First-line immunotherapy for advanced non-small cell lung cancer: current progress and future prospects
2024-02-29JingyiWangLinWu
Jingyi Wang, Lin Wu
1The Second Department of Thoracic Oncology, Hunan Cancer Hospital/The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410013, China; 2Xiangya Lung Cancer Center, Xiangya Hospital, Central South University, Changsha 410008, China
Global Cancer Statistics 2022 reported the prevalence and high mortality rate of lung cancer.Notably, non-small cell lung cancer (NSCLC) accounts for the majority of the histologic types1.Precision therapy for lung cancer has progressed rapidly and immune checkpoint inhibitors (ICIs) have become a leading research topic.Indeed, ICI therapy has been shown to improve the prognosis of lung cancer patients.ICI monotherapy or combination therapy has now become the first-line standard treatment option for patients with driver gene-negative advanced NSCLC2.Despite the clear progress being made in immunotherapy, many issues still need to be further explored,such as the selection of optimization strategies and the identification of efficacy-related biomarkers.Herein we will summarize the current status of first-line immunotherapy for NSCLC,discuss the research progress with respect to immunotherapy biomarkers, and clarify the challenges and future directions of first-line immunotherapy for NSCLC.
Mechanism of action underlying ICIs
The ICIs that have been studied intensively include programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitors.PD-L1 is a co-regulatory molecule expressed on tumor cells and inhibits T cell-mediated cell death.T-cells express PD-1 (a negative regulator), which binds to ligands, including PD-L1 (CD274) and PD-L2 (CD273).PD-1/PD-L1 is a negatively regulated signaling pathway for T-cell activation.By blocking this pathway PD-1/PD-L1 inhibitors reactivate suppressed T-cells, enhance recognition of tumor antigens and kill tumor cells.CTLA-4 is another co-stimulatory molecule that negatively regulates T-cell activation.CTLA-4 inhibitors effectively block the binding of CTLA-4 to B7 molecules and restore the activity of the co-stimulatory CD28-B7 signaling pathway.Thus, the inhibitory effect on T cell activation is weakened and the infiltration of tumor-specific T cells is increased2,3.
Current status of first-line immunotherapy for advanced NSCLC
Immunotherapy has changed the landscape of first-line treatment for patients with advanced NSCLC.We have summarized immunotherapy regimens approved by the U.S.Food& Drug Administration (FDA) and/or the Chinese National Medical Products Administration (NMPA) for first-line treatment of advanced NSCLC.
ICI monotherapy
Currently, pembrolizumab, atezolizumab, or cemiplimab monotherapy is recommended as first-line treatment for advanced NSCLC with high PD-L1 expression and negative driver genes regardless of histologic type (squamous or non-squamous; Table 1)2.
Evidence for monotherapy with the PD-1 inhibitor, pembrolizumab, comes primarily from the KEYNOTE-024 and KEYNOTE-042 studies.The KEYNOTE-024 study included305 patients with advanced NSCLC and a PD-L1 tumor proportion score (TPS) ≥ 50%.The study showed that pembrolizumab significantly improves the objective response rate[ORR (44.8%vs.27.8%)], prolongs progression-free survival[PFS (median, 10.3vs.6.0 months; HR = 0.50)], and overall survival [OS (median, 26.3vs.13.4 months; HR = 0.62)] compared to chemotherapy4.The KEYNOTE-042 study expanded the enrollment criteria to PD-L1 TPS ≥ 1%, the results of which suggested that pembrolizumab significantly reduces the risk of death compared to chemotherapy.The subgroup analysis, however, suggested that patients with PD-L1 and a TPS ≥ 50% were the primary population to benefit5.
