF401, a potent and selective FGF19-FGFR4 signaling inhibitor, has shown promising results: either FGF401 monotherapy or combined therapy with spartalizumab has shown safety in patients with FGFR4/KLB-positive tumors, including HCC.Preliminary clinical efficacy has also been observed152.
In contrast, BA pathway antagonists, particularly inhibitors of BA transporters (e.g., ASBT), have also shown therapeutic potential in preclinical studies.ASBT inhibition increases colonic BA accumulation and decreases the BA pool, thus potentially alleviating liver pathogenesis in cholestatic liver disease and NASH animal models153.Similarly, another study has demonstrated the ability of ASBT inhibitors, such as SC-435 and A4250, to modulate BA metabolism and ameliorate liver histology in mice with sclerosing cholangitis154,155.In humans, the ASBT inhibitor odevixibat has shown promising results in alleviating pruritus symptoms and decreasing serum BA in a phase 3 trial of cholestatic liver diseases156.In contrast,the use of an ASBT inhibitor SHP626 (volixibat) has shown limited therapeutic benefits in patients with NASH in a phase 2 trial157.
Therapeutic strategies targeting microorganism-mediated BA metabolism
Probiotics,prebiotics,and diet
Probiotics are live microorganisms that offer a wide range of health benefits after being consumed.For instance, a mouse study has demonstrated the prophylactic effect ofLactobacillus rhamnosus GGagainst liver fibrosis by inhibiting hepatic BA synthesis and enhancing BA excretion122.Lactobacillus eosinophilhas potential in alleviating mouse NAFLD through modulating the microorganism-mediated FXR/FGF15 signaling pathway158.Similarly,Lactobacillus brevishas been found to alleviate HCC progression in miceviainfluencing the interplay among the gut microbiota, BAs, and NOTCH1 signaling127.Given that probiotics modulate BA metabolism, several clinical trials have investigated the potential of using probiotics, particularly variousLactobacillusstrains, in the treatment of liver diseases.For instance, the effects of a mixture containing 3Lactobacillus plantarumstrains on the BA profile, plasma lipids, and other associated biomarkers is being evaluated through a dose-dependent regimen in a cohort of overweight participants (NCT05378230).
Beyond probiotics, prebiotics—substrates that stimulate the growth and activity of for beneficial gut bacteria—also confer health benefits in humans.In general, prebiotics are more advantageous in managing metabolic conditions than probiotics and specific BA agonists or antagonists, because they stimulate BA production and activate associated receptors in a more natural, controlled manner159.These characteristics makes prebiotics preferable candidates for alleviating metabolic conditions without inducing serious adverse effects.In addition, emerging research highlights the influence of diet on cancer development160-162.Personalized dietary interventions tailored to the gut microbiota in each individual could therefore be feasible for preventing chronic liver diseases and HCC.By optimizing the diet to promote the growth of beneficial commensal organisms and modulate BA metabolism, personalized dietary interventions may restore intestinal homeostasis and support liver health163.A compelling example is Pu-erh tea, which has been shown to decrease BSH enriched bacteria and BSH activity.This modulation of BA metabolism, characterized by the accumulation of conjugated BAs in the ileum,attenuates hypercholesterolemia and might have potential as a dietary intervention influencing HCC risk164.
Fecal microbiota transplantation(FMT)
FMT refers to the transfer of fecal material from a healthy donor to a recipient to restore a balanced gut microbiota165.FMT is currently clinically approved for treatingClostridium difficileinfection166, and has shown promising potential against various intestinal and extra-intestinal diseases167,168.FMT modulates both the BA profile and the gut microbiota.In a recent clinical trial, researchers investigating the effects of oral capsule FMT have observed enhanced microorganism-mediated BA metabolism: specifically, the study reported significant decreases in stool TCA levels in patients receiving FMT with respect to their baselines.Moreover, the BA profiles of FMT recipients began to resemble those of the donors169.Another clinical trial in obese patients has revealed that, after FMT,Bacteroides ovatusandPhocaeicola doreiare positively correlated with unconjugated BAs, whereasBifidobacterium adolescentis,Collinsella aerofaciens, andFaecalibacterium prausnitziiare positively correlated with secondary BAs170.These promising results have led to further ongoing clinical trials aimed at exploring the application of FMT in HCC.For instance, NCT05750030 is a phase IIa pilot study testing the safety and efficacy of combining FMT with atezolizumab plus bevacizumab in patients who previously did not respond to immunotherapy for advanced HCC.Similarly, NCT05690048 is investigating whether FMT might overcome resistance to atezolizumab/bevacizumab in the context of HCC.Despite limited evidence regarding its specific efficacy in HCC, FMT has shown potential in enhancing liver homeostasis and influencing the hepatic immune microenvironment.However,further comprehensive studies are warranted to ascertain its effectiveness and safety specifically in the treatment of HCC.
