In situ confined vertical growth of Co2.5Ni0.5Si2O5(OH)4 nanoarrays on rGO for an efficient oxygen evolution reaction
2024-01-27YngMuXioyuPeiYunfengZhoXueyingDongZongkuiKouMioCuiChnggongMengYifuZhng
Yng Mu,Xioyu Pei,Yunfeng Zho,Xueying Dong,Zongkui Kou,Mio Cui,Chnggong Meng,Yifu Zhng,*
a State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,Dalian,116024,China
b State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,Wuhan University of Technology,Wuhan,430070,China
Keywords: Co2.5Ni0.5Si2O5(OH)4@rGO Vertical grown nanoarrays Geometric and electronic structure regulation Metal-support interactions Oxygen evolution reaction
ABSTRACT Rational design of oxygen evolution reaction (OER) catalysts at low cost would greatly benefit the economy.Taking advantage of earth-abundant elements Si,Co and Ni,we produce a unique-structure where cobalt-nickel silicate hydroxide [Co2.5Ni0.5Si2O5(OH)4]is vertically grown on a reduced graphene oxide (rGO) support(CNS@rGO).This is developed as a low-cost and prospective OER catalyst.Compared to cobalt or nickel silicate hydroxide@rGO (CS@rGO and NS@rGO,respectively) nanoarrays,the bimetal CNS@rGO nanoarray exhibits impressive OER performance with an overpotential of 307 mV@10 mA cm-2.This value is higher than that of CS@rGO and NS@rGO.The CNS@rGO nanoarray has an overpotential of 446 mV@100 mA cm-2,about 1.4 times that of the commercial RuO2 electrocatalyst.The achieved OER activity is superior to the state-of-the-art metal oxides/hydroxides and their derivatives.The vertically grown nanostructure and optimized metal-support electronic interactions play an indispensable role for OER performance improvement,including a fast electron transfer pathway,short proton/electron diffusion distance,more active metal centers,as well as optimized dualatomic electron density.Taking advantage of interlay chemical regulation and the in-situ growth method,the advanced-structural CNS@rGO nanoarrays provide a new horizon to the rational and flexible design of efficient and promising OER electrocatalysts.
1.Introduction
Green and sustainable energy issues have received a lot of attention recently,especially after the carbon neutrality initiative (CNI).Thus,various technologies in the field of energy storage and conversion,including solar cells,CO2reduction,water splitting,rechargeable metalair batteries,N2reduction and so on,have inspired vigorous research effort[1–4].The key half-reaction,oxygen evolution reaction(OER)is at the heart of the above electrochemical conversion process.Owing to the four-electron transfer mechanism,OER kinetics are considered to be relatively sluggish [5].Currently,Ru-and Ir-based oxides exhibit ideal reactivity to accelerate such thermodynamically upward OER behavior.Nevertheless,their high cost and scarcity seriously limit widespread application [6,7].Consequently,the exploration of high-efficiency and inexpensive electrocatalysts is important,especially if they can use earth-abundant materials.
Recently,non-precious-metal-based OER catalysts have attracted much interest.The (hydro)oxides,carbides,nitrides,phosphides and chalcogenides of transition metals in the Fourth period have been widely investigated.In particular,Fe/Co/Ni/Mn-based materials have emerged as potential alternatives [8–11].Among them,layered hydroxides,oxyhydroxides and layered double hydroxide (LDH) stand out with remarkable OER performance.This is attributed to the edge-sharing octahedral MO6layered structure with more active sites on the surface[10,12].However,owing to the strong van der Waals’ forces between layer and layer,the two-dimensional (2D) nanosheets are inclined to undergo severe aggregation,leading to significant loss of active sites.Therefore,it is essential to tune the interlayered composition and structure through covalent and non-covalent interactions,for the purpose of increasing surface area,boosting electrolyte penetration,and exposing ideal crystal facets,to further improve OER activity and stability[13,14].
Transition metal silicates (TMSs) contain the ubiquitous element Si,and display some unique advantages,such as low-cost raw ingredients,flexible microstructure and robust SiO4tetrahedral skeleton.They have demonstrated great potential in the field of catalysis,energy storage,adsorption,and drug delivery[15–24].For example,Mai et al.prepared various TMSs-based materials,including nickel silicate/rGO,copper silicate hydrate/rGO,manganese silicate/GO and so on,and these TMSs-based composites showed good capacity and cycle stability in a Li-ion battery[25–28].Recently,our groups have also made some efforts to apply TMSs in electrochemical energy storage.For example,various 3D TMSs/C (Zn,Mn,Ni-silicates) were derived from bamboo leaves and they exhibited improved electrochemical performance as electrode materials for hybrid supercapacitors [29–33].Cheng et al.synthesized Co2SiO4nanobelts/GO,Co2SiO4nanobelts@MnSiO3and Co2SiO4nanobelt-on-nanobelt/rGO composites to improve the electrochemical properties of Co2SiO4in alkaline electrolyte [34–36].Considering the favorable properties of TMSs-based materials in the field of supercapacitors,it raises the question of their performance in the electrocatalysis process.This has aroused considerable interest.Qiu et al.[37,38]first demonstrated that carbon nanotubes compositing with P–incorporated nickel–cobalt silicate hydroxide showed some OER activity (440 mV @ 10 mA cm-2).Kang et al.[39]prepared layered amorphous cobalt phyllosilicate (ACP) as efficient OER catalysts and it showed an overpotential of 367 mV @ 10 mA cm-2.Although the OER activity of these TMSs is far from the level desired,there is a lot of potential to design new architectures based on TMSs to improve their OER performance.From our studies,the poor conductivity of TMSs mainly restricts their performance in various ways in electrochemistry.Dong et al.demonstrated that rGO could greatly improve the specific capacitances of TMSs in alkaline electrolyte[40–43].Thus,coupling TMSs with a conductive support is an effective strategy to accelerate ion and molecular diffusion,especially in electrocatalytic applications[44,45].The improved conductivity is beneficial for fast proton/mass transfer process,so the electron/reagent can reach the active centers more easily.Constant effort is still needed for rational design and fabrication of TMSs-based electrocatalysts to offer more accessible active centers and a fast proton/mass transfer process.To achieve the optimal active centers,the vertical growth of TMSs nanoarrays on rGO support is considered as an effective tool.