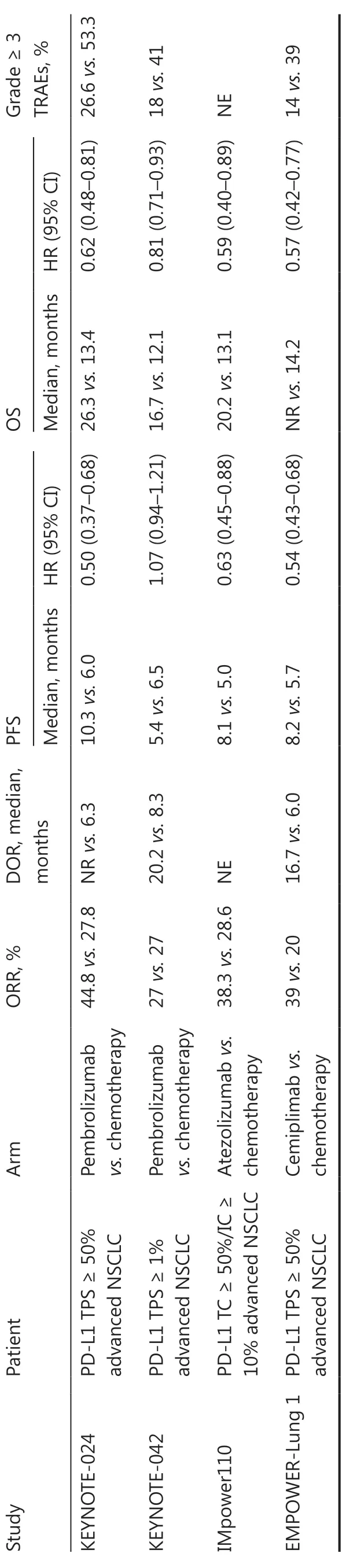
Table 1 Summary of clinical trials of first-line ICIs monotherapy for advanced NSCLC
The IMpower110 study showed that among advanced NSCLC patients with high PD-L1 expression [tumor cell(TC) ≥ 50% or tumor-infiltrating immune cell (IC) ≥ 10%],the PD-L1 inhibitor, atezolizumab, significantly improved the ORR (38.3%vs.28.6%), PFS (median, 8.1vs.5.0 months;HR = 0.63), and OS (median, 20.2vs.13.1 months; HR =0.59)6.In 2020 the U.S.FDA approved atezolizumab for firstline monotherapy in metastatic NSCLC with a PD-L1 TC ≥50% or an IC ≥ 10%.
The PD-1 inhibitor, cemiplimab, was approved by the U.S.FDA for first-line treatment of metastatic NSCLC with a PD-L1 TPS ≥ 50% based on the EMPOWER-Lung 1 study.Specifically, cemiplimab significantly improved the ORR (39%vs.20%) and prolonged the PFS (median, 8.2vs.5.7 months;HR = 0.54) and OS [median, not reached (NR)vs.14.2 months; HR = 0.57] compared to chemotherapy7.
In conclusion, for patients with advanced NSCLC and high PD-L1 expression, ICI monotherapy provides significant clinical benefits and changes the treatment pattern; however, the clinical benefits of immune monotherapy in NSCLC patients with low or no PD-L1 expression are not significant.Therefore,immuno-combination therapy is vital for further expanding the population that will benefit and optimizing the efficacy of immunotherapy.
ICIs combined with chemotherapy
ICIs, in combination with chemotherapy, are the guidelinerecommended first-line standard for driver gene-negative advanced NSCLC independent of the level of PD-L1 expression (Table 2).
The KEYNOTE-189 study enrolled patients with advanced non-squamous NSCLC who were treated with pembrolizumab in combination with pemetrexed and platinum.Compared tothe chemotherapy-only group, the combination therapy group had improved ORR (48.3%vs.19.9%), PFS (median, 9.0vs.4.9 months; HR = 0.49), and OS (median, 22.0vs.10.6 months;HR = 0.56)8.The KEYNOTE-407 study further established the efficacy of pembrolizumab in combination with paclitaxel/nanoparticle albumin-bound (nab)-paclitaxel and carboplatin as first-line therapy in patients with advanced squamous NSCLC9.In combination with chemotherapy, pembrolizumab is recommended as the preferred first-line treatment option for non-squamous and squamous NSCLC2.

