Construction of all-organic low dielectric polyimide hybrids via synergistic effect between covalent organic framework and cross-linking structure
2024-01-27WnjingZhoZhoyngWeiChonghoLuYizhngTongJingshuHungXinwuCoDenShiRoertLiWeiWu
Wnjing Zho,Zhoyng Wei,Chongho Lu,Yizhng Tong,Jingshu Hung,Xinwu Co,**,Den Shi,Roert K.Y.Li,Wei Wu
a Key Laboratory of Polymer Processing Engineering of Ministry of Education,Guangdong Provincial Key Laboratory of Technique and Equipment for Macromolecular Advanced Manufacturing,School of Mechanical and Automotive Engineering,South China University of Technology,Guangzhou,510640,China
b Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials,Ministry of Education Key Laboratory for the Green Preparation and Application of Functional Materials,Hubei Key Laboratory of Polymer Materials,Faculty of Materials Science and Engineering,Hubei University,Wuhan,430062,China
c Department of Materials Science and Engineering,City University of Hong Kong,Tat Chee Avenue,Kowloon,Hong Kong Special Administrative Region of China
d Jihua Laboratory,Foshan,528200,China
Keywords: Polyimide Covalent organic framework All-organic Cross-linking structure Dielectric property Hybrid film
ABSTRACT Polyimide(PI)is a promising electronic packaging material,but it remains challenging to obtain an all-organic PI hybrid film with decreased dielectric constant and loss without modifying the monomer.Herein,a series of allorganic PI hybrid films were successfully prepared by introducing the covalent organic framework (COF),which could induce the formation of the cross-linking structure in the PI matrix.Due to the synergistic effects of the COF fillers and the cross-linking structure,the PI/COF hybrid film containing 2 wt% COF exhibited the lowest dielectric constant of 2.72 and the lowest dielectric loss(tan δ)of 0.0077 at 1 MHz.It is attributed to the intrinsic low dielectric constant of COF and a large number of mesopores within the PI.Besides,the cross-linking network of PI prevents the molecular chains from stacking and improves the fraction of free volume(FFV).The molecular dynamics simulation results are well consistent with the dielectric properties data.Furthermore,the PI/COF hybrid film with 5 wt% COF showed a significant enhancement in breakdown strength,which increased to 412.8 kV/mm as compared with pure PI.In addition,the PI/COF hybrid film achieve to reduce the dielectric constant and thermal expansion coefficient (CTE).It also exhibited excellent thermal,hydrophobicity,and mechanical performance.The all-organic PI/COF hybrid films have great commercial potential as next-generation electronic packaging materials.
1.Introduction
With the fast development of microelectronic products and the 5G industry,materials with low dielectric constant have drawn great attention in the electronic packaging field.The low dielectric constant can solve the problems of signal crosstalk,delay,and loss caused by the reduction of chip size [1–3].Polyimides (PIs) are widely used in the electronics industry for a variety of interconnect and electronic packaging applications due to their outstanding insulation,good chemical and thermal stability,excellent mechanical performance,and adhesion performance [4,6,7].However,the relatively high dielectric constant of traditional PIs is far from the requirements of electronic devices,and 17% of the total loss in electronic devices derives from the tan δ of the insulating material [8].Therefore,it is important to decrease the dielectric constant and suppress the tan δ of PIs simultaneously.
As for the dielectric constant,there have been three main strategies to decrease the dielectric constant of PIs.The first one is the introduction of lower polarity groups or large volume atoms such as fluorinated substituents.The second method is the incorporation of low dielectric constant fillers into PIs.The last one is the introduction of porous structures into PIs due to the dielectric constant of air being just 1.0[9].For the first method,Bei et al.[10]found that the dielectric constant decreased with an increase in the benzene ring number in the pendant group due to the free volume of the polyimides become larger.Chen et al.[11]prepared novel fluorinated aromatic polyimides and found polyimides showed the intrinsic low dielectric constant (low-k) due to the introduction of the bulky triphenyl methane side groups and the tortuous backbone structures.For the latter two methods,the introduction of low dielectric constant mesoporous nanofillers [12,13]such as polyhedral oligomericsilsesquioxane(POSS)and mesoporous silica in PI matrix has become a research hotspot.Shi et al.[14]introduced the octa (aminopropyl) silsesquioxane(OAPrS)into the PI matrix to obtain the OAPrS/PI composite films.The result showed that OAPrS/PI composites with the addition of 3.9 wt% OAPrS had a dielectric constant of 2.64 at 1 MHz.Kurinchyselvan et al.[15]found that PI/amine-functionalized mesoporous silica (PI/FMCM-41) composites with 7 wt% filler loading showed a dielectric constant of 2.21(1 MHz).However,it will lead to a significant deterioration in the tensile strength and elongation at break of PI composites,which were decreased by 40% and 69%,respectively.Wang et al.[16]obtained an ultralow dielectric constant of 1.84 by introducing a silica colloid solution into fluorinated polyimide (FPI) at the expense of mechanical properties.However,the method of reducing the dielectric constant of PI by adding inorganic nanoporous fillers will inevitably weaken the mechanical properties.Furthermore,inorganic nanoporous fillers have poor compatibility with the polymer matrix,so surface modification of fillers is usually required,which increases the complexity and cost of preparation.
Covalent organic framework(COF)is a new kind of porous crystalline material composed of light elements (C,N,O,etc.) through strong covalent bonds [17,18].Recently,COFs have become a research hotspot because of their intrinsic properties,including uniform pore sizes,tailorable surface functional groups,ordered channel structure,and excellent thermal and chemical stability [19–21].Zhang et al.[22]reported PI-COF has a wide range of applications such as adsorption and separation,drug delivery,energy storage,photocatalysis et al.Kuehl et al.[23]also reported a detailed study of the reactions of two dimensional polyimide-linked covalent organic frameworks.However,there are few studies on dielectric properties of PI/COF for electronic packaging.Evans et al.[1]reported that two-dimensional COFs had an ultra-low dielectric constant of 1.6,making them promising low-k dielectric materials.Purushothaman et al.[24]showed the dielectric constant of PI film with 4 wt% COF-LZU-1 was reduced to 1.36,which demonstrated that COFs had great potential in the microelectronic industry.
