Growth hormone promotes the reconstruction of injured axons in the hypothalamo-neurohypophyseal system
2024-01-24KaiLiZhanpengFengZhiweiXiongJunPanMingfengZhouWeizhaoLiYichaoOuGuangsenWuMengjieCheHaodongGongJunjiePengXingqinWangSongtaoQiJunxiangPeng
Kai Li ,Zhanpeng Feng ,Zhiwei Xiong ,Jun Pan,Mingfeng Zhou,Weizhao Li,Yichao Ou,Guangsen Wu,Mengjie Che,Haodong Gong,Junjie Peng,Xingqin Wang,Songtao Qi ,Junxiang Peng
Abstract Previous studies have shown that growth hormone can regulate hypothalamic energy metabolism,stress,and hormone release.Therefore,growth hormone has great potential for treating hypothalamic injury.In this study,we established a specific hypothalamic axon injury model by inducing hypothalamic pituitary stalk electric lesions in male mice.We then treated mice by intraperitoneal administration of growth hormone.Our results showed that growth hormone increased the expression of insulin-like growth factor 1 and its receptors,and promoted the survival of hypothalamic neurons,axonal regeneration,and vascular reconstruction from the median eminence through the posterior pituitary.Altogether,this alleviated hypothalamic injury-caused central diabetes insipidus and anxiety.These results suggest that growth hormone can promote axonal reconstruction after hypothalamic injury by regulating the growth hormone-insulin-like growth factor 1 axis.
Key Words:arginine vasopressin;growth hormone;hypothalamo-neurohypophyseal system;hypothalamus;injury;insulin-like growth factor 1;oxytocin;regeneration
Introduction
Hypothalamic syndrome is a disorder caused by injury to the hypothalamus.It is characterized by severe morbid obesity,multiple endocrine abnormalities,memory impairment,attention deficit and reduced impulse control,and increased risk of cardiovascular disease and metabolic disorder (Müller et al.,2022).Central diabetes insipidus (CDI),due to impaired secretion of arginine vasopressin (AVP) from the posterior pituitary,is characterized by polyuria,polydipsia,low urine specific gravity (USG),and decreased serum vasopressin levels (Christ-Crain et al.,2019).Most cases of acquired CDI result from destruction of the neurohypophysis,which can be caused by space-occupying lesions that destroy vasopressin neurons (by squeezing or infiltration),or injury due to surgery,especially after the removal of tumors near the hypothalamus(such as craniopharyngiomas,germ cell tumors,gliomas,Rathke’s cysts,and Langerhans cell hyperplasia) (Müller et al.,2019;Angelousi et al.,2021;Yang et al.,2022).In our previous study (Zhou et al.,2022),we established a CDI model by pituitary stalk electrical lesion (PEL) surgery in mice.The model initially revealed manifestations of CDI syndrome in mice,but how to further treat CDI and improve its prognosis remains a great challenge.
Growth hormone (GH) is secreted in a pulsatile mode with peaks and troughs (higher at night than during the day)mediated by a reduction in tonic hypothalamic somatostatin secretion (Veldhuis et al.,2008;Cen et al.,2022) through a complicated functional network termed the ‘GH–insulin-like growth factor (IGF) axis’.Following GH–GH receptor binding,several intracellular signaling pathways are activated,including the Janus kinase (JAK)–signal transducer and activator of transcription (STAT;particularly STAT5) pathway.Activation of STAT5 induces STAT5 dimerization and nuclear translocation,which promotes the transcription of various genes,such as those encoding IGF1 and IGF2 (Ranke and Wit,2018).
GH holds great potential in treating many neurological disorders including peripheral neuromuscular injury,traumatic brain injury,neonatal hypoxia,and stroke (Ong et al.,2018;Wright et al.,2020).In addition to its classical effects on growth and metabolism (Furigo et al.,2019;Al-Massadi et al.,2022),GH is therapeutically implicated in a number of neurorestorative effects within the central nervous system(CNS),including enhanced neurogenesis,angiogenesis,and axonal regeneration (Martinez-Moreno et al.,2019;Jung et al.,2021).Numerous clinical and preclinical studies have shown that GH plays a role in promoting IGF-1 expression,enhancing vascular endothelial growth factor levels,promoting synaptic plasticity,fostering proliferation of neural progenitors in the peri-infarction area,and supporting cognitive and motor function in the hippocampus.(Feeney et al.,2017;Jung et al.,2017).Other studies have shown that GH can function through the phosphorylated STAT5 (p-STAT5)/IGF-1 pathway(Ashpole et al.,2015;Carter-Su et al.,2016;Ranke and Wit,2018).However,this pathway is progressively attenuated in the CNS during development (Ashpole et al.,2015),while in adults,the liver responds primarily to this pathway,as IGF-1 appears to be mostly secreted from the liver (Ranke and Wit,2018).
As GH exhibits rhythmic pulse secretion under physiological conditions,the method of peripheral intraperitoneal (i.p.)administration of GH has been shown to be superior to continuous administration,as previously reported (Walser et al.,2014).The hypothalamus has a weaker blood-brain barrier than other brain areas,thus a stronger response to GH is observed in the hypothalamus (Wasinski et al.,2021).Considering that previous studies have shown that GH can regulate hypothalamus-related energy metabolism,stress,and hormone release (Furigo et al.,2019;Quaresma et al.,2021),GH treatment has great potential for functional improvements after hypothalamic injury.