in situvertically grown metal silicate hydroxide Co2.5Ni0.5Si2O5(OH)4nanosheets on a conductive rGO support (denoted as CNS@rGO) have been developed by interlayered chemical regulation and anin situgrowth process.CNS@rGO with vertically grown nanostructures and optimized metal-support electronic interactions exhibits favorable OER activity and stability.It is superior to monometallic nanohybrids (CS@rGO and NS@rGO)and physically mixed materials(m-CNS@rGO,m-CS@rGO and m-NS@rGO).The achieved OER activity is even higher than the values of most transition metal oxide/hydroxide relevant catalysts.The improved OER performance of CNS@rGO is mainly attributed to the vertically grown nanostructure and the optimized metal-support electronic interactions.This work may lead to a new direction for rational and flexible design of practical electrocatalysts.
2.Materials and methods
2.1.Preparation of functionalized graphene oxide (GO)
The original GO nanosheet was synthesized via a modified Hummer's method[46]and the details are shown in SupportingInformation.
2.2.Preparation of SiO2@rGO template
The SiO2@rGO precursor was synthesized by a modified St¨ober method [40].First,0.15 g hexadecyltrimethylammonium bromide(CTAB,Macklin,99%) was dissolved into 70 mL ethanol and 10 mL deionized water.A 5 mL GO suspension(9.5 mg/mL)was well-dispersed into 70 mL ethanol and 20 mL deionized water under agitation(15 min,room temperature) and then ultrasonic treatment (15 min,room temperature),repeated three times.Then,the above two solutions were mixed by vigorous stirring for 10 min,followed by adding 3 mL concentrated ammonium hydroxide(NH3⋅H2O,Aladin)into the solution.Under constant stirring,1 mL tetraethylorthosilicate (TEOS,Aladin,98%)was added dropwise to ensure the slow hydrolysis of TEOS and to be uniformly combined onto the GO surface.This is of vital importance for furtherin-situgrowth specific nanostructures.Indeed,this kind of combination seems to be a chemical reductive process such that GO was converted into reduced graphene oxide(rGO),proved by some results in Supporting Information (Fig.S1).After agitation for 4 h,dark powders were obtained and washed with deionized water and ethanol through vacuum filtration,and then freeze dried(around-45°C).
For the next preparation of comparative samples,pure SiO2powder was also synthesized by the same process above without adding the GO suspension.
2.3.Preparation of vertically grown CNS@rGO electrocatalyst
In the final procedure,bimetal isin-situvertically grown on the surface of the rGO support forming metallic hydroxyl silicate through the simple hydrothermal treatment.First,0.04 g SiO2@rGO template was uniformly dispersed in 8 mL ethanol and 1 mL deionized water with 5 min ultrasonic treatment.Then,0.7 mmol cobalt (II) chloride hexahydrate (CoCl2⋅6H2O,Aladin) and 0.15 mmol nickel (II) chloride hexahydrate (NiCl2⋅6H2O,Aladin) were dissolved in the above suspension and stirred for 15 min,followed by the addition of NH3⋅H2O(4 mL).To obtain the ideal proportion of bimetal electrocatalysts,the added amount of CoCl2⋅6H2O was varied with 0.35 mmol,1.1 mmol and 1.4 mmol,denoted as CNS@rGO-1 CNS@rGO-2 and CNS@rGO-3,respectively.After being well mixed,the reaction mixture was transferred to a 50 mL autoclave at 180°C for 24 h.After the hydrothermal reaction,the black powders were obtained and washed with deionized water and ethanol through vacuum filtration,and then freeze dried.The resulting product was Co2.5Ni0.5Si2O5(OH)4@rGO,denoted as CNS@rGO.The corresponding contents of Co and Ni were calculated from Inductive Coupled Plasma Emission Spectrometer(ICP)results(Table S1).
To further illustrate the structure-activity relationship,in particular the essential role of metal-support electronic interactions for enhanced electrocatalytic performance,the compared monometallic nanoarrays Co3Si2O5(OH)4@rGO and Ni3Si2O5(OH)4@rGO were prepared by the same process above containing only one metal in the solution,denoted as CS@rGO and NS@rGO,respectively.
The vertically grown nanoarray with interlayer covalent interaction is significant in electrocatalytic performance improvement.The physical mixed samples were developed to prove this point.Metal silicates were first synthesized through the same hydrothermal reaction above using pure SiO2powder as silicon source rather than SiO2@rGO composite,and then ultrasonic mixing the same amount of GO(9.5 mg/mL,5 mL)with each metal silicate powder,marking “m-” in front of the names of the three samples.Thus they were m-CS@rGO,m-NS@rGO and m-CNS@rGO,respectively.
2.4.Material and electrochemical characterizations
All materials and electrochemical characterizations are presented in detail in SupportingInformation.