Table 2 Summary of clinical trials of first-line ICIs combined with chemotherapy for advanced NSCLC
The IMpower130 study compared atezolizumab with chemotherapy and chemotherapy alone as a first-line treatment option in patients with advanced non-squamous NSCLC.The combination therapy group had significant improvement in PFS (median, 7.0vs.5.5 months; HR = 0.64)and OS (median, 18.6vs.13.9 months; HR = 0.79).Therefore,the U.S.FDA approved atezolizumab in combination with nab-paclitaxel plus carboplatin for first-line treatment of metastatic non-squamous NSCLC10.
The EMPOWER-Lung 3 study evaluated the efficacy of cemiplimab in combination with platinum-doublet chemotherapy in advanced NSCLC as first-line treatment.The study showed that the median PFS and OS were significantly longer in the cemiplimab combination chemotherapy group than the chemotherapy alone group [median (m)PFS, 8.2vs.5.0 months, HR = 0.56; mOS: 21.9vs.13.0 months, HR = 0.71]11.The U.S.FDA approved cemiplimab plus platinum-doublet chemotherapy as a first-line treatment option for patients with advanced NSCLC.
In addition, Chinese self-developed PD-1/PD-L1 inhibitors have achieved remarkable success in clinical studies of ICIs combined with chemotherapy for the first-line treatment of advanced NSCLC.Based on the CameL12and CameL-sq13studies, the NMPA approved the PD-1 inhibitor, camrelizumab, in combination with pemetrexed/paclitaxel and carboplatin for first-line treatment of advanced non-squamous/squamous NSCLC.Camrelizumab combined with chemotherapy significantly improved the ORR (non-squamous, 60.5%vs.38.6%; squamous, 64.8%vs.36.7%) and prolonged the PFS(non-squamous: median, 11.3vs.8.3 months, HR = 0.60;squamous: median, 8.5vs.4.9 months, HR = 0.37) and OS(non-squamous: median, NRvs.20.9 months, HR = 0.73; squamous: median, NRvs.14.5 months, HR = 0.55).Based on the ORIENT-1114and ORIENT-1215studies, the NMPA approved the PD-1 inhibitor, sintilimab, in combination with pemetrexed/paclitaxel and carboplatin for first-line treatment of advanced non-squamous/squamous NSCLC.The sintilimab/chemotherapy combination group significantly prolonged the PFS compared to the chemotherapy group (non-squamous:median, 8.9vs.5.0 months; HR = 0.482; squamous: median, 5.5vs.4.9 months; HR = 0.536).Based on the RATIONALE 30416and RATIONALE 30717studies, the NMPA approved the PD-1 inhibitor, tislelizumab, in combination with pemetrexed/gemcitabine and carboplatin for first-line treatment of advanced non-squamous/squamous NSCLC.Tislelizumab in combination with chemotherapy met the primary study endpoint,i.e., a significant prolongation of the PFS compared to standard chemotherapy alone (non-squamous: median, 9.7vs.7.6 months; HR = 0.645; squamous: median, 7.6vs.5.5 months;HR = 0.524).The NMPA approved the PD-L1 inhibitor, sugemalimab, in combination with pemetrexed/paclitaxel and carboplatin for first-line treatment of metastatic non-squamous/squamous NSCLC based on the GEMSTONE-302 study18.Sugemalimab in combination with chemotherapy significantly improved the ORR (63.4%vs.40.3%) and prolonged the PFS(median, 9.0vs.4.9 months; HR = 0.48) and OS (median,22.8vs.17.7 months; HR = 0.67) compared to chemotherapy alone.The NMPA approved the PD-1 inhibitor, toripalimab,in combination with pemetrexed and carboplatin for firstline treatment of advanced non-squamous NSCLC based on the CHOICE-01 study19.Compared to chemotherapy alone,toripalimab in combination with chemotherapy significantly improved the ORR (65.7%vs.46.2%) and prolonged the PFS(median, 8.4vs.5.6 months; HR = 0.49) and OS (median, not reachedvs.17.1 months; HR = 0.69).The NMPA approved the PD-1 inhibitor, penpulimab, in combination with paclitaxel and carboplatin for first-line treatment of advanced squamous NSCLC based on the AK105-302 study20.Specifically, immune combination therapy significantly prolonged the PFS compared to the chemotherapy-only group (median, 7.6 monthsvs.4.2 months; HR = 0.44).