The CNS (including the cerebral cortex,hippocampus,spinal cord,and optic nerve) and peripheral nervous system exhibit structural plasticity and self-repair compensatory capabilities under injury and other pathological conditions (Peng et al.,2022;Varadarajan et al.,2022).Upon axonal injury,improving the survival of injured neurons and promoting axon regeneration has always been a major concern (Adams and Gallo,2018;Varadarajan et al.,2022).In an optic nerve injury model,the death rate of retinal ganglion cells was reportedly as high as 80% (Jacobi et al.,2022).After pituitary stalk injury,there is also a large amount of neuronal death (Zhou et al.,2019).Using a hypothalamic PEL-induced injury model,in this study,we preliminarily explored the effect and mechanism of GH treatment in magnocellular neurons,providing new avenues for the treatment of hypothalamic syndrome.
Methods
Animals
Male C57BL/6 mice (license No.SCXK [Yue] 2021-0057),weighing 21 g (18–23 g),aged 8–10 weeks,and male Sprague-Dawley rats (for RNA-seq and electron microscopy,license No.SCXK [Yue] 2021-0041),weighing 220 g (200–260 g) were purchased from the Experimental Animal Center of Southern Medical University (Guangzhou,China) and included in this study.All animals were housed individually in metabolic cages (DXL-XS,FENGSHI,Suzhou,China) under temperaturecontrolled conditions (20°C,40% humidity) with a daily 12-hour dark/light cycle.Male mice were used as their hormone pattern differs from females,and could affect the results.Animals were randomly allocated to sham surgery,3 days post-PEL,7 days post-PEL,and 10 days post-PEL groups (n=5 per group).At each time point,animals were treated with normal saline or GH.All animal experiments were approved by the Animal Ethics Committee of Southern Medical University (approval No.NFYY-2020-0737) on July 10,2020.All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE)guidelines (Percie du Sert et al.,2020).
Hypothalamus injury model and GH treatment
PEL surgery was performed in mice according to a previous report (Zhou et al.,2022).Briefly,mice were deeply anesthetized with 75 mg/kg sodium pentobarbital (Micxy reagent,Chengdu,Sichuang,China) by i.p.injection.After skin preparation and disinfection,the mouse head was fixed on a stereotaxic frame (RWD Life Science Inc.,Shenzhen,China).A bone window (1.9 mm wide) was then opened halfway through the bregma.A three-dimensional (3D)printed electrocautery knife (0.8 mm wide and 0.5 mm thick) was inserted along the sagittal midline until it reached the skull base (more than 6 mm below the skull surface).A cathodic current of 0.3 mA was applied for 30 seconds with a constant power output (53500,UGO Basile,Gemonio,Italy).The depth of the in-feed was also 6 mm in the control group,but no current was applied.After surgery,all mice were returned to their original metabolic cages and their daily water consumption (DWC),daily urine output (DUV),and specific gravity of urine (USG) were monitored for 7 days.After surgery,an i.p.injection of 0.7 mg/kg of recombinant human growth hormone (rhGH) (Jintropin,Changchun,Jilin,China) was given twice daily as a pulsatile GH treatment until sacrifice,with a dose of 20 mg/kg rhGH given before sacrifice to fully activate STAT5 (Walser et al.,2014;Zhang et al.,2014;Furigo et al.,2019;Sanchez-Bezanilla et al.,2022;Figure 1A).
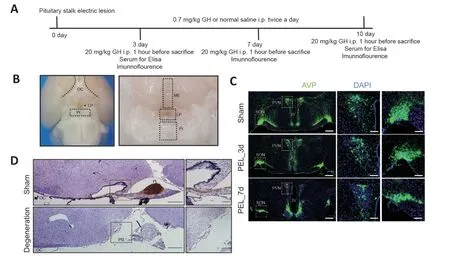
Figure 1 |Change in the hypothalamo–neurohypophyseal system of the hypothalamic pituitary stalk electrical lesion model.
Marble burying test
The marble burying test was tested at 3 and 7 days postsurgery.A standard polycarbonate mouse cage (26 cm ×48 cm × 20 cm;ZOSEN,Guangzhou,Guangdong,China)was used for the embedded bead test.Corn cob litter was distributed evenly at 5 mm thick by pressing another cage of the same size into the surface of the litter.Standard glass marbles (various styles and colors,15 mm in diameter,5.2 g in weight) were gently placed on the surface of the litter and arranged in five rows with four marbles in each row.One mouse was placed in the corner of the cage containing the marbles and the filter top cover was placed on the cage.Food and water were not given during the test.The mouse was leftundisturbed in the cage for 30 minutes.The number of marbles buried was recorded;the marble was marked as buried if twothirds of its surface area was covered by litter (Angoa-Pérez et al.,2013;Vestlund et al.,2020).