3.Results and discussion
The vertically oriented CNS@rGO nanoarrays are developed by interlayer chemistry regulation and thenin-situgrowth progress,as shown in Fig.1a.First,an SiO2cover layer uniformly associates to both sides of GO surfaces,through the hydrolysis-condensation reaction between TEOS and the surface functional groups of the GO layer.In detail,the inert GO plane with perfect hexagonal structure can be activated by the covalently bonded [-Si-O-Si-]nunits,through regulating the inert interlayer van der Waals interactions by electron perturbation[13].This brings about the new 2D SiO2@rGO plane with more suitable electronic states in return.Because of the strong metal-support electronic interaction,the bimetal cobalt-nickel precursor adsorbs on the anionically charged 2D surface of SiO2@rGO templates,and the coprecipitation reaction happens during the hydrothermal process.The covalently linked units[SiO4]2-tetrahedral can not only assist the growth of Co,Ni silicates in the direction vertical to the rGO substrate,but also effectively keep the layered bimetal nanosheet away from aggregation[47].
X-ray diffraction(XRD)analysis is performed to gain insights into the structure and chemical composition of CNS@rGO nanoarrays.As shown in Fig.1b,the bimetal CNS@rGO nanoarrays has a similar crystal structure to both of the monometallic CS@rGO (No.21–0872) and NS@rGO(No.49–1859).The constant diffraction peaks of CNS@rGO do not show evident shifts and phase impurities,indicating lattice matching between Ni and Co atoms in CNS@rGO[48].Since the atomic radii of Co and Ni are similar,coprecipitation reaction is more likely to occur under hydrothermal treatment.Simultaneous growth bimetals can rely on mutual lattice-substitution effects[49].The basic diffraction peak of GO(001) plane (2θ=11.9°) transforms into a broader peak in SiO2@rGO,indicating the formation of amorphous substrate with sp3hybrid carbon atoms,as shown in Fig.S1.This kind of lattice transformation proves that the interlayered support (rGO) is in a reduced state [50],which is confirmed by Fourier-transform infrared spectra (FTIR) analysis discussed in the following.An emerging low crystallinity occurs in SiO2@rGO during the interlayer covalent regulation,indicating a long-range disordered structure.The inert hexagonal GO plane is activated in this process [51].Low crystallinity is still prominent in the vertically grown nanoarrays CNS@rGO,CS@rGO and NS@rGO(Fig.1b),suggesting that their structure is also long-range disordered and unsaturated.In brief,vertically oriented nanoarrays are expected to induce and expose more unsaturated active centers.This significant structural advantage is further proved through comparing their OER performance with physical mixture samples,which contain the interfacial structure without the covalent force.
FTIR is performed to provide more structural information for these vertically grown nanoarrays to illustrate the vital role of covalently integrated “[SiO4]2-bridge” and metal-support electronic interactions,as shown in Fig.1c and Fig.S2.The deformation vibration of hydroxyl groups can be observed in all the samples with a broad band around 3400 cm-1[52].Vibrations of CO,CC and two types of epoxy C–O bonds locate at 1729,1618,1420 and 1066 cm-1,affirming the existence of oxygen functionalities on the initial GO nanosheet[43].Compared to the initial GO ingredient,SiO2@rGO exhibits significantly enhanced C–H vibrations in the range of 3000–2800 cm-1.This can be specified as the terminal alkyl-groups of the grafted [-Si-O-Si-]nunits [53],as shown in Fig.S2.The strong and broad band of Si–O–Si(C)at 1069 cm-1,as well as the Si–O stretching and bending vibrations at 956,793 and 455 cm-1,indicate the formation of covalently bonded[-Si-O-Si-]nunits [28].The seemingly disappeared CO vibrations in SiO2@rGO further prove the covalent linkage(-Si-O-C)between TEOS and the oxygen functionalities on the GO support[53].The electronic perturbation would be introduced to the interlayered rGO support during this covalent regulation progress,resulting in the electron deficient surface appearing on the SiO2@rGO template.As revealed in previous work,TEOS containing active terminal groups is inclined to covalently bond to oxygen-functional groups and self-assemble into a monolayer on the surface of the target support,resulting in an electrophilic surface with abundant silanol groups [42].Accordingly,the electrophilic surface of SiO2@rGO provides new opportunities for further vertical growth of metal nanoarrays.In comparison to SiO2@rGO,all three nanohybrids exhibit the lower wavenumbers of Si–O and Si–O–Si vibrations,implying the increase of bond lengths and electronic density.It can be confirmed that the support serves as the electron receiver no matter it is in the mono-/bi-metal coordinated structure [54].More detailed insights into the differences of electronic effects can be obtained by comparing the position of Si–O–Si(C)and Si–O bonds in the three nanoarrays.As displayed in Fig.1c,the vibrations of CS@rGO locate in the lowest wavenumbers,indicating more electron aggregation on Si–O bonds,while the highest position of NS@rGO represents the least electron accumulation.Notably,CNS@rGO with a middle wavenumber reflects a moderate electronic intensity,suggesting that there are interactions between lattice Co and Ni atoms consistent with the XRD results[55].Based on the above results,on the one hand,anin-situvertically grown bimetal CNS@rGO is not simply two phases blending with electrons adding together,but a co-growing dual-atomic plane.On the other hand,the appropriate metallic state would be of benefit for balancing the adsorption/desorption behavior in electrocatalysis.
Raman spectroscopy of the three samples (CNS@rGO,CS@rGO,NS@rGO)is used to study and confirm the stability of rGO substrate,as shown in Fig.1d.All samples show two typical bands located at around 1601 (G-band) and 1348 cm-1(D-band),which correspond to in-plane vibration of polyaromatic sp2carbon atoms and activation resonance of sp3carbon atoms,respectively[56].The lattice-defect or graphitization degree of the rGO support is reflected by the intensity ratio of D and G bands (ID/IG) [57].As shown in Fig.1d,the negligible changes of ID/IGsuggest that growing metal silicates would not damage the structure of the interlayered rGO support,and rGO have already reached its highest defect level during the interlayer covalent regulation process [50].No matter that mono/bi-metal is involved in the second hydrothermal step,metal precursors react directly to the “[SiO4]2-bridge” rather than the oxygen functionalities of the rGO support,as indicated by the unchanged graphitization.Therefore,it can be shown that nanoarrays can bein-situgrown on the rGO support by a “[SiO4]2-bridge”.The stable rGO conductive substrate not only contributes to the geometric structural stability of these vertical nanoarrays,but also benefits the electronic regulation between metal and support.