As one eminent representative of COFs,the COF synthesized by 2,5-dimethoxyterephthaldehyde(DMTP)and 1,3,5-tris(4-aminophenyl)benzene (TAPB) has higher porosity and crystallinity than other types of COFs,which makes it a potential low-k material for dielectrics.In this work,the COF condensed from TAPB and DMTP was doped into the PI matrix to realize the low dielectric constant,low dielectric loss,and excellent comprehensive properties of PI/COF hybrid films.The reaction between the amino groups on the surface and the PAA molecular chains on COF could serve as a cross-linking point.The molecular dynamics simulation was used to confirm the change in the molecular structure of the PI hybrids.In addition,the mesoporous pores of COF could result in many nanopores in the PI hybrid films.The synergistic effects of COF and the cross-linking structure on the dielectric properties,water absorption,mechanical properties,and thermal stability of PI/COF hybrid films were analyzed in detail.
2.Experimental
2.1.Materials
4,4'-(4,4′-isopropylidenediphenoxy)bis-(phthalic anhydride)(BPADA),and 2,2-bis [4-(-aminophenoxy)phenyl]hexafluoropropane (HFBAPP),2,3,3′,4′-biphenyltetracarboxylic dianhydride (α-BPDA),4,4'-(9-fluorenylidene)dianiline (BAFL) were purchased from Bepharm (Shanghai China).1,3,5-tris(4-aminophenyl)benzene (TAPB),2,5-dimethoxyterephaldehyde (DMTP),1,4-dioxane,butanol,and methanol were bought from Maclean (Shanghai,China).N,N-dimethylacetamide (DMAc) and acetic acid was supplied by Tianjin Fuyu Fine Chemical Co.,Ltd.(Tianjin,China).
2.2.Synthesis of the COF
The COF was synthesized successfully based on a reported method[25,26].1,4-dioxane,butanol,and methanol were mixed to prepare a cosolvent system with a volume ratio of 4:4:1.Then 0.3 mmol of TAPB and 0.45 mmol of DMTP were added to 45 ml of the above-mixed solution,followed by ultrasonication for 1 h to completely dissolve.Subsequently,0.5 ml of acetic acid(12 mol/L)was introduced into the above solution.Then,the solution was reacted in an oven at 70°C for 24 h after adding 4.5 ml of acetic acid.Finally,the mixed solution was filtered and washed with tetrahydrofuran and acetone to obtain COF,and the resultant COFs were dried in a vacuum drying oven at 40°C for 12 h.
2.3.Preparation of the PI/COF hybrid films
The synthesized diagram of the all-organic PI/COF hybrid films is presented in Scheme 1.All processes were under nitrogen atmosphere.The fluorinated PI was synthesized by quaternary copolymerization of dianhydrides (BPADA and α-BPDA)and diamines(BAFL and HFBAPP).
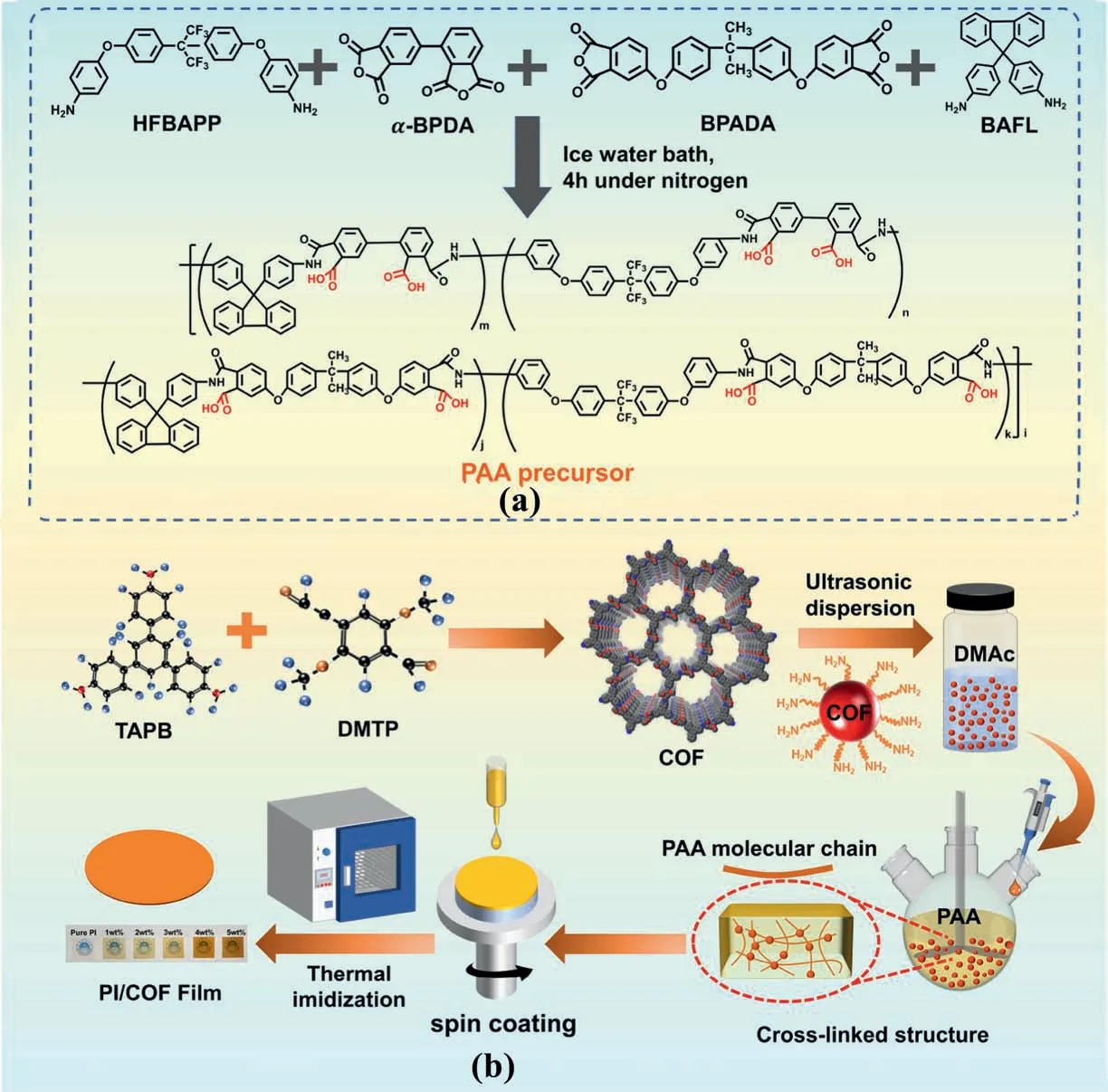
Scheme 1. Synthesis route of PI/COF hybrid films.