Immunofluorescence staining and immunohistochemical staining
As previously described (Yang et al.,2022;Zhou et al.,2022;Li et al.,2023),mice were deeply anesthetized with 75 mg/kg sodium pentobarbital and the brains were processed as either frozen sections (30 µm) or paraffin-embedded (3µm) sections.Cryosections were blocked for 1 hour at 37°C with 5% nonspecific antigenic goat serum containing 0.5%Triton X-100.Cryosections were incubated with primary antibodies (Additional Table 1) at 4°C overnight.The next day,cryosections were incubated for 1 hour at 37°C with the corresponding secondary antibodies conjugated to Alexa-488 or Alexa-594 (Additional Table 2).After staining with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich,Shanghai,China),all sections were slowly mounted onto glass slides and coverslips were placed onto the mounting medium.Fluorescence images were captured with a confocal microscope (LSM980,Zeiss,Oberkochen,Germany) using ZEN software (blue version,version 3.2).For paraffin sections,the procedure was the same as for cryosections,but images were captured with a fluorescence microscope(bx63,Olympus,Tokyo,Japan).Sodium citrate was used for immunohistochemical antigen retrieval.Sections were blocked with 5% nonspecific antigenic goat serum,incubated overnight at 4°C with primary antibodies (Additional Table 1),incubated for 1 hour at 37°C with the corresponding secondary antibodies (Additional Table 2),and subsequently treated with diaminobenzidine (ZSGB-BIO,ZLI-9017,Beijing,China) for 3 minutes at 37°C.Labeled cells were counted using ImageJ (version 1.6.0) software (National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012).For a specific cell count,the data were expressed as the number of cells/section.For double staining,data were expressed as the ratio of double-positive to single-positive cells.ImageJ software was used to measure mean fluorescence intensity in target cells.
p-STAT5 staining
Mice were given i.p.injections of 20 mg/kg rhGH to activate STAT5 before sacrifice.One hour later,mice were sacrificed and the brains removed.Harvested brains were fixed overnight,dehydrated,and sliced.The sections were incubated in 1% hydrogen peroxide with 1% sodium hydroxide(pH >13) for 20 minutes,washed with phosphate buffered saline (PBS) three times,then incubated with 0.3% glycine for 10 minutes,and 0.03% sodium dodecyl sulfate for 10 minutes.Samples were blocked with 5% goat serum for 1 hour at 37°C,incubated with a primary antibody for p-STAT5 (Additional Table 1) for 40 hours at 4°C,and incubated with secondary antibodies (Additional Table 2) for 90 minutes at 37°C(Quaresma et al.,2021).
Western blot assay
Mice were deeply anesthetized with sodium pentobarbital,perfused with normal saline at 4°C,and 100 µm brain slices cut using a vibrating blade microtome (Leica Vt1000s,Wetzlar,Hesse,Germany).The supraoptic nucleus (SON),paraventricular nucleus (PVN),and median eminence (https://portal.brain-map.org/) were carefully separated under a stereomicroscope (Leica EZ4 HD).The tissue was lysed with radio immunoprecipitation assay buffer (Solarbio,Beijing,China) (50 mM Tris-HCl pH 8.0,1 mM ethylene diamine tetraacetic acid pH 8.0,5 mM dithiothreitol,2% sodium dodecyl sulfate) containing protease inhibitors and phosphate protease inhibitors.Protein concentrations were determined using a bicinchoninic acid protein assay kit (Beyotime Biotechnology,Beijing,China).Protein samples were then separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore Corporation,Billerica,MA,USA).Membranes were blocked with 5% bovine serum albumin and incubated with primary antibody (Additional Table 1)overnight at 4°C.Membranes were then reacted with the corresponding horseradish peroxidase-conjugated secondary antibody (Additional Table 2) for 1 hour at 37°C.Protein bands were detected by chemiluminescence using Immobilon Enhanced Chemiluminescence Ultra Western horse radish peroxidase substrate (Millipore Corporation,Billerica,MA,USA).ImageJ software was used to measure the grayscale value for statistics.
Serum oxytocin content measurements
At different time points post-PEL,mice were deeply anesthetized with sodium pentobarbital (75 mg/kg,i.p.).Blood samples were collected from the right ventricle and separated by centrifugation at 1200 ×gfor 10 minutes at 4°C for serum.Oxytocin (OXT) content was detected following the manufacturer’s instructions using the OXT enzyme linked immunosorbent assay kit (74-OXYHU-E01,ALPCO,Selam,NH,USA).
Electron microscopy
Rats were deeply anesthetized with sodium pentobarbital(75 mg/kg),followed by perfusion with 0.1 M phosphate buffer (pH,7.4) and a buffer containing 2.5% glutaraldehyde.Tissue embedding and electron microscopy were performed as described.Electron microscopy was performed using a transmission electron microscope (Hitachi H-7500,Tokyo,Japan).
RNA transcriptome sequencing of hypothalamic tissues
Following surgery,three rats from each group (Sham and PEL) were sacrificed either 1 day or 7 days post-PEL.The hypothalamic AVP nucleus and median eminence tissue were obtained under a stereomicroscope using forceps.RNA preparation,library construction,and RNA transcriptomic sequencing of hypothalamic tissue were performed in accordance with our previous study (Zhou et al.,2022).
Trend analysis and functional enrichment analysis of differentially expressed genes
The original RNA sequencing data can be accessed from the public Gene Expression Omnibus database (GSE167904).Short Time-series Expression Miner (STEM;http://www.cs.cmu.edu/~jernst/stem/) was used for trend analysis following thetime axis of Sham,PEL_1d,and PEL_7d.The genes in profile5 were selected for Kyoto Encyclopedia of Genes and Genomes Gene Ontology (KEGG) analysis.
Statistical analysis
The sample size was determined based on effect sizes from previous studies (Charan and Kantharia,2013;Sun et al.,2022;Zhou et al.,2022).The data were submitted to Kolmogorov-Smirnov and Shapiro-Wilk normality tests to assess distribution.One-way or two-way analysis of variance(ANOVA) followed by Tukey’s multiple comparison test or Bonferroni’s multiple comparison test were performed using GraphPad Prism (version 7.02 for Windows,GraphPad Software,San Diego,CA,USA,www.graphpad.com).A twotailed unpairedt-test was used for single comparison between two groups.The error bars in all figures represent mean ±standard error of the mean (SEM).A value ofP<0.05 was considered statistically significant.