X-ray photoelectron spectroscopy (XPS) analysis was carried out to gain further insight into the elemental composition and electronic states of the electrocatalysts,especially the optimized metal-support electronic interactions,as is seen in Fig.2.The XPS survey spectra reveal the main components of the three samples(CNS@rGO,CS@rGO,NS@rGO),containing C,O,Si,and the corresponding mono-/bi-metal Co,Ni elements(Fig.2a).The C 1s orbital can be deconvoluted into three subpeaks,as defined into C––O,C–O,and C–C/C––C bonds,indicating the existence of functionalized rGO support (Fig.2b) [56].The unchanged position and intensity further suggest the structural stability of the interlayered rGO support,in good agreement with the Raman results.High-resolution Si 2p spectrum shows that Si–O bands in CNS@rGO locate between CS@rGO and NS@rGO (Fig.2c),indicating the moderate electron aggregation on the support.It may further be inferred that some donated electrons from lattice Co atoms to support may be attracted by lattice Ni atoms owing to their slightly higher electronegativity.CNS@rGO still shows a slightly higher electron state than NS@rGO,reflecting the dominant role of lattice Co atoms in electron donating.Therefore,CNS@rGO exhibits the optimized metal-support electronic interactions.This can effectively increase the electron density of electrocatalysts,agreeing well with the FTIR results.From another point of view,if stoichiometric Co and Ni species form two phases without interactions,both would transfer electrons directly to the support,and then the Si–O bonds in bimetal composites would aggregate more electrons than both its monometallic components.Fig.2d displays the high-resolution Co 2p spectra of CS@rGO and CNS@rGO.Co 2p1/2(798.4 eV) and Co 2p3/2(782.2 eV) peaks of CNS@rGO locate at a higher binding energy,indicating the optimized electron structure of Co with more electron depletion and higher oxidation state [58].Considering further the high-resolution Ni 2p spectra in Fig.2e,CNS@rGO has a slightly negative shift of Ni 2p1/2(874.6 eV) and Ni 2p3/2(857.2 eV) in contrast to NS@rGO,suggesting the slightly increased electron accumulation on Ni atoms[59].Therefore,in a CNS@rGO nanohybrid,electrons of Co atoms not only transfer to Si–O bonds of the support but also are partially attracted by lattice Ni atoms,contributing to more unsaturated Co centers.O 1s XPS spectra are further used to confirm the results above,as shown in Fig.2f.The two pairs of fitting peaks are assigned to the C–O and CO vibrations in the rGO support,as well as Si–O and M -O vibrations in metal silicate hydroxide,respectively [60].In these three samples,the constant C–O and CO bands indicate the invariable nature of the functionalized rGO support,agreeing well with the discussion above.The relatively high M -O intensity in CNS@rGO suggests the existence of more coordinate metal atoms.In brief,the vertically grown bimetal nanoarrays contain the optimized metal-support electronic interactions increasing the electron density of active metal centers (Lewis acid)effectively.Based on the Lewis acid-base theory,these kinds of sites are capable to adsorb and activate more H2O (Lewis base) molecules[61].In addition,the differences of the dual-atomic electronic density also boost the H2O molecule cleavage and the intermediate conversion,so that a favorable OER performance is induced in CNS@rGO nanoarrays.
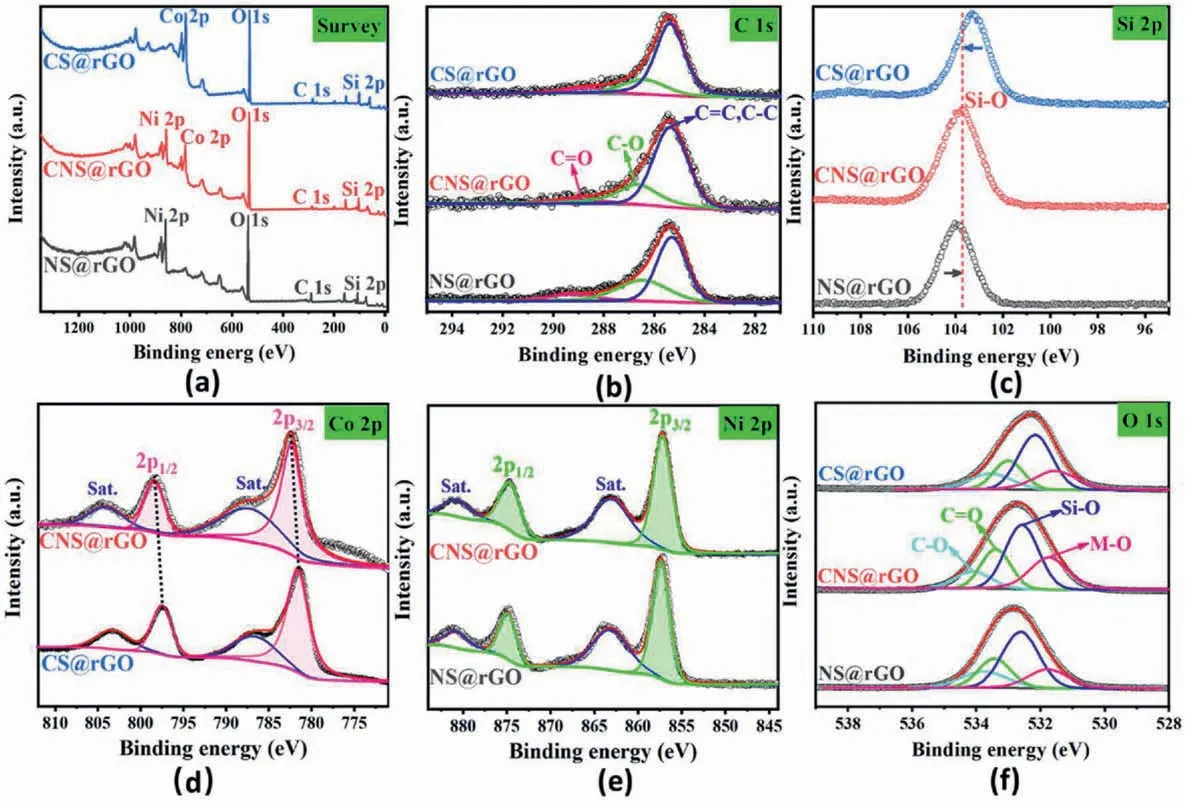
Fig.2.Electronic structure characterizations of CS@rGO,NS@rGO and CNS@rGO: Full scan XPS spectrum (a),C 1s(b),Si 2p (c),Co 2p (d),Ni 2p (e),and O 1s (f)XPS spectrum.