ICIs combined with anti-angiogenic therapy
ICIs combined with anti-angiogenic therapy have also shown promising applications in first-line treatment of advanced NSCLC (Table 3).
The IMpower150 study showed that patients with advanced non-squamous NSCLC treated with atezolizumab plus BCP (ABCP) had significant improvement in the PFS(median, 8.3vs.6.8 months; HR = 0.62), OS (median,19.2vs.14.7 months; HR = 0.78), and ORR (63.5%vs.48.0%) compared to bevacizumab plus carboplatin plus paclitaxel (BCP).The ABCP four-drug combination regimen was approved by the U.S.FDA for first-line treatment of metastatic non-squamous NSCLC based on the results of IMpower15021.
今年年初,一组以陶俑、青铜器等文物形象为蓝本绘制的表情包在网上引发网友热议。形态各异的文物在一位美术老师的笔下纷纷化身为或是骑着“皮皮怪”、或是跳着“海草舞”的卡通形象,不少网友将这组形象称为“1000岁的文物表情包”。而创作这组表情包的是来自浙江温岭的一位美术老师。这位美术老师在接受采访时表示,自己完全没想到这组表情包会受到关注。在南京博物院历史馆,陈列着不少形态各异的陶俑、青铜器等文物。今年暑假期间,这位美术老师到南京博物院参观,关注到很多文物表情丰富,十分有趣。出于兴趣爱好,她在现场进行了速写,把一些文物画了下来,这也成了她后来创作文物表情包的素材来源。
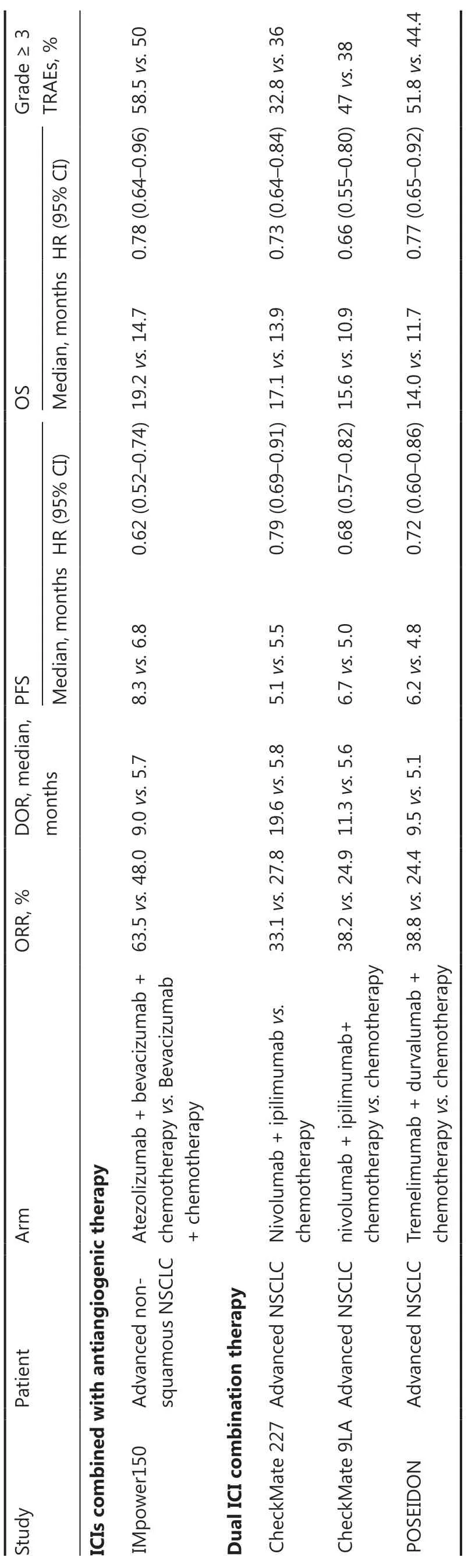
Table 3 Summary of clinical trials of first-line ICIs combined with anti-angiogenic therapy or dual ICI combination therapy for advanced NSCLC
Dual ICI combination therapy
Studies have reported positive results related to the first-line treatment of advanced NSCLC with dual ICI combination therapy (PD-1/PD-L1 inhibitors combined with CTLA-4 inhibitors; Table 3).