Engineered bacteria
Advances in genetic engineering have opened exciting possibilities to create specialized bacteria with therapeutic properties.Bacteria such asE.coliandLactococcus lactishave been used as vehicles for delivering therapeutic recombinant proteins,yet their abilities to persist and survive in the human gut are limited.Engineered bacterial strains are thus required to serve as more effective tools.For example, a study has successfully modified a strain ofClostridium sporogenesto heterologously express genes fromClostridium scindensresponsible for BA conversion; this modification enabled the recombinant bacteria to synthesize DCA and LCA48.Hence, developing engineered bacteria with enhanced ability to modulate BA metabolism and the gut microbiota may be feasible to treat liver diseases including HCC.
Immunotherapy
Immunotherapy has pioneered a new treatment paradigm in HCC, setting a novel therapeutic benchmark.In particular, the FDA approval of the combination of atezolizumab (anti-PD1) and bevacizumab (anti-VEGF), the first-line treatment for advanced HCC, marked a major milestone171.Studies increasingly indicate that the interplay between the gut microbiota and the liver influences the tumor microenvironment in HCC, thus resulting in varied responses to immunotherapy172,173.Notably, differences in gut microbiota composition have been observed between patients with HCC who are responders and non-responders to immunotherapy174, thus suggesting a potential role of microorganisms as prognostic biomarkers for immunotherapy efficacy175.Furthermore, several bacterial strains stimulate anti-tumor responses to immune checkpoint inhibitors176-180.Moreover, BA profiles can be used to distinguish responders and non- responders to immunotherapy in HCC.In responders, elevated levels of secondary BAs, including UDCA, TUDCA, UCA, and MDCA, have been found to be accompanied by increases inLachnoclostridium,Lachnospiraceae, andVeillonella, and a decrease inPrevotella 9124.Gut microorganisms associated with both TIME and BA metabolism have shown good performance in discriminating 5-year survival (AUC 81%)181.These findings thus underscore the potential of research on liver cancer microbiota in immunotherapy to identify novel therapeutic approaches for managing HCC.
In summary, the diverse therapeutic strategies addressing the intricate interplay between dysregulated gut microbiota and the BA axis have promise in combating HCC.Animal experiments have demonstrated the potential therapeutic efficacy of various microbiota-targeting strategies, such as probiotics, prebiotics, dietary intervention, and FMT in managing HCC.Preclinical studies onLactobacillusstrains have indicated their ability to inhibit liver fibrosis and slow HCC progression.Prebiotics and dietary interventions, which are preferred because of their controlled and natural effects on BA metabolism, have promise in managing liver diseases without causing significant adverse effects.Although combining FMT with immunotherapy is safer and may improve efficacy beyond that of monotherapy in various cancer types182, its efficacy and safety in HCC treatment require further validation in human clinical trials.Figure 2 provides a visual representation of the effects of dysregulated gut microbiota on the BA axis and their contributions to HCC, along with potential therapeutic interventions.Further research and clinical trials are crucial to fully realize the potential of these strategies in the treatment and prevention of HCC.