The Brunauer Emmett Teller (BET) specific surface area and porous structures of the vertically grown nanoarrays CNS@rGO,CS@rGO and NS@rGO were characterized by the N2adsorption method.As plotted in Fig.3a,all three electrocatalysts show classical type IV isotherms with an H3-type hysteresis loop,representing the existence of a mesoporous structure [30].According to the pore size distribution curves,NS@rGO exhibits the lowest percentage of mesopores around 3.5 nm among the three nanoarrays.This may be explained by the geometric structural differences of the vertically grown metal silicate hydroxides.NS@rGO contains larger-sized nanosheets vertically grown on the rGO support,resulting in a decreased interface binding between them,and thus the low mesopore porosity would be owing to the low connectivity of NS@rGO.Comparing the three electrocatalysts,an increase of surface area can be observed with the decreased size of the vertically grown nanoarrays.The specific surface area is calculated to be 285,215 and 92 m2/g for CNS@rGO,CS@rGO and NS@rGO,respectively.It can be concluded that the more interconnected nanoarrays grown on the rGO support,the greater the increase in the specific surface areas.The stable “[SiO4]2-bridge” may also contribute to the increased surface areas through effectively preventing the layered-and-layered aggregation of nanosheets.Therefore,CNS@rGO contains a significantly increased surface area and mesopore content.This will provide more accessible surface for effective electrolyte penetration.
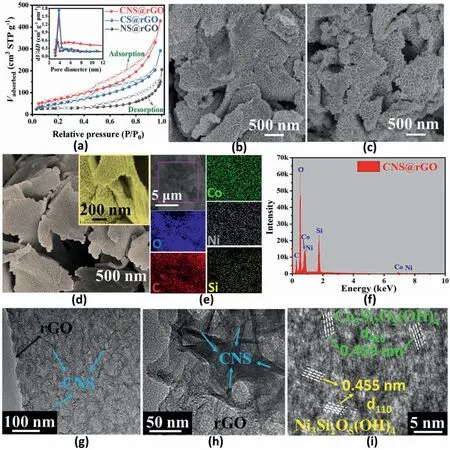
Fig.3.N2 adsorption-desorption isotherms;inset:BJH pore-size distribution(a).SEM images of vertically grown nanoarrays CS@rGO(b),NS@rGO(c)and CNS@rGO(d).SEM element maps (e) and corresponding EDS images (f) of CNS@rGO.TEM images of CNS@rGO with different magnifications (g,h,i).
A scanning electron microscopy (SEM) image shows the vertical nanoarray morphology in these samples.Initially,the thickness of the original GO nanosheet evidently increases with the aid of interlayer covalent regulation,generating the homogeneous SiO2cover layers on its surface,as shown in Fig.S3.The 2D topological structure of SiO2@rGO templates may be further expanded throughin-situgrown metal silicate hydroxides,resulting in the favorable vertically distributed nanostructures,as can be seen from the resulting nanoarrays of CS@rGO(Fig.3b and S4a),NS@rGO(Fig.3c and S4b)and CNS@rGO(Fig.3d and S5a).None of the CS@rGO and NS@rGO structures can be observed in CNS@rGO.The size ofin-situvertically grown nanosheets in CNS@rGO is much smaller than the monometallic nanoarrays (CS@rGO and NS@rGO).The physical mixed samples contain a smooth GO plane and an isolated metal silicate sphere (Fig.S6),which is in sharp contrast to the vertically grown samples.Metal silicate is inclined to be packaged by the thin GO plane,indicative of the Van der Waals force between them.Thus,CNS@rGO nanoarrays is a‘co-growing’of the bimetal components instead of a simple two-phase physical blending,agreeing well with the XPS results.Moreover,the smaller sized nanoarrays have more contact with support,and thus,CNS@rGO exhibits a more interconnected interface,in line with the BET analysis.Such a novel nanostructure is expected to expose more active phases and to shorten proton/electron diffusion length.This plays an important role in the surface reaction of OER[62].In addition,energy dispersive X-ray spectrum(EDS)mapping analysis indicates a coincided and uniform distribution of Si,O,C,Co and Ni throughout the CNS@rGO(Fig.3e and f),and only metal Co or Ni is evenly distributed in CS@rGO and NS@rGO,as shown in Fig.S7 and Fig.S8.