The CheckMate 227 study compared the efficacy and safety of the PD-1 inhibitor, nivolumab, plus the CTLA-4 inhibitor,ipilimumab, with chemotherapy alone for first-line treatment of advanced NSCLC.Specifically, a significant median OS benefit of nivolumab plus ipilimumab over chemotherapy alone in patients with a PD-L1 ≥ 1% (17.1vs.14.9 months;HR = 0.79,P= 0.007) was reported, which met the primary study endpoint22.The U.S.FDA approved nivolumab plus ipilimumab for first-line treatment of advanced NSCLC with a PD-L1 TPS ≥ 1%.
The CheckMate-9LA study was a phase III clinical study that determined the efficacy and safety of first-line nivolumab plus ipilimumab combined with two cycles of chemotherapy versus chemotherapy alone for advanced NSCLC.Combination treatment significantly prolonged the PFS (median, 6.7 monthsvs.5.0 months; HR = 0.68) and OS (median, 15.6 monthsvs.10.9 months; HR = 0.66) compared to chemotherapy23.The U.S.FDA approved nivolumab plus ipilimumab combined with chemotherapy (2 cycles) for first-line treatment of advanced NSCLC.
The efficacy and safety of the PD-L1 inhibitor, durvalumab, plus the CTLA-4 inhibitor, tremelimumab, in combination with chemotherapy and chemotherapy alone in first-line treatment of metastatic NSCLC were compared in the POSEIDON study.Dual immunotherapy combined with chemotherapy significantly prolonged the PFS (median, 6.2 monthsvs.4.8 months; HR = 0.72) and OS (median, 14.0 monthsvs.11.7 months; HR = 0.77) compared to chemotherapy alone.Subgroup analyses showed that OS was similar in the squamous cell histology group and other subgroups24.The U.S.FDA approved the tremelimumab plus durvalumab regimen in combination with platinum-based chemotherapy as first-line treatment option for patients with metastatic NSCLC.
Novel immunotherapy
A series of emerging ICIs are currently under development.The ICIs will become an important direction for future research and include inhibitors targeting novel immune checkpoints, such as the T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), lymphocyte activation gene-3 ( LAG-3), T cell immunoglobulin and mucin domaincontaining protein (TIM-3), bi-/tri-specific antibody-targeted therapy, and tumor vaccines25.