The same molar ratio of two diamines(BAFL,HFBAPP)was dissolved in a certain amount of DMAc by mechanical stirring in nitrogen.Next,the three-necked flask containing the diamine solution is put into an icewater bath.Then,the same molar amount of two dianhydrides(α-BPDA and BPADA) were added to the diamine solution.DMAc was dropped into the mixed solution to dilute it to the 15 wt% solid content.Finally,the mixed solution was kept stirred for 4 h.At the same time,a certain amount of COF was added to 5 ml of DMAc solution and sonicated for 4 h.After 4 h of reaction,a certain amount of COF solution was added into the three-necked flask and stirred for 1 h.
The PAA/COF glue without bubbles was spin-coated on a glass plate,and the obtained film was put into an oven for thermal imidization.The following is the heating procedure: 80°C/2 h,100°C/1 h,150°C/1 h,200°C/1 h,250°C/1 h,and 300°C/1 h.
2.4.Simulation methodology
2.4.1.Model of PI and PI/COF
The pure PI and PI/COF hybrid films are constructed and simulated by the Materials Studio package using the COMPASS force field.The Amorphous Cell module is used to construct the system,and then the Geometry Optimization and Dynamics are analyzed by the Forcite module.First,four kinds of polymer repeat units (BAFL,HFBAPP,α-BPDA,and BPADA)are constructed and minimized by the Smart method.Then the polymer builder module is used to create a PI chain of 20 repeating units with minimization by the Smart method.The atomic positions of 2D COF are obtained from the experimental data.The pore size of COF is 33 Å and unit cell parameters ofa=b=37∙6695 Å,c=3∙7589 Å,α=β=90∘,and γ=120∘.The 3D amorphous systems with periodic boundary conditions and containing pure PI chains and PI/COF composites are built by using the Amorphous Cell module.Here,the COF contents of the hybrid models are about 1 wt%,2 wt%,3 wt%,4 wt%,and 5 wt%.
2.4.2.Simulation procedures
After minimization is performed,these optimum conformations are further confirmed by molecular dynamics (MD) running an NPT ensemble and an NVT ensemble.The time step used in all MD simulations is set to 1.0 fs.The van Der Waals and Coulombic nonbonding interactions are calculated by the Ewald summation method.
2.5.Characterization
Scanning electron microscopy (SEM,FEI Quanta 200) and transmission electron microscopy(TEM,Philips Tecnai)were used to observe the morphology of COFs and PI/COF hybrid films.The Fourier-transform infrared spectrum (FT-IR) was used to analyze the functional groups using a Nicolet IS50 FTIR instrument.The crystal phase of the samples was measured by X'Pert3 POWDER X-ray diffractometry with CuKαradiation (λ=0.154 nm).Gel permeation chromatography (GPC) was analyzed by Waters 1515,and the weight-average molecular weight(Mw)of pure PI is about 195,660 (Fig.S2).The thermal decomposition temperatures corresponding to 10% weight loss(T10)and the char residues at 800°Cwere characterized by a TG209F3 thermogravimetric analyzer.Glass transition temperature was acquired from a dynamic thermomechanical analyzer (DMA242,Netzsch,Germany) under a tension mode.The scanning rate is 10°C/min with a load frequency of 1 Hz in nitrogen.The CTEs of the PI/COF hybrid films were measured by a Netzsch TMA402F1 thermomechanical analyzer at a heating rate of 5°C/min and a temperature range of 40–250°C.A Dataphysics OCA40 Micro-Surface contact angle measuring instrument was used to test the contact angle of PI/COF hybrid films.The optical properties of PI films were measured by a Hitachi U3900 spectrophotometer,and the thickness of the films was about 25 μm.The dielectric constant and dielectric loss were obtained from a Stabilizer WK6500B precision impedance analyzer in a frequency range between 102and 107Hz.The breakdown strength was measured using a CS99 instrument under a direct-current voltage ramp.The tensile mechanical performance of PI/COF films was measured with an Instron 5566 universal testing machine.The final data is the average of at least five samples.
3.Results and discussion
3.1.Synthesis and characterization of COF
Fig.1 shows the morphology and structure of COF.The TEM images of as-prepared COFs are displayed in Fig.1a–c.The prepared COF has a spherical shape with a rough surface,and its diameter is about 1 μm.At a higher magnification (Fig.1d),it showed that the large area regular hexagonal channel structure is arranged along the radial direction of COF spheres[27].Previous study have demonstrated that the 2D COF layers can form rod-like crystallites through π-π stacking,which can further self-assemble to form hollow COF spheres [28].Therefore,it is speculated that the COF spheres in this work may be aggregates of rod-like crystallites.Moreover,the EDX elemental mapping images in Fig.1d–g confirm that COF contains only C,O,and N elements.