Transmission electron microscopy(TEM)was performed to gain more insight into overall structural information.At low magnification of CNS@rGO (Fig.S5b),CS@rGO (Fig.S4c) and NS@rGO (Fig.S4d),all three samples inherit the flake structure of the rGO support forming flat nanosheets,which also supports the conclusion above that the structure of interlayered rGO support will not be damaged regardless of growing mono-/bi-metal components.Further enlarged images for CNS@rGO(Fig.3g and h),CS@rGO(Fig.S4e)and NS@rGO(Fig.S4f)display thein situvertically grown nanoarrays.The size of vertically grown nanosheets in CNS@rGO is much smaller than CS@rGO and NS@rGO,agreeing well with the SEM analysis.The high-resolution TEM(HR-TEM)image reveals the lattice fringes of CNS@rGO(Fig.3i).The lattice spacing of 0.455 and 0.459 nm corresponds to the(110)and(020)plane of Ni3Si2O5(OH)4and Co3Si2O5(OH)4,respectively.TEM findings provide intuitive evidence that metal silicate hydroxides canin-situvertically grow on both sides of the rGO support[63].Such highly exposed nanostructures contribute to the reactant proximity,intermediate conversion,as well as gas product migration.
In order to illustrate the role of vertically grown bimetal nanoarrays for improved OER behavior,a linear sweep voltammetry (LSV) test is conducted on the relevant samples,including CNS@rGO,CS@rGO,NS@rGO as well as their corresponding physical mixed samples m-CNS@rGO,m-CS@rGO,m-NS@rGO,under 5 mV s-1sweep rate,in 1 mol L-1KOH electrolyte (Fig.4a and b).At an overpotential @ 10 mA cm-2,all the vertically grown nanoarrays deliver a better catalytic property than the corresponding physical mixed samples modulated by Van der Waals interactions.Thus it can be concluded that vertically grown nanoarrays with covalently regulated interface play the predominant role in OER activity improvements.Both of the Co-containing samples (CS@rGO and m-CS@rGO) perform better than monometallic Ni electrocatalysts(NS@rGO and m-NS@rGO),suggesting the significant role of Co atoms in the OER process with a more electron-donating effect.Bi-metal synergetic catalysts(CNS@rGO and m-CNS@rGO)exhibit more desirable activity than all the monometallic samples.This is highly related to the optimized metal-support electronic interaction.The influence of bi-metal ratio on OER performance is further illustrated(Fig.S9),and CNS@rGO is identified as the most active type.Although Co has a better electron-donating ability,the appropriate amount of Co can promote the electrocatalyst to reach best performance.The vertically grown bi-metal CNS@rGO nanoarrays exhibit the best catalytic property with the overpotential of 307 mV @ 10 mA cm-2in 1.0 M KOH,outperforming most of the non-precious metal catalysts reported to date,including various of the transition metal oxide/hydroxides as summarized in Table S2 and Fig.4g.They even outperform the commercial IrO2/C,with overpotentials in the 320–350 mV range[64–66].Catalytic activity at high current density should be considered when developing more practical catalysts.CNS@rGO achieve a reasonably low overpotential (446 mV) @ 100 mA cm-2,which is better than the standard RuO2catalyst (630 mV) [67],as shown in Fig.4c.Excepting the geometric activity above,it is necessary to evaluate the intrinsic activity of electrocatalysts more accurately,so the activity of these nanoarrays is also normalized by intrinsic metrics,including both specific activity[electrochemical active surface areas (ECSA) normalization]and turnover frequency (TOF).Fig.4d shows the ECSA-normalized LSV curves.CNS@rGO still has excellent performance compared to the two monometallic samples(CS@rGO and NS@rGO).However,the specific activity of NS@rGO is better than CS@rGO contrary to the geometric-area based trend.So it can be confirmed that there are positive interactions between lattice Co and Ni centers in CNS@rGO,and the electrochemical activity of per Ni centers is higher than per Co centers in monometallic nanoarrays(CS@rGO and NS@rGO).The calculated TOF of the three samples is shown in Fig.4e.The TOF value of CNS@rGO at overpotential of 307 mV is 4.6239E-05 s-1,much higher than CS@rGO(1.02169E-06 s-1)and NS@rGO (1.81089E-06 s-1),proving the point of the above ECSA-normalized analysis.Because TOF reflects the activity of each active metal site and the vertically grown nanoarrays expose a large number of active metal centers,its TOF may be smaller than some bulk catalysts with fewer active sites.Through analyzing the electronic and geometric structures of each sample,it can be revealed that both optimized metal-support electronic interactions and vertically grown interfacial layers play an indispensable role for OER performance improvements [68].In order to confirm the critical role of optimized metal-support electronic interactions and vertically grown nanostructure,more detailed investigations on electrocatalytic properties will be performed.
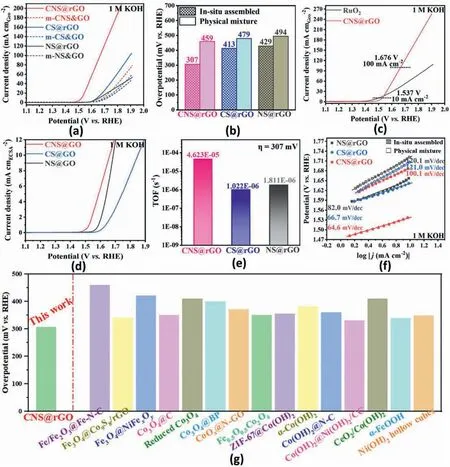
Fig.4.(a)LSV polarization curves of different vertically grown nanoarrays(CNS@rGO,CS@rGO,NS@rGO)and their corresponding physical mixed samples through Van der Waals interactions(m-CNS@rGO,m-CS@rGO,m-NS@rGO),(b)comparing analysis of overpotential@10 mA cm-2,(c)LSV curves of RuO2 and CNS@rGO.(d)ECSA-normalized LSV polarization curves.(e)TOF calculated by normalizing the current at η=307 mV.(f)Tafel slopes.(g)Comparison of the OER overpotential of vertically grown nanoarrays CNS@rGO with previously reported transition metal oxide/hydroxides electrocatalysts @ 10 mA cm-2.