Research progress of biomarkers for immunotherapy
PD-L1
The NCCN guidelines recommend that all patients with advanced NSCLC undergo immunohistochemistry (IHC)testing for PD-L1 expression prior to receiving first-line therapy if clinically feasible to assess the availability of an ICI regimen2.Several prospective clinical trials have demonstrated a correlation between high PD-L1 expression and the efficacy of first-line immunotherapy.Based on the Keynote 024 study, pembrolizumab was approved for first-line treatment of patients with advanced NSCLC and a PD-L1 TPS ≥ 50%.The Keynote 042 study expanded the indication for pembrolizumab to include patients with a PD-L1 TPS ≥ 1%, but subgroup analyses suggested that the primary population of benefit would be PD-L1 patients with a TPS ≥ 50%.The subsequent IMpower110 and EMPOWER-Lung 1 studies obtained similar results.PD-L1 expression has been shown to have predictive value in exploratory analyses of many NSCLC clinical trials.Some patients with negative PD-L1 expression also benefit from PD-1/PD-L1 inhibitor therapy.With the advent of immunologic combination therapy strategies, the predictive value of PD-L1 has decreased.Subgroup analyses of several clinical studies have shown that patients with advanced NSCLC benefit from first-line immunocombination therapy independent of PD-L1 expression status8-21,23,24.Imperfections in PD-L1 as a biomarker can be attributed to various factors.PD-L1 expression is heterogeneous, varies within tumors, may be inconsistent in sections of the same tumor sample, and may even change with treatment.Differences in assay methods and interpretation of results exist across assays and need to be standardized26.Although PD-L1 is not a perfect biomarker,PD-L1 expression is currently the best available biomarker for assessing a patient’s suitability for receiving a PD-1/PD-L1 inhibitor, and remains an essential guide.
Tumor mutational burden (TMB)
Others
In recent years, emerging blood-based biomarkers based on liquid biopsies have attracted much attention in the investigation of biomarkers for predicting immune efficacy in NSCLC,including circulating free tumor DNA (ctDNA), circulating non-coding RNAs (microRNAs), and peripheral blood immune cell populations.The evolution of epigenetic biomarkers and the gut microbiota are also receiving attention27.
Challenges and future directions of first-line immunotherapy for NSCLC
Immunotherapy has changed the landscape of first-line treatment for advanced NSCLC, but also faces many challenges.There are no head-to-head comparative studies between immunotherapy regimens, and the optimal treatment paradigm remains to be clarified.The effective management of immune-related adverse events (irAEs) must be emphasized independent of the type of immunotherapy.Although various biomarkers, including PD-L1 and the TMB, have some predictive value in many clinical trials, neither are ideal biomarkers with respect to efficacy.Fewer irAEs have been identified as predictive biomarkers.Although first-line immunotherapy has improved the survival prognosis of patients with advanced NSCLC, only a fraction of patients have experienced long-term benefits after receiving immunotherapy.The mechanism of acquired resistance to immunotherapy remains unclear and the mode of treatment after resistance warrants further study.In addition, the higher treatment cost of ICIs is a current problem.
Emerging ICIs should be further developed in the future and additional optimal combination therapy modalities should be validated.For example, immunotherapy combined with anti-angiogenic therapy and de-chemotherapy modalities have good prospects for application in the first-line treatment of patients with advanced NSCLC.Immunotherapy combined with radiotherapy in advanced NSCLC also deserves further exploration, especially the choice of radiotherapy modality, the optimal dose, and the sequence of radiotherapy and immunotherapy need to be clarified.In addition, the search for the best biomarkers to predict ICI efficacy and irAEs should continue.Comprehensive prediction and dynamic monitoring models should be further constructed to achieve individualized precision immunotherapy.Furthermore, elucidating the mechanism underlying immune resistance and exploring strategies to overcome immune resistance are also directions for future research.
Grant support
This study was supported by the Hunan Lung Cancer Clinical Medical Research Center (Grant No.2023SK4024 to LW), the Hunan Science and Technology Innovation Program (Grant No.2021SK51121 to LW), and the Hunan Cancer Hospital Climb plan (Grant No.ZX2020005-5 to LW).
Conflict of interest statement
No potential conflicts of interest are disclosed.
猜你喜欢
杂志排行
Cancer Biology & Medicine的其它文章
- Circulating cell-free mtDNA as a new biomarker for cancer detection and management
- Microbiome changes in esophageal cancer: implications for pathogenesis and prognosis
- Deep insight into the B-cell associated tertiary lymphoid structure and tumor immunotherapy
- Cancer-educated neutrophils promote lung cancer progression via PARP-1-ALOX5-mediated MMP-9 expression
- Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma
- Perspective on new cell-free DNA technologies for early cancer detection