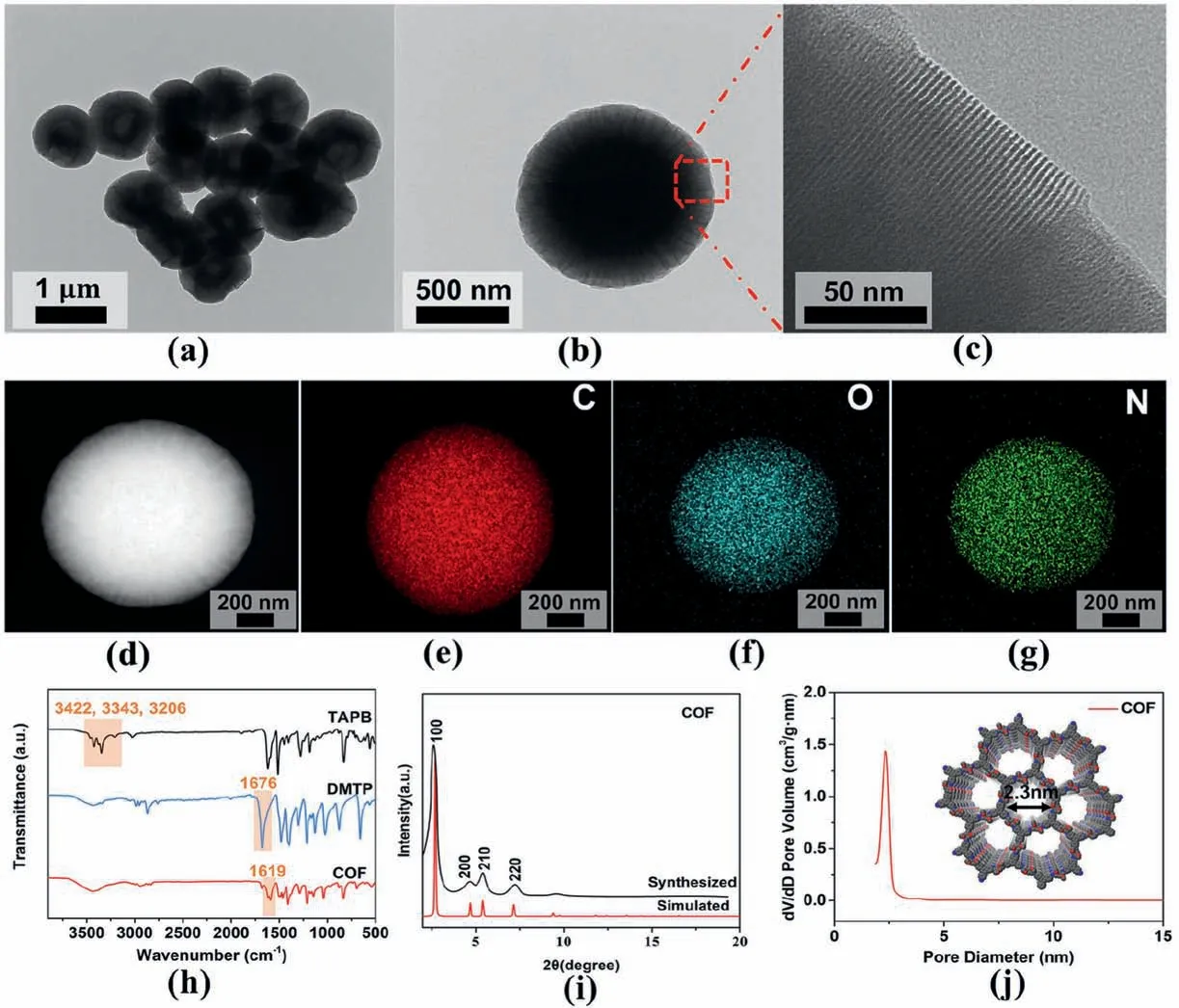
Fig.1.(a–c)TEM images of COF,and(d–g)EDS-TEM elemental mapping of C,O,N for COF,(h)FTIR patterns of TAPB,DMTP and COF,(i)XRD patterns of COF,(j)Pore size distribution of COF (Inset: the crystal structures of COF viewed through planes).
The FTIR spectra of DMTP,TAPB,and COF are shown in Fig.1h.The absorption bands of TAPB are at 3422,3343,and 3206 cm-1,which correspond to the stretching vibration of the N–H functional group.The peak at 1676 cm-1is the characteristic of the CO stretching band in DMTP.The characteristic absorption of CN groups in COF is at 1619 cm-1.In the FTIR spectrum of COF,the stretching vibration peaks of the aldehyde group and amino group become weakened,which indicates that DMTP has been reacted with TAPB[29].The results proved that COF was successfully synthesized.The detailed crystallographic structure of COF was displayed in Fig.1i.There is a strong peak at 2.63°,which can be attributed to the(100)reflecting surface of COF.The diffraction peaks at 4.67°,5.46°,and 7.25°can be designated as the reflection planes of(200),(210),and (220),respectively [30].The porosity of COF was investigated by BET desorption analysis,as shown in Fig.S1a.A steep increase in nitrogen uptake of COF at low relative pressure indicates the micropore structure of COF.In addition,the pore size distribution of COF shows a main pore width centered at 2.3 nm(Fig.1j).The mass ratios and thermal stability of the COF were measured using thermogravimetric analysis(TGA,Fig.S1b).The result shows that the synthesized COF has high thermal stability up to 400°C,which meets the temperature requirement for the thermal imidization of PIs.
3.2.Microstructure and optical properties of PI/COF hybrid films
Imidization and the structure of the PI hybrid films were analyzed by ATR-FTIR analysis.As shown in Fig.2b,the characteristic peaks at around 1787,1724,and 742 cm-1can be observed,which represent the asymmetric stretching vibration,the symmetric stretching vibration,and the bending vibration of the CO group of PI,respectively.Besides,the absorption peaks that occurred at 1376 cm-1can be attributed to the stretching vibration of the C–N group [9].In addition,the absorption peak of the C–F stretching vibration can be found at 1176 cm-1,which reveals the fluorine atom was successfully introduced[25].All the results indicate the completion of thermal imidization.