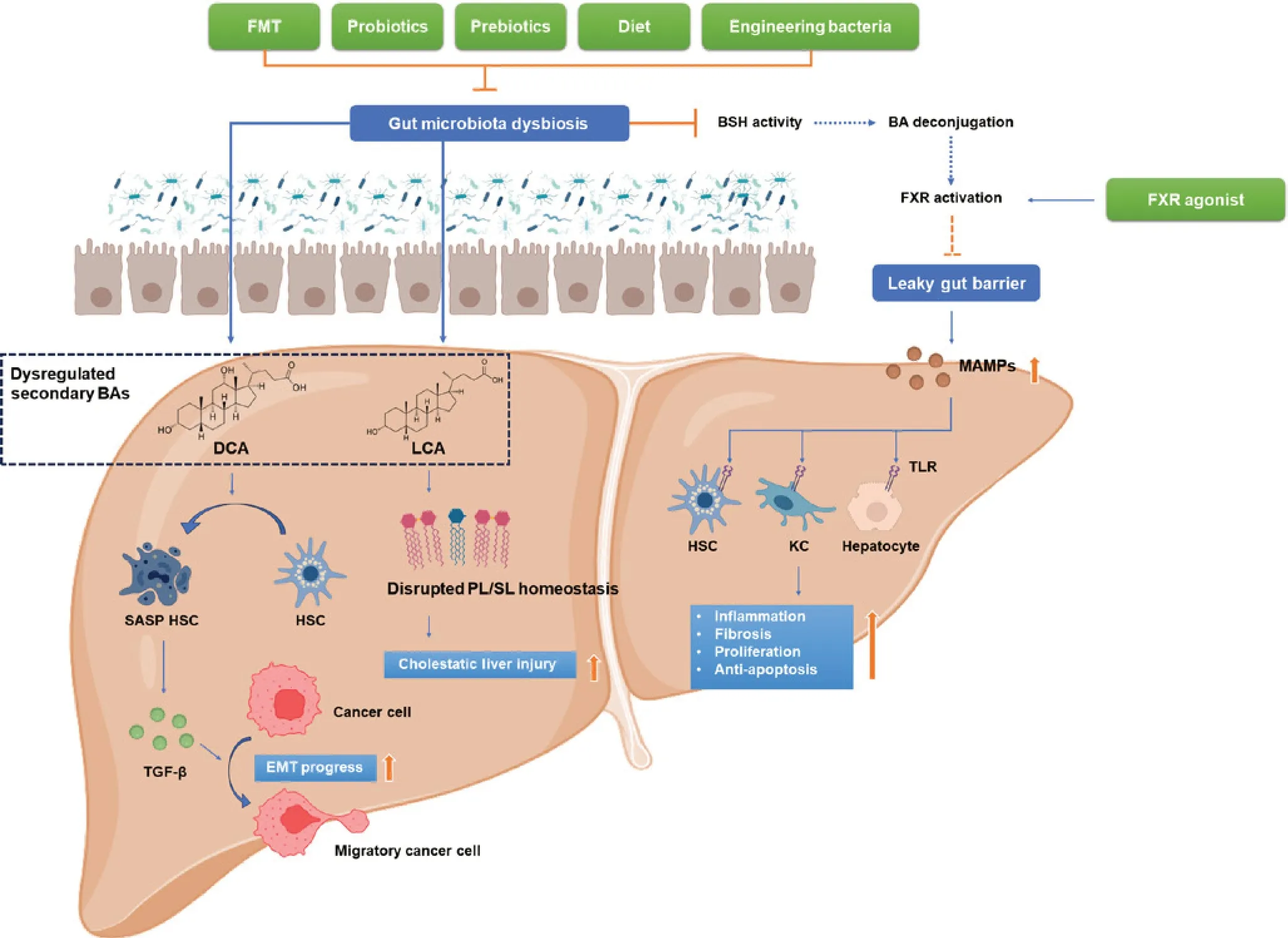
Figure 2 Selected evidence of dysregulation of the gut microbiota-bile acid axis contributes to hepatocellular carcinoma.Dysbiosis of the gut microbiota in HCC contributes to the dysregulation of secondary BA production.DCA induces SASP within quiescent HSCs, thus increasing secretion of cytokines including TGF-β, promoting EMT in cancer cells, and enhancing metastasis.LCA disrupts phospholipid/sphingolipid homeostasis and exacerbates cholestatic liver injury.Dysbiosis also impairs BSH activity, decreases primary BA deconjugation and FXR activation, and results in a compromised gut barrier.With impaired gut barrier function, MAMPs translocate to the liver via the portal vein and activate TLR on HSCs, KCs, and hepatocytes.This cascade intensifies liver inflammation, fibrosis, cell proliferation, and anti-apoptosis, thus facilitating hepatocarcinogenesis.Therapies, including FMT, probiotics, prebiotics, dietary intervention, and engineered bacteria, may alleviate gut microbiota dysbiosis as adjunctive treatments for HCC.Additionally, FXR agonists may improve gut barrier function, thereby decreasing MAMP toxicity and exerting anti-HCC effects.BAs, bile acids; BSH, bile salt hydrolase; DCA, deoxycholic acid; FMT, fecal microbiota transplantation; FXR, farnesoid X receptor; HSC, hepatic stellate cells; KC, Kupffer cells; LCA, lithocholic acid; MAMP, microorganism-associated molecular pattern; PL, phospholipids; SASP, senescence-associated secretory phenotype; SL, sphingolipids.Figure generated with BioRender.com.