Results
Central diabetes insipidus after hypothalamic pituitary stalk injury
We established a hypothalamic-PEL model using a 3D-printed knife combined with electrolytic injury,and by adopting a transcranial parietal approach,which did not interfere with the anterior pituitary or pituitary portal system.Hypothalamic injury was verified after the procedure using low-magnification microscopy.The results confirmed that the procedure had little effect on other areas of the ventral hypothalamus (Figure 1B).Post-operatively,AVP neurons showed a decrease in nerve fibers and neuronal number over time (Figure 1C).At 10 days post-PEL,the mice showed a massive loss of axons in the median eminence (Figure 1D).Trend analysis demonstrated the significance of the JAK–STAT pathway in rats(Additional Figure 1A–C).Electron microscopy in rats showed that 1 week after the pituitary stalk was destroyed,increased vesicles appeared in neuronal cell bodies (Additional Figure 1D).Moreover,fragments per kilobase million (FPKM) of AVP and OXT also showed a decreasing trend post-PEL (Additional Figure 1EandF).
To evaluate the condition of the mice,we monitored three biological parameters related to the water-electrolyte system(mainly related to AVP),including DWC,DUV,and USG.We also determined whether repetitive,compulsive-like behavior was present in the marble burying test (Angoa-Pérez et al.,2013).The first day was classified as the acute phase of CDI,with high DWC,high DUV,and low USG.From post-operative days 2 to 3,the second phase of CDI was characterized by low DWC,low DUV,and high USG.The third phase of CDI was defined from day 4 and continued to be characterized by high DWC,high DUV,and low USG (Figure 2A–C).During the second phase,the mice showed a strong threat removal state.During the third phase,the mice exhibited reduced resistance to potential threats (Figure 2DandE).Moreover,serum levels of OXT by enzyme-linked immunosorbent assay indicated that GH treatment promoted the release of OXT to physiological levels (Figure 2F).Due to the positive effect of GH treatment on the water-electrolyte system and anti-anxiety behavior,we further evaluated the target of GH by examining p-STAT5 levels,which reflects activation of the GH receptor.
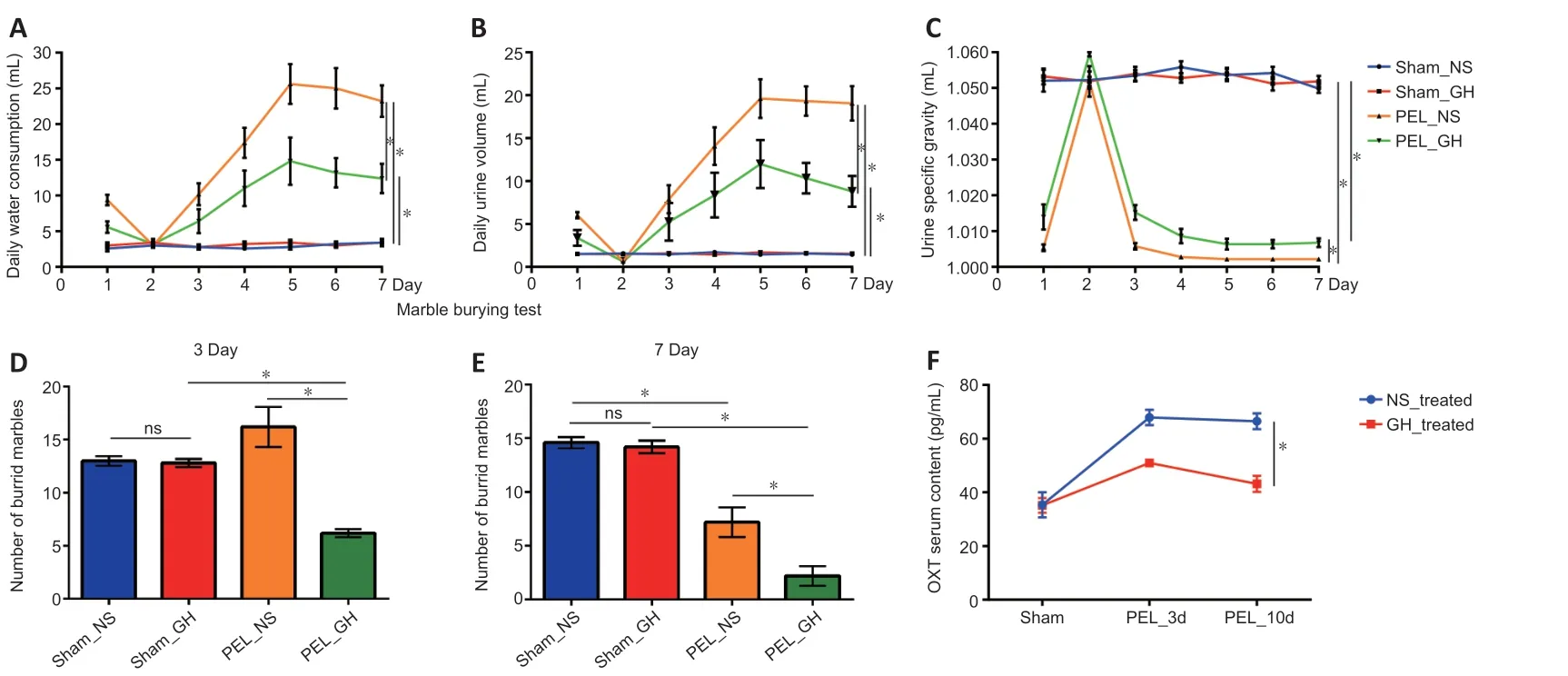
Figure 2 |Central diabetes insipidus and changes in anxiety state post-pituitary stalk electrical lesion.