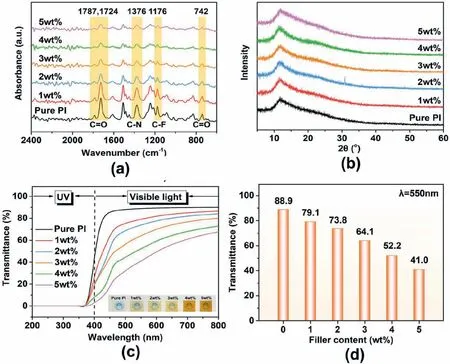
Fig.2.(a)ATR-FTIR spectra,(b)XRD patterns of pure PI and PI/COF hybrid films with various filler content,(c)UV–Vis spectra of the PI films(Inset:photograph of the PI/COF hybrid films with different COF contents),and (d) the transmittance of the prepared polyimide films under 550 nm wavelength.
The XRD patterns were utilized to explore the molecular packing preferences of PI films.As shown in Fig.2b,the patterns of all PI/COF films exhibited a wide diffraction peak at around 2θ=12°,and there is no obvious crystal absorption peak.The result indicates the addition of COF has little effect on the amorphous aggregation structure of PI films,and the COFs are homogeneously dispersed in PI networks through covalent bonding.The XPS spectra of COF and the PI/COF hybrid films with 0 wt%,2 wt%,5 wt% filler content were shown in Fig.S3.The peak at 283 eV was identified as C 1s,that at 397 eV was identified as N 1s,that at 530 eV was identified as O 1s,and the peak at 686 eV corresponds to F 1s [5].Compared with the spectra of COF,the fluorine element was introduced on the surface of PI/COF hybrid films.The result showed that COF and PI/COF hybrid films had the same elements(C,N,and O).The ultraviolet–visible light (UV–Vis) spectra of the PI films are shown in Fig.2c and d.The curves show that the light transmittance of the PI/COF hybrid films gradually decreases with COF content increasing in the visible light region.Fig.2d shows the transmittance of the prepared PI films under a 550 nm wavelength since people are most sensitive to wavelengths of 550 nm[31].Compared with pure PI,the transmittance of PI/COF hybrid films with 5 wt% COF loading decreased from 88.9% to 41.0% at the 550 nm light wavelength.The inserted photo in Fig.2c exhibits that the color of PI/COF hybrid films deepens with the increase of COF content.It is attributed to the formation of intermolecular charge transfer complexes(CTC)in PI molecule chains[32].In the case of pure PI,the presence of trifluoromethyl groups has strong steric hindrance and electron absorption effects that inhibit the formation of intermolecular CTC[33].However,the incorporation of COFs contributes to a stronger CTC effect due to their electron-rich diamine groups and strong electron-donating ability.Therefore,the stronger CTC effect will lead to a deeper color.
3.3.Thermal properties of PI/COF hybrid films
The thermal properties,including the temperature corresponding to 10% weight loss(T10),the char residues at 800°C,the value of CTE,and the glass transition temperature(Tg)of PI/COF hybrid films,are shown in Fig.3 and Table 1.As expected,theT10values and the char residues at 800°C of all PI/COF hybrid films are higher than 543°C and 62%,respectively.It further confirmed the excellent thermal stability of PI/COF hybrid films due to rigid imide rings and the enhancement of intermolecular interaction forces between benzene rings and imide rings[27].In comparison with pure PI,a slight increase is observed for theT10values and the char residues at 800°C of PI/COF hybrid films.In addition,theTgvalue of PI hybrid film with 5 wt% COF increases from 278.9 to 282.8°C in comparison with pure PI.The enhancement in thermal stability is attributed to the cross-linking structure,which restricts the movement of molecular chains.
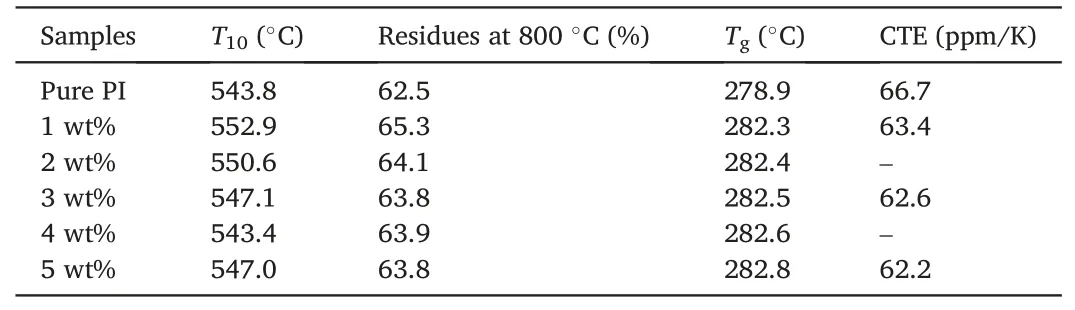
Table 1Thermal properties of PIs.

Fig.3.(a) TGA and (b) DTG curves of PI composites with different filler content,(c) DSC heating curves and (d) CTE and glass transition temperature curves of PI composites.
The CTE of interlayer dielectrics is another important factor to ensure the reliability of electronic devices.When the CTE of interlayer dielectrics is close to that of copper (17 ppm/K),electronic devices demonstrate higher reliability [34].According to Fig.3d and Table 1,pure PI film exhibits larger CTEs due to the easy change of bond length and bond angle with the increase of temperature.However,compared with the CTE values of pure PI(66.7 ppm/K),the CTE of PI/COF hybrid films decreased to 62.2 ppm/K when the content of COF loading reached 5 wt%.This can be owed to the fact that the cross-linking network restricts segment movement,resulting in a reduced CTE value[35,36].The result showed the introduction of the crosslink structure could effectively reduce CTE.
3.4.Hydrophobicity performance of PI composites
Hydrophobicity is an important factor for interlayer dielectrics because the absorption of water significantly affects the dielectric properties.The water contact angle (WCA) and surface morphology can indicate the hydrophobicity of the PI/COF hybrid films.In Fig.4a,the WCA of the PI/COF hybrid films reaches the highest value of 99.4°with the addition of 3 wt% COF content,which is increased by 20.6% as compared to that of pure PI.The average surface roughness(Ra)of the PI/COF hybrid films increases as COF content increases (Fig.4d–f),which demonstrates that the increase in the surface roughness of the film leads to an improvement in hydrophobicity [33].In addition,the incorporation of COF contributes to the formation of the hydrophobic cross-linking networks in the PI matrix,which makes the surface more hydrophobic[37].However,when the COF content exceeds 3 wt%,the WCA begins to decrease because the excessive COF will lead to excessive amino groups,which damage the hydrophobicity of PI/COF hybrid films[33].