Tafel slopes are determined solely by the OER reaction kinetics,which also reflect the intrinsic activity of the aforementioned samples.When compared to the physical mixed catalysts,the Tafel slopes of vertically grown nanoarrays decrease significantly,as shown in Fig.4f.The high-performance CNS@rGO catalyst also exhibits the lowest slope(64.6 mV/dec),which is smaller than both the monometallic nanohybrids (CS@rGO 66.7 mV/dec and NS@rGO 82.0 mV/dec),indicating its superior OER reaction kinetics.Additionally,the high Tafel slopes in physical mixed samples are nearly equal,indicating a similar reaction approach and rate-controlling steps [39].Therefore,the advanced interface containing vertically grown nanosheets more readily provides a fast electron transfer pathway,using the electronic perturbation along the plane of the rGO support more efficiently.Optimized metal-support electronic interactions may further increase the dual-atomic electron density,possessing more suitable electronic states for rapid electron/mass migration,confirming the FTIR and XPS results.In the OER process,according to previous research,the Tafel slope can assess the rate-determining process[69].The 1st,2nd and 3rd elementary reaction steps correspond to the Tafel slope values with 120,60 and 40 mV dec-1,respectively.In the case of vertically grown nanoarrays,the second elementary reaction is recognized as the rate-determining step with the slopes close to 60 mV dec-1,which differs from the physical mixed catalysts(the first elementary reaction step).
Nyquist plots are used to investigate the electron-transfer kinetics of catalysts,which are evaluated at the open circuit potential.In the highfrequency region (Fig.5a),each of the vertically grown nanoarrays shows nearly linear EIS curves without a semicircle,implying fast electron-transfer kinetics for the OER process [70].Compared to NS@rGO,lattice Co atoms in CS@rGO are inclined to donate more electrons to the support,so as to accelerate the reaction kinetics.In addition,CNS@rGO shows the highest slope in the Nyquist plot,indicating that this more suitable electronic state is more valuable for fast mass transfer.Hence,it can be concluded that CNS@rGO exhibits excellent electron transport behavior owing to the optimized metal-support electronic interactions.

Fig.5.(a)Nyquist plots,capacitance current differences(Δj)plotted against scan rates(b),and(c)Chronopotentiometry experiments of the commercial RuO2,and asprepared CNS@rGO,CS@rGO and NS@rGO nanohybrids@10 mA cm-2.LSV polarization curves before and after 1000 CV cycles of CNS@rGO(d),CS@rGO(e),and NS@rGO (f);the inset shows corresponding CV curves at the first and thousandth cycles.
The ECSA of each nanoarray is measured by double-layer capacitance(Cdl),and this is recorded by cycling the electrode in non-Faradaic regions at different scan rates.The values of ECSA reflect the reactive surface of the catalyst.Cdlis twice the slope of capacitance current (Δj)vs.scan rate.As demonstrated in Fig.S10,the anodic (janodic) and cathodic current (jcathodic) of each nanoarray is detected at 1.15 V (vs.RHE),deriving from Δj=janodic-jcathodic[71].Fig.5b shows that the ECSA of CNS@rGO is larger than both monometallic nanohybrids(CS@rGO and NS@rGO),indicating more active sites for the OER process.These vertically grown nanoarrays with large specific surface area are helpful in providing more active sites.However,the optimized metal-support electronic interactions seem to contribute more to the enhanced activity[72].
Chronopotentiometry experiments are applied to evaluate the durability of CNS@rGO,CS@rGO and NS@rGO.CNS@rGO shows the best stability decrease of only 5% after an 18 h long-term test.This is almost comparable to the commercial RuO2with a 3.7% decline.CS@rGO and NS@rGO decrease rapidly at the very beginning,and undergo around 12% and 14% drop off after a 14 h stability test (Fig.5c).The great durability of CNS@rGO is further proved by a non-decreased LSV polarization curve after 1000 CV cycles (Fig.5d).CS@rGO performs with better stability than NS@rGO(Fig.5d and f),which may be attributed to more electron transfer effects from lattice Co atoms to rGO supports,possessing stronger metal-support interactions.SEM morphology images of CNS@rGO (Fig.6a and S11a) and CS@rGO (Fig.6b and S11b) after stability testing are in good accordance with the observations before the measurements.In spite of some polymer adhesive covered on their surface,vertically grown nanoarrays are still well preserved without migrating and/or aggregating into a large agglomerate.The remarkable dissociation of nanoarrays from the rGO support can be discovered in NS@rGO (Fig.6c and S11c),which may also reflect our suggestion above.NS@rGO possesses relatively weak metal-support interactions because of less electron transfer from lattice Ni atoms to the rGO support,resulting in the poor structural stability.An XRD pattern of CNS@rGO after stability testing further confirms the structural stability of the bimetal nanoarrays,as shown in Fig.S12.As a result,the particular structural advantages (vertically grown nanoarrays) has benefit for improving the stability,although metal-support electronic interaction seems to contribute more,as demonstrated by bi-metal CNS@rGO which has the best durability.