Distribution characteristics and functions of GH-responsive cells
In sham-operated mice,p-STAT5 was highly expressed in the hypothalamus,mainly in the periventricular nucleus and nuclei in the base of the skull,such as the SON,PVN,suprachiasmatic nucleus,tuberal nucleus,and arcuate nucleus(Figure 3A).In the SON,p-STAT5-positive cells were mainly OXT neurons (25.23%),AVP neurons (24.73%),and some astrocytes (6.57%) (Figure 3B–D).In the PVN,p-STAT5-positive cells were mainly OXT neurons (16.61%),with only a small percentage of AVP neurons (3.94%) and some astrocytes(2.76%) (Additional Figure 2A–C).Moreover,65.25% of OXT neurons in the PVN were p-STAT5 positive,and 86.7%of OXT neurons in the SON were p-STAT5 positive;few AVP neurons and astrocytes were p-STAT5 positive (Figure 3DandAdditional Figure 2C),indicating the importance of GH for OXT.In addition,RNA sequencing in rats showed that a large number of GH-related genes were upregulated at 1 day post-PEL and downregulated by 7 days post-PEL based on KEGG pathway analysis (Additional Figure 2D–H).
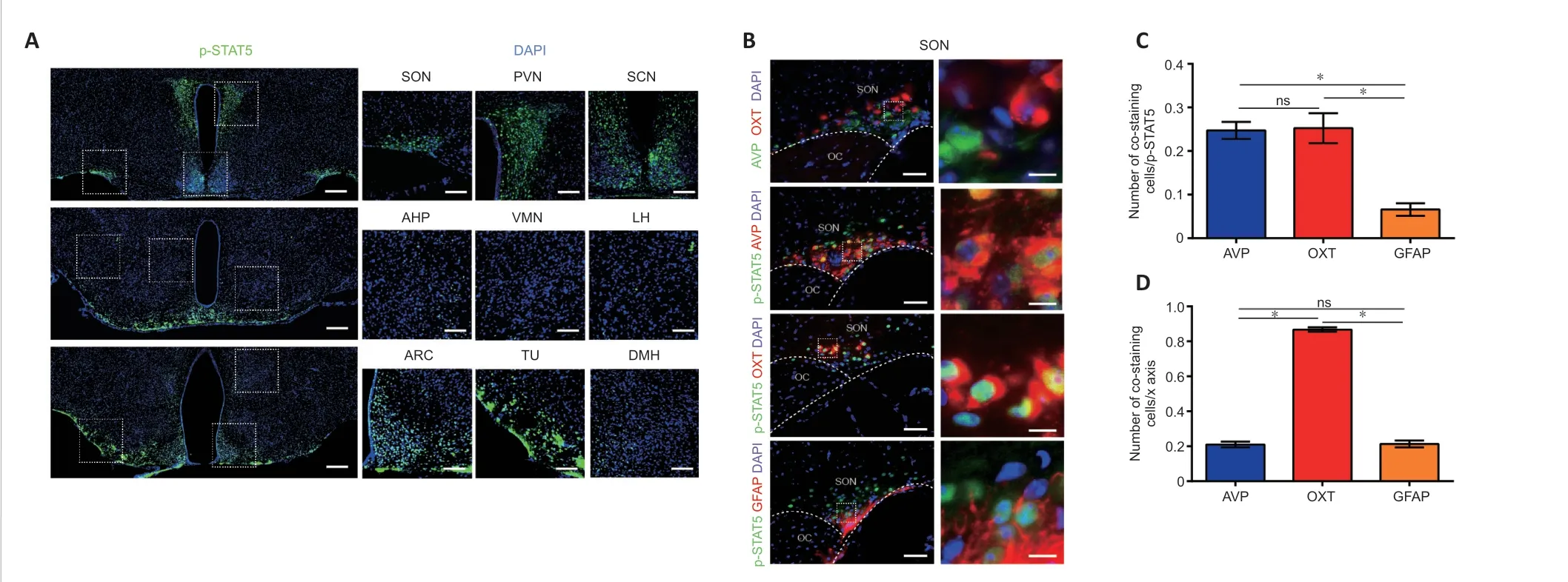
Figure 3 |Normal condition of growth hormone-reactive nucleus and cell types.
The effect of GH treatment on PEL
After GH treatment,the number of AVP-surviving neurons did not increase significantly in the SON or PVN by 7 days post-PEL.However,the proportion of p-STAT5-positive AVP neurons was decreased as early as 3 days post-PEL in the SON,suggesting that GH may not have a direct effect on AVP neurons (Figure 4AandB).In addition,more AVP neurons survived in the PVN at 3 days post-PEL and more p-STAT5-positive AVP neurons survived by 7 days post-PEL (8.31%to 37.86%;Additional Figure 3AandB).In the SON,the number of OXT neurons did not increase either with GH treatment post-PEL,but the proportion of p-STAT5-positive cells was increased at 7 days post-PEL (Figure 4CandD).This suggests that GH mainly acts on OXT neurons,while in the PVN,slightly more OXT neurons survived by 7 days post-PEL with GH treatment (Additional Figure 3CandD).In total,more p-STAT5-positive magnocellular neurons survived after GH treatment.In the SON,AVP neurons tended to spread ventrally,while OXT neurons tended to spread dorsally.In the PVN,AVP and OXT neurons tended to show an intermediate distribution.It seems that OXT neurons somehow support AVP neurons.As previously reported,neuronal activation promotes survival after axonal injury (Zhang et al.,2019).