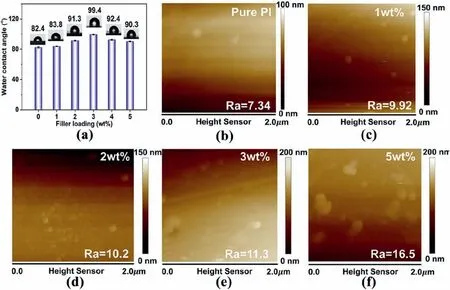
Fig.4.Water contact angle on the surfaces of (a) PI/COF hybrid films (Insert are the water contact angle images),and (b–f) AFM images of the PI hybrid films.
3.5.Dielectric properties and electric breakdown strength of the PI/COF hybrid films
The frequency dependences of the dielectric constant of pure PI and PI/COF hybrid films with different COF loadings are shown in Fig.5a.The dielectric constant shows little change in the frequency range of 102–107Hz,which indicates PI hybrid films have a stable dielectric constant.As shown in Fig.5c,pure PI has a dielectric constant of 3.22 at 1 MHz at room temperature,and the introduction of COF into the molecular chain of PI leads to a significant decrease in its dielectric constant.When COF reaches 2 wt% loading,the dielectric constant of the PI/COF hybrid film decreases to its lowest value of 2.72 at 1 MHz.As shown in Fig.5b,it demonstrates that the tan δ decreased first and increased with the COF content increasing.The PI/COF hybrid film with 2 wt% COF obtains the lowest dielectric loss of 0.0077.In addition,the tan δ of all PI/COF hybrid films is lower than 0.02 in the frequency range of 102–107Hz,which has met the application requirements of tan δ should be less than 0.05 in this field [38].
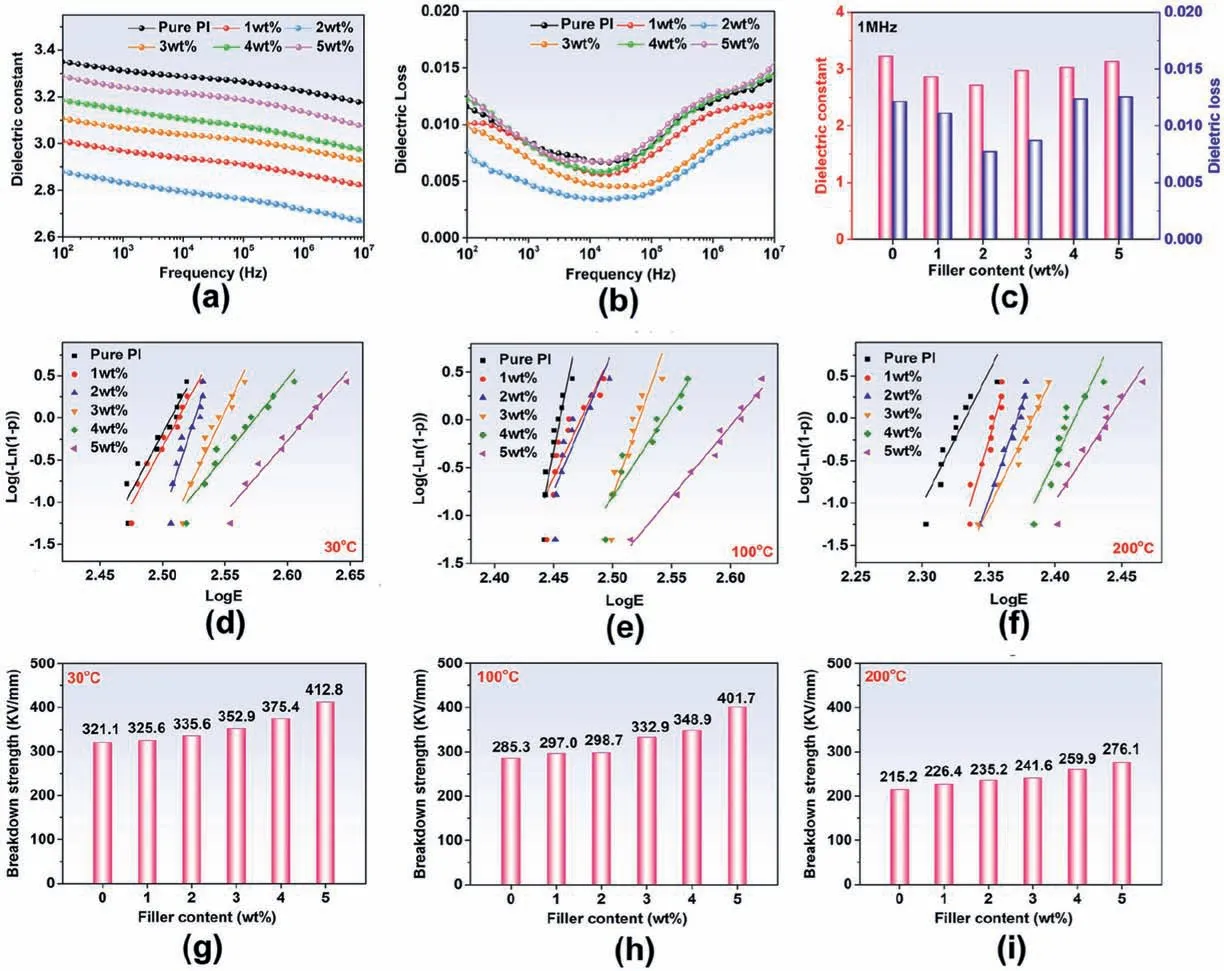
Fig.5.(a)Dielectric constant and(b)dielectric loss of PI hybrid films as a function of frequency;(c)dielectric constant and dielectric loss with different filler content under 1 MHz.Weibull statistic plots and deduced characteristic of dielectric breakdown strength of PI hybrid films of (d) 30 °C,(e) 100 °C,and (f) 200 °C;and Comparison of characteristic breakdown strength of PI composites (g) 30 °C,(h) 100 °C,and (i) 200 °C.