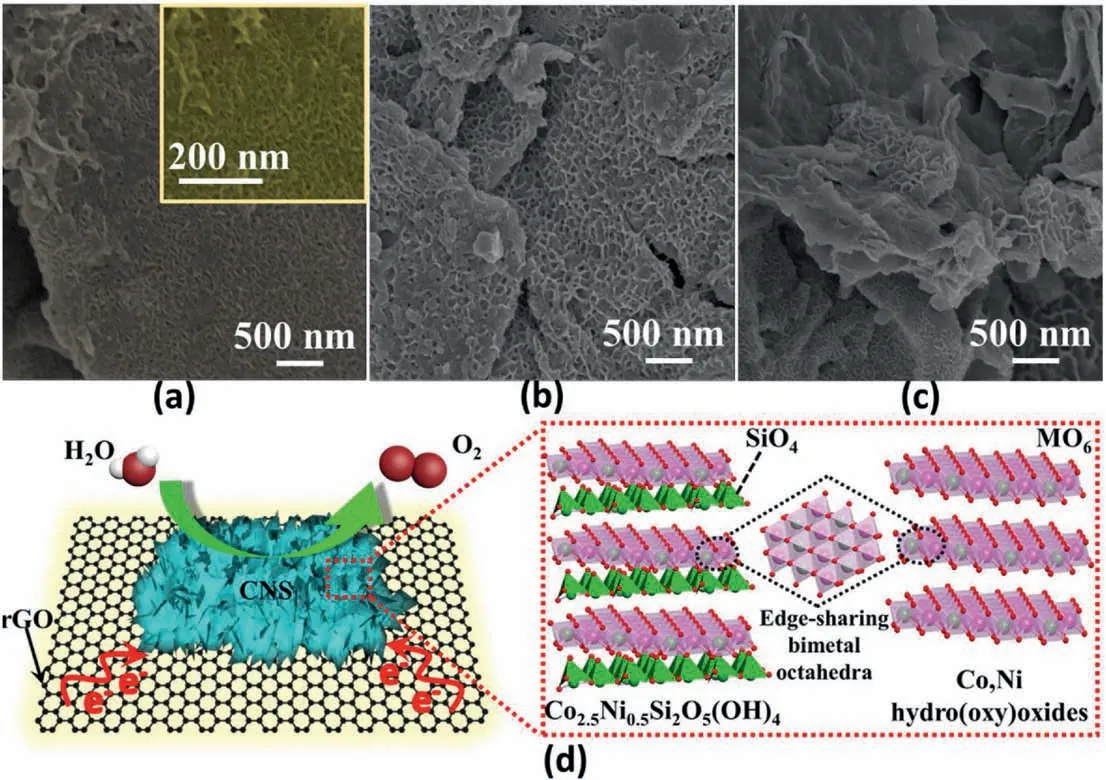
Fig.6.SEM images of CNS@rGO(a),CS@rGO(b)and NS@rGO(c)after durability measurements.(d)Schematic illustration of the geometric and electronic effects of CNS@rGO electrocatalyst with enhanced OER performance.
The above results suggest that CNS@rGO nanoarrays exhibit fascinating OER performance.Fig.6d illustrates that both the vertically grown nanostructure and the optimized metal-support electronic interactions of CNS@rGO contribute to the high OER performance.(1) Thein-situvertically grown nanoarrays contain the stable “[SiO4]2-bridge”,which significantly prevents the aggregation of bi-metal nanosheets.This feature provides a large surface area with more exposed active centers,and this facilitates the migration of various reactants and products.(2)Thein-situvertically grown nanoarrays with interconnected interface makes for a short proton/electron diffusion distance.The simultaneously introduced electron perturbation provides a fast electron transfer pathway along the rGO support,thus improving the OER kinetic process effectively.(3) Optimized metal-support electronic interactions modify the electronic configuration and surface chemistry of catalysts,generating more unsaturated Co centers with high oxidation states and different electronic density between lattice Co and Ni atoms.The above characteristics are of benefit for H2O molecular adsorption,cleavage and intermediates conversion.Vertically grown CNS@rGO nanoarrays exhibit enhanced OER stability and activity resulting from such geometric and electronic structural advantages.
4.Conclusion
In summary,an advanced CNS@rGO electrocatalyst has been synthesized by interlayer chemistry regulation and then anin-situgrowth progress,comprising of vertically grown bimetal nanosheets on an rGO support.Compared with CS@rGO and NS@rGO nanoarrays,CNS@rGO further assembles the fine architectures,and presents favorable OER performance.The overpotential can be achieved at 307 mV @ 10 mA cm-2and 446 mV @ 100 mA cm-2,which is 1.4 times that of RuO2@100 mA cm-2.First,vertically grown CNS@rGO contains an interconnected interface and maximum active site exposure.This makes a great contribution to the enhanced OER performance.Secondly,lattice Co and Ni atoms optimize the electron transfer to the rGO support,providing more unsaturated Co centers and electronic density differences in dual-atoms.Finally,electron perturbation in the support promotes electron conduction and a multi-electron transfer process.This work provides a new approach to designing TMS architectures with suitable OER activity.Taking advantage of earth-abundant elements (O,Si,Ni and Co) in the catalyst,this work may open a new way to meet the industrial demand for OER progress.
Declaration of competing interest
None
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (DUT21LK34) and Natural Science Foundation of Liaoning Province(2020-MS-113).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2022.04.002.
杂志排行
Namo Materials Science的其它文章
- Advancing the pressure sensing performance of conductive CNT/PDMS composite film by constructing a hierarchical-structured surface
- Water-based synthesis of nanoscale hierarchical metal-organic frameworks:Boosting adsorption and catalytic performance
- An overview of recent progress in the development of flexible electrochromic devices
- Wearable and stretchable conductive polymer composites for strain sensors:How to design a superior one?
- Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review
- Synergistic coupling of 0D–2D heterostructure from ZnO and Ti3C2Tx MXene-derived TiO2 for boosted NO2 detection at room temperature