Figure 4 |Expression pattern of p-STAT5 post-pituitary stalk electrical lesion in the supraoptic nucleus.
Therefore,we detected nuclear expression of c-fos,a protein expressed in active neurons (Zhang et al.,2021),and found an increased portion of c-fos positivity in the GHtreated group (Figure 4E,FandAdditional Figure 3E,F).Unfortunately,GH treatment only modestly promoted the survival of AVP and OXT neurons.Considering that astrocytes,as nutritive cells in the CNS,are highly associated with IGF-1 expression (Chesik and De Keyser,2010),we investigated the GH responsiveness of astrocytes.The number of astrocytes in the GH-treated group was greater at 3 days and less at 7 days post-PEL than in the untreated group,although treatment with GH did not change the increasing trend over time.The number of p-STAT5-positive astrocytes in the GH-treated group decreased,which might be associated with inhibition of inflammation by suppression of astrocyte migration (Figure 4G,H,andAdditional Figure 3G,H).
OXT neurons have IGF-1 autocrine or paracrine abilities
As GH mainly promotes the expression of IGF-1 (Bianchi et al.,2017;Ranke and Wit,2018),we next examined the expression pattern of IGF-1.In OXT neurons,the fluorescence intensity of IGF-1 was significantly higher than in other cells,suggesting that OXT neurons produce IGF-1 in large quantities (Figure 5AandB).This potential IGF-1 secreting ability of OXT neurons was improved with GH treatment (Figure 5C–F).In addition,the distribution of OXT in nerve fibers of the median eminence did not correlate with IGF-1 (Figure 5G).This suggests that OXT neurons mainly release IGF-1 by autocrine or paracrine signaling through the cell body instead of secreting IGF-1 from axons (Pristerà et al.,2019).AVP did not co-stain with IGF-1 in the sham-operated group,and co-stained cells in the SON were mainly distributed on the dorsal side (Additional Figure 4AandB),indicating that AVP neurons mainly obtain nutrition from other cells.Only a few astrocytes were positive with IGF-1 (Additional Figure 4C).Altogether,these results show that OXT neurons mainly release IGF-1 from the SON and PVN.
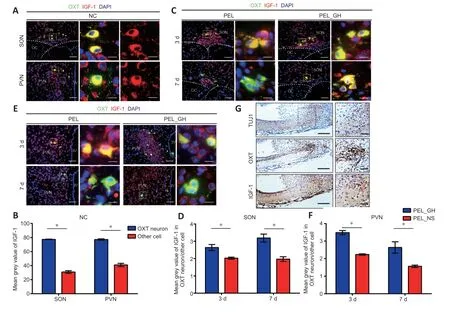
Figure 5 |Insulin-like growth factor 1 may act in an autocrine or paracrine manner.
Insulin-like growth factor 1 receptor is abundantly expressed in the posterior pituitary system
The expression of IGF-1 was upregulated in the nucleus after injury,as shown by western blotting,and the expression of IGF-1 was further promoted by GH treatment (Figure 6A).At the same time,western blot results also suggested an upregulation of IGF-1 receptor (IGF-1R) expression in the nucleus after injury,but GH treatment did not appear to lead to a significant change in the total amount.Notably,GH treatment promoted the retention of IGF-1R at the axonal end of neurons (Additional Figure 5AandB).At 3 days postsurgery,GH did not significantly change IGF-1R expression in AVP and OXT neurons (Figure 6BandC) but seemed to promote IGF-1R expression in the longer-term,as seen at 7 days (Additional Figure 5C).Lastly,the results of RNA-seq in rats suggested that RNA expression of Igf1r was upregulated at 1 day post-PEL and downregulated at 7 days post-PEL,while Igf2r did not change markedly (Additional Figure 5DandE).

Figure 6 |Expression of insulin-like growth factor 1 receptor post-pituitary stalk electrical lesion in the supraoptic nucleus.
GH promotes axonal regeneration in the hypothalamoneurohypophyseal system
At 10 days post-PEL,axons in the GH-treated group could regenerate back to the posterior pituitary across the lesioned end,whereas axons in the non-GH-treated group could not cross the lesion area (Figure 7A).In addition,axon end vascularization was better with GH treatment,thereby providing a better environment for hormone release,whereas the non-GH treatment group showed atrophy of the posterior pituitary vascular network (Figure 7B).After 10 days of GH treatment,AVP neurons mostly co-stained with OXT neurons in the PVN (Additional Figure 6A),indicating that GH treatment changes the pattern of AVP and OXT expression in magnocellular neurons.In addition,within the area of regenerated axons,we observed β-amyloid expression,which was mainly distributed on the surface of nerve fibers promoting neurite outgrowth (Zhou et al.,2012).Furthermore,GH treatment promoted the retention of glial fibrillary acidic protein-positive cells in the median eminence (Additional Figure 6B).Lastly,PEL mainly injured the hypothalamo-hypophyseal system in the hypothalamus,while merely injured the pituitary (Additional Figure 7).