The reason for the reduction of the dielectric constant and dielectric loss of the PI/COF hybrid films can be explained by the Debye equation,as shown in Eq.(1)[39].
where ε is the relative dielectric constant of PI/COF films,αeand αdstand for the electronic and distortion polarization in the molecule,Nis the number of dipoles,μ repersents the orientation polarization related to the dipole moment,kbis the Boltzmann constant,andTis the temperature.For the PI/COF hybrid films,a certain degree of a cross-linking network can reduce the dipole orientation by limiting the mobility of the polymer chain,which will effectively prevent the electron cloud in PI/COF from being polarized,so the αe,αdand μ were reduced.Therefore,appropriate cross-linking degree can reduce intermolecular friction work by reducing dipole rotation in PI matrix,leading to reduced dielectric loss values of PI/COF hybrid films [40].Moreover,the rigid cross-linking structure in PI/COF can prevent the stack of molecules and improve the FFV,thus theNis decreased which leads to lower dielectric constant [34].However,when the COF content is higher than 2 wt%,both the dielectric constant and dielectric loss increase.This is because the excessive COF in the PI matrix will become impurities,which will increase the tan δ and the dielectric constant[41].In addition,the excessive cross-linking reduces the FFV of PI hybrid films resulting in the reduction of the dielectric constant,which confirms by molecular dynamic simulation.The fractional free volume(FFV)of PI/COF hybrid films was further investigated by molecular dynamics simulation as shown in Fig.6.The blue and gray areas corresponded to the free volume and the occupied volume,respectively.FFV was calculated to explain the effect of COF on the dielectric constant of PI hybrid films[42–44].
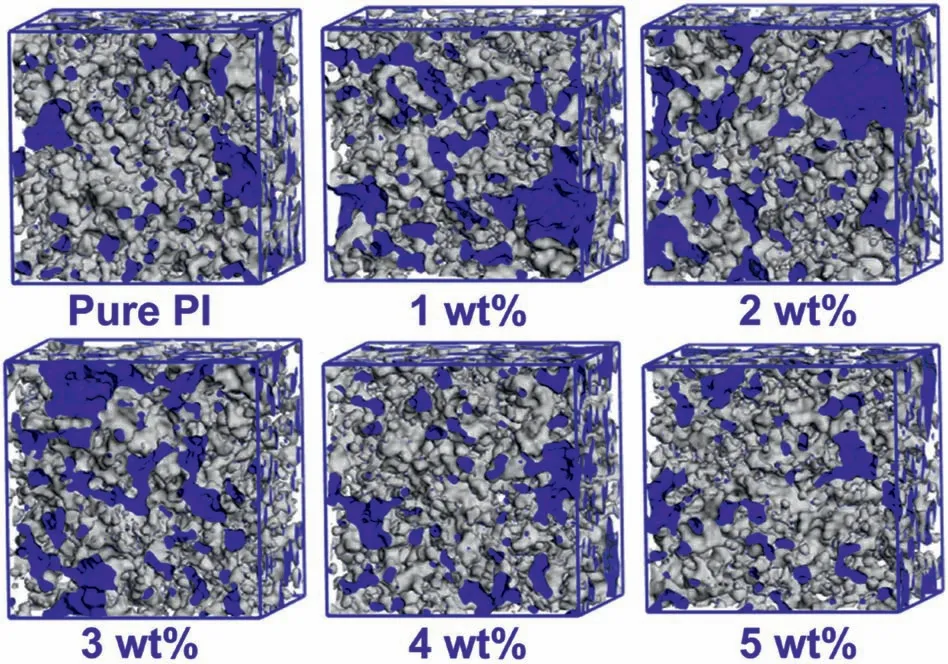
Fig.6.Simulation models of PI/COF hybrid films with different COF content.
whereVT,VF,andV0represent the total,free,and occupied volumes of the models,respectively.The occupied volume,free volume,and total volume as well as the calculated FFV,calculated density,and measured density for different systems are listed in Table 2.The calculated FFV increases as the COF content increased to 2 wt% and then decreased,which is consistent with the dielectric constant results.Moreover,the decrease in density also confirms the increase in FFV of PI/COF hybrid films.It could be inferred that the reduction of the dielectric constant is due to the increase of FFV caused by a certain degree of cross-linking of COF.
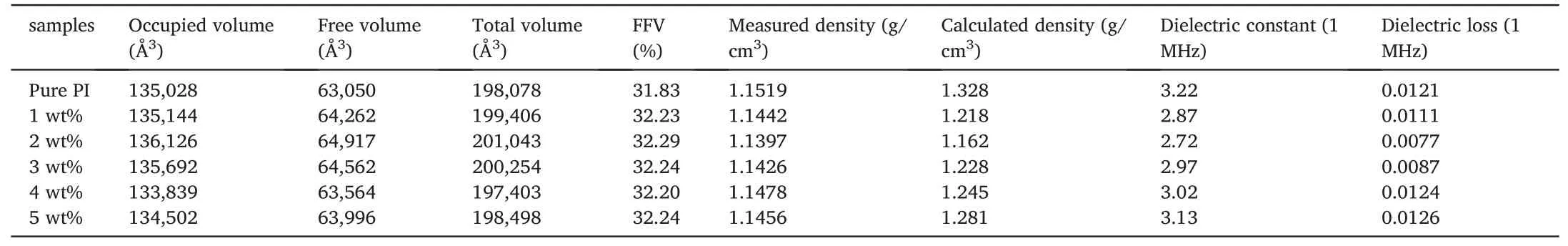
Table 2The occupied volume,free volume,and total volume of simulation cells as well as calculated FFV,calculated density and measured density for PI/COF hybrid films with different fillers loading.