Conclusions and future perspectives
The intricate interplay among BAs, gut microbiota, and HCC has illuminated a novel pathway for understanding the complex pathogenesis of this malignancy and exploring innovative therapeutic approaches.The effects of the gut microbiota on BA metabolism, and vice versa, requires deeper exploration of how microbial dysbiosis contributes to HCC initiation and progression.
With the emergence of new analytical tools and strategies,including metagenomics and metabolomics, insights are rapidly being gained into the metabolic interactions between the gut and liver, as well as the signaling pathways that regulate HCC development.The modulation of the gut microbiota and BA profiles is a novel approach to treating HCC.This strategy has immense promise and clearly defines the next frontier in HCC research.Research efforts should focus on: 1) elucidating the crosstalk among the gut microbiota, BAs, and host immune responses in HCC, particularly how microbial dysbiosis and BA dysregulation contribute to chronic inflammation and immune evasion, which are hallmarks of cancer progression; 2) investigating the role of the gut microbiota and BA in modulating the efficacy and adverse effects of existing HCC treatments, such as chemotherapy, immunotherapy, and targeted therapy; 3) exploring the potential of using engineered probiotics to target and modulate BA pathways for HCC prevention and treatment; and 4) understanding the effects of lifestyle factors, such as diet and exercise, on the liver-BA-gut microbiota axis and its relevance to HCC risk and progression.
Several limitations warrant consideration.The translation of preclinical findings into effective clinical therapies presents a substantial challenge.Clinical trials are required to validate the efficacy, safety, and long-term effects of therapeutic strategies targeting BAs and the gut microbiota in HCC prevention and treatment.Furthermore, the microbiome is highly individualized, and is influenced by genetics, diet, environment,and other factors.Therefore, the development of personalized interventions based on an individual’s unique microbiome composition and BA metabolism would require careful consideration and validation.Moreover, the liver-BA-gut microbiota axis is a dynamic entity with intricately intertwined individual components.To effectively intervene in HCC and achieve meaningful therapeutic outcomes, comprehensive treatments must address all these interconnected components collectively.Finally, ethical considerations surrounding FMT and engineered bacteria therapies must be carefully addressed.
In conclusion, the relationship between BAs and the gut microbiota in HCC is an exciting and evolving field of research.By targeting these pathways, novel therapeutic approaches may be uncovered to halt or slow HCC progression, and ultimately improve prognosis and quality of life for patients with HCC.Continued exploration of the BA-gut microbiota interaction in HCC is expected to yield valuable insights that will guide the development of innovative therapies in the fight against this deadly disease.
Grant support
This work was supported by Fujian Provincial Natural Science Foundation project (2020J01122587), National Natural Science Foundation of China (82103355, 82102255,and 82222901), RGC Theme-based Research Scheme (T12-703/19-R), and Research grants Council-General Research Fund (14117422 and 14117123).
Conflicts of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Yang Song: conceptualization, literature review, and writing the manuscript.
Harry CH Lau: revision of the manuscript.
Jun Yu and Xiang Zhang: supervision and revision of the manuscript.
杂志排行
Cancer Biology & Medicine的其它文章
- Circulating cell-free mtDNA as a new biomarker for cancer detection and management
- Microbiome changes in esophageal cancer: implications for pathogenesis and prognosis
- Deep insight into the B-cell associated tertiary lymphoid structure and tumor immunotherapy
- First-line immunotherapy for advanced non-small cell lung cancer: current progress and future prospects
- Cancer-educated neutrophils promote lung cancer progression via PARP-1-ALOX5-mediated MMP-9 expression
- Perspective on new cell-free DNA technologies for early cancer detection