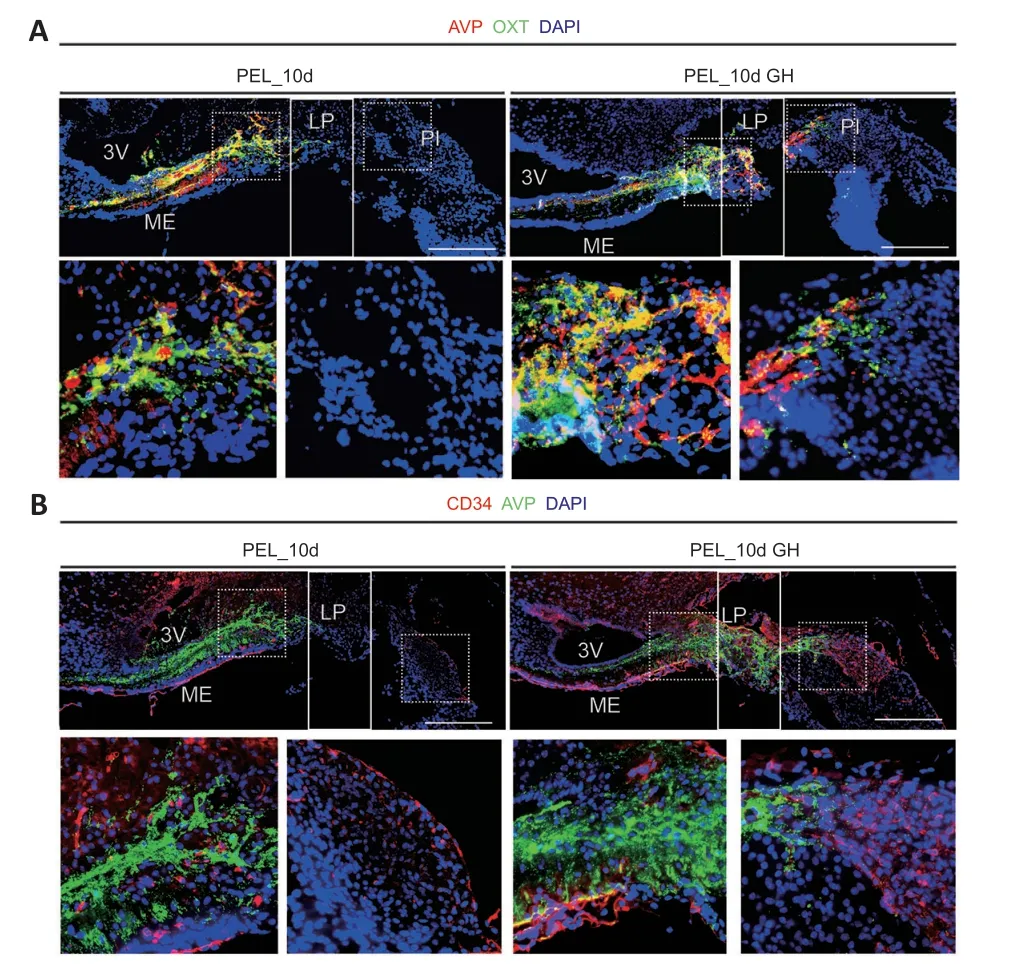
Figure 7 |Growth hormone treatment promoted axon regeneration.
Discussion
Structural repair is often the basis of functional repair(Anderson et al.,2018).However,how to regenerate and reconstruct unmyelinated nerve fibers associated with endocrine neurons in the hypothalamus remains unexplored.Following hypothalamic-neurohypophyseal injury,mice experience CDI,obesity,and anxiety,indicating a loss of AVP and OXT (Klockars et al.,2017;Lawson,2017;Aulinas et al.,2019;Christ-Crain et al.,2019).The main factors affecting the failure of repair after injury include cell death,clearance of neuronal debris,decreased intrinsic growth potential,and the presence of inhibitory factors (Sofroniew,2018).Studies have shown a role of the GH/IGF-1 axis in regeneration following axonal injury in different models (Bei et al.,2016;Raimondo et al.,2019;Hanwright et al.,2022).Regarding functional recovery following peripheral nerve injury,GH treatment in rats and the delivery of IGF-1 by different materials in rabbits after peripheral nerve injury promote muscle proliferation and neuromuscular junction redistribution (Tuffaha et al.,2016;Lopez et al.,2019;Raimondo et al.,2019;Hanwright et al.,2022).Findings from Bei et al.(2016) suggested that genetic strategies of AAV-assisted overexpression of IGF1 after injury promote long-distance regeneration of retinal axons after both optic nerve and optic tract injury models.Following spinal cord injury,combinatorial strategies of osteopontin (OPN)/IGF-1 treatment maximized functional recovery through OPNsensitized corticospinal neuronal signaling responses to IGF-1 (Liu et al.,2017).Our study shows a method to promote axonal regeneration following hypothalamic magnocellular neuronal injury,and preliminarily reports the mechanism underlying the effect of GH treatment on increasing intrinsic growth capacity.