The dielectric breakdown strength(Eb)is usually analyzed by a twoparameter Weibull statistic described as follows[45]:
wherePrefers to the cumulative electrical failure probability,β is a shape parameter,E is experimental breakdown strength,Ebrepresents the electric breakdown strength at P(E)=63.28%,and it is called characteristic breakdown strength.As shown in Fig.5d–i,Ebincreases with the increasing filler content at the same temperature.TheEbof the PI/COF hybrid film with 5 wt% COF loading obtains the highest breakdown strength of 412.8 kV/mm at 30°C,which increases by 28.6% as compared to that of pure PI.The fundamental reason for the increasing breakdown strength may be originated from the increase in crosslinking degree by introducing the COF,which restricts the chain motion of films under highly applied electric fields,resulting in the enhancement of the breakdown strength of all films[46].When the temperature rises,theEbof the PI/COF hybrid films decreases significantly under the same filler loading.The theory of thermal breakdown and its theoretical formula are described as follows[47]:
WhereUbis the thermal breakdown voltage;β andT0correspond to the coefficient of heat emission of material and temperature;d and S are the length and cross-section of the breakdown channel;α is the temperature coefficient of resistance.The formula shows thatUbis related to temperature.When the temperature rises,the breakdown voltage decreases exponentially.However,the breakdown strength improved with COF content,even at high temperatures.The characteristic breakdown strength of the PI/COF hybrid film with 5 wt% COF increases from 215.2 to 276.1 kV/mm at 200°C as compared to pure PI.It indicates that the cross-linking structure can significantly enhance the breakdown strength of film even at high temperatures.
3.6.Mechanical properties of the PI composites
Fig.7a shows the tensile stress versus strain of PI/COF hybrid films filled with various content of COF.The average tensile strength and elongation at break of the PI/COF hybrid film obtained from the tensile test are shown in Fig.7b.It is observed that the pure PI exhibits a typical brittle failure with an elongation at break of 7.6%.With the increase of COF content,both the tensile strength and the elongation at break of the PI/COF hybrid films increase simultaneously.Compared with pure PI,the tensile strength of PI film with 4 wt% COF improves by 12.9%,and the elongation at break also increases by 45.3%,respectively.It indicates that COF incorporation exerts a certain strengthening and toughening effect.When the COF content exceeds 4 wt%,the tensile strength and elongation at break decrease slightly.Overall,all the PI/COF hybrid films have good mechanical performance.
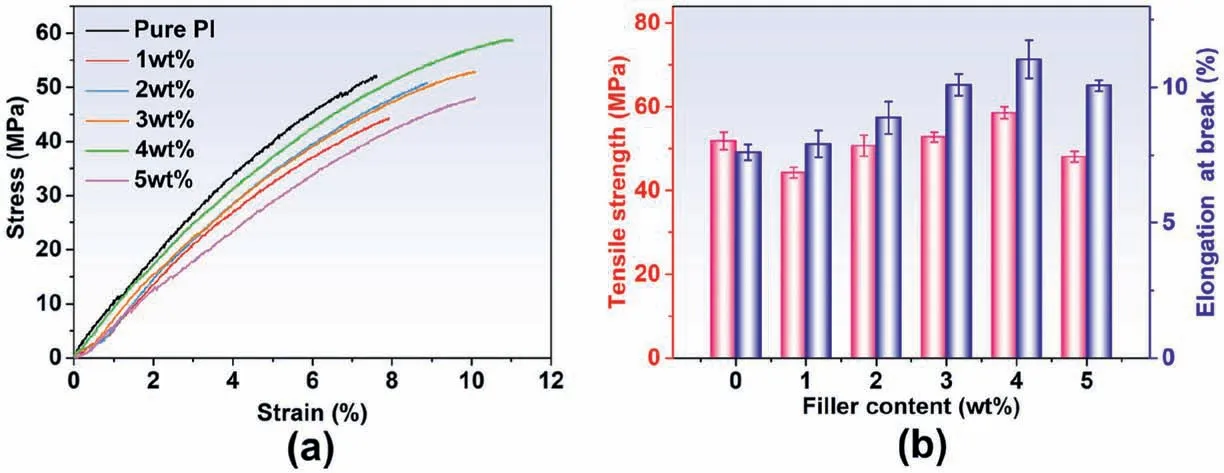
Fig.7.Tensile stress versus strain curves of(a)PI/COF hybrid films;(b)maximum stress and elongation at break of PI/COF hybrid films as a function of filler content.
4.Conclusions
In this work,the novel all organic cross-linkable polyimides were successfully synthesized by introducing covalent organic framework(COF).The cross-linking films with 2 wt% COF showed the lowest dielectric constant of 2.72 and a significantly reduced tan δ of 0.0077 at 1 MHz.Moreover,while reducing the dielectric constant and tan δ,the film shows other excellent comprehensive properties.PI/COF hybrid films exhibited superior thermal stability(Tg>282°C,T10%>543°C),and the CTE of 5 wt% PI/COF was decreased by 6.7% as compared with pure PI.The hydrophobicity and mechanical properties are also improved due to the introduction of crosslinking networks.Moreover,the breakdown strength of 5 wt% PI/COF film increased from 321.1 kV/mm of pure PI to 412.8 kV/mm.Thus,the outstanding comprehensive performances make the PI/COF hybrid films potential candidates for next-generation electronic packaging materials.These encouraging results revealed that the synergistic effect of cross-linking structure and COF can reduce the dielectric constant of polyimide films while improving their overall performance.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This work was supported by National Natural Science Foundation of China(52103029 and 51903075).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2023.02.002.
杂志排行
Namo Materials Science的其它文章
- MXene-based flexible pressure sensor with piezoresistive properties significantly enhanced by atomic layer infiltration
- Synergistic coupling of 0D–2D heterostructure from ZnO and Ti3C2Tx MXene-derived TiO2 for boosted NO2 detection at room temperature
- Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review
- Wearable and stretchable conductive polymer composites for strain sensors:How to design a superior one?
- An overview of recent progress in the development of flexible electrochromic devices
- Water-based synthesis of nanoscale hierarchical metal-organic frameworks:Boosting adsorption and catalytic performance