Although most studies in adults have found that GH/IGF-1 mainly exerts its regulatory role through the secretion of IGF-1 from the liver (Ranke and Wit,2018),some recent studies have shown that the CNS,especially the hypothalamus,show responsiveness to GH (as shown by staining of p-STAT5)(Wasinski et al.,2021).GH in the arcuate nucleus regulates energy metabolism through agouti-related protein neurons(Furigo et al.,2019),while there are few studies on the other neuronal types in the hypothalamus.In the present study,we found that the effects of GH on the hypothalamusneurohypophyseal system were mainly concentrated in OXT neurons.Further,GH appeared to promote growth factor responsiveness by improving neuronal excitability,similar to modulating resynaptic inputs to promote retinal ganglion cell axon regeneration after axotomy (Zhang et al.,2019).Following activation of STAT5,IGF-1 also acts in an autocrine or paracrine manner in the CNS;for example,dopaminergic neurons regulate electrical signaling through autocrine regulation of IGF-1 (Pristerà et al.,2019).The present study suggests that IGF-1 might also function in a paracrine or autocrine manner in OXT neurons.Although there are a variety of neurons in the SON and PVN that express IGF-1,OXT neurons synthesize more IGF-1 than other cells,suggesting its importance to OXT neurons.At the same time,the distribution area of IGF-1R and IGF-1 was concomitant,as previously reported (Dyer et al.,2016).A study on optic nerve injury found that increased neuronal activity increases the retention of IGF-1R on axons and promotes axonal regeneration (Zhang et al.,2019).Moreover,GH may also promote neuronal IGF-1R retention by increasing neuronal activity in the posterior hypothalamic-pituitary axis as c-fos increases.In summary,the GH/IGF-1 axis promotes functional recovery of magnocellular neurons and improves the viability of neuronal self-regeneration.In past research on CNS regeneration after injury,the existence of a glial scar makes it difficult for axon regeneration across the damaged end;thus a nodule forms at the axon end (Dorrier et al.,2021).In our previous study on rats,we also found formation of a lobe-like structure in the median eminence after magnocellular neuron axonal injury.Neurogenesis of the median eminence was involved in functional reconstruction of the structure,promoting neurovascular reconstruction of the endocrine unit (Ou et al.,2022).In our present study,GH treatment appeared to inhibit scar formation,promote axonogenesis,and regenerate nerve fibers from magnocellular neurons back to the posterior pituitary,thereby providing a better environment for the release of AVP and OXT (Chauvet et al.,1995).Nonetheless,the scar components in the lobe-like structure are still unknown.At a minimum,astrocytes or tanycytes might participate in regeneration in the median eminence,which is quite different from the cells that participate in regeneration following spinal cord injury (Chauvet et al.,1995;Chen et al.,2023;Zhao et al.,2023).However,more research is needed to investigate how GH can change the components of the lobelike structure.After all,this is the first study to show axonal regeneration back to the neurohypophysis.
Although neural lobe-like neurovascular contact regions developed after intrahypothalamic transection of the hypothalamo-neurohypophysial tract,the capillaries were not fenestrated (Dellmann and Carithers,1992).This demonstrates that promoting axonal regeneration back to the posterior pituitary with fenestrated capillaries is a significant undertaking.Moreover,studies have shown that OXT plays a crucial role in the formation of the posterior pituitary gland during development,and a lack of OXT can disrupt posterior pituitary gland formation (Anbalagan et al.,2018).The effect of GH on OXT neurons might promote this structural reconstruction.At the same time,GH treatment promoted angiogenesis and reduced vascular atrophy in the posterior pituitary caused by axon degeneration of magnocellular neurons.
As the effect of hormones in axonal regeneration is complex,more studies regarding the injury of endocrine neurons are needed.Several studies have shown the effect of hormones in axonal regeneration: OXT promoted nerve recovery in a rat sciatic nerve damage model (Gümüs et al.,2015);a gonadotropin-releasing hormone agonist was used to promote sciatic nerve recovery (Hernández-Jasso et al.,2020);testosterone promoted the regeneration of motor neuron injury (Chew and Sengelaub,2020);estrogen improved the regeneration of axons after subcortical axon injury (Xia et al.,2020);neuronal androgen receptor was required for activity dependent enhancement of peripheral nerve regeneration(Ward et al.,2021);and lastly,GH and prolactin can both activate STAT5 (Silveira et al.,2019),which makes the condition in both sexes more complicated.
Our study demonstrates that GH can partially alleviate the concomitant hypothalamic syndrome following hypothalamic injury.In the hypothalamo-pituitary system,GH primarily alters the balance of AVP and OXT in magnocellular neurons.This then restores functional homeostasis after injury through the IGF-1/IGF-1R pathway.The limitations of our study are that we did not exclude other effects of GH within the niche,and that STAT5/IGF-1/IGF-1R might not be the only pathway induced by GH.
Author contributions:KL and ZF designed the experiments,generated data,and drafted the manuscript;KL,ZX,JP,MZ,WL,YO carried out the majority of the experiments;GW,MC,HG,JP,XW contributed to sample collection;JP and SQ designed the experiments,supervised the experiments,and co-wrote the manuscript.All authors approved the final version of this manuscript.
Conflicts of interest:We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:José Donato Jr,The University of Chicago,Universidade de São Paulo,Brazil.
Additional files:
Additional Figure 1:The potential ability of JAK-STAT pathway in rats.
Additional Figure 2:Baseline growth hormone reactiveness pattern of hypothalamic nucleus.
Additional Figure 3:The expression pattern of p-STAT5 post-PEL in the PVN.
Additional Figure 4:The expression pattern of IGF-1 in AVP neurons and astrocytes.
Additional Figure 5:The expression of IGF-1R post-PEL.
Additional Figure 6:The other characteristics of magnocellular neurons 10 days post-PEL.
Additional Figure 7:The change of hypothalamo-hypophyseal system post-PEL.
Additional Table 1:The primary antibodies used in this study.
Additional Table 2:The secondary antibodies used in this study.
Additional file 1:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
