3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
2024-01-24YizeQiYaoZhouJiyangLiFangyuanZhuGengniGuoCanWangManYuYijieWangTengfeiMaShanwuFengLiZhou
Yize Qi ,Yao Zhou ,Jiyang Li ,Fangyuan Zhu ,Gengni Guo ,Can Wang ,Man Yu,Yijie Wang,Tengfei Ma,,Shanwu Feng,Li Zhou
Abstract Methamphetamine addiction is a brain disorder characterized by persistent drug-seeking behavior,which has been linked with aberrant synaptic plasticity.An increasing body of evidence suggests that aberrant synaptic plasticity is associated with the activation of the NOD-like receptor family pyrin domain containing-3 (NLRP3) inflammasome.3′-Deoxyadenosin,an active component of the Chinese fungus Cordyceps militaris,has strong anti-inflammatory effects.However,whether 3′-deoxyadenosin attenuates methamphetamine-induced aberrant synaptic plasticity via an NLRP3-mediated inflammatory mechanism remains unclear.We first observed that 3′-deoxyadenosin attenuated conditioned place preference scores in methamphetamine-treated mice and decreased the expression of c-fos in hippocampal neurons.Furthermore,we found that 3′-deoxyadenosin reduced the aberrant potentiation of glutamatergic transmission and restored the methamphetamine-induced impairment of synaptic plasticity.We also found that 3′-deoxyadenosin decreased the expression of NLRP3 and neuronal injury.Importantly,a direct NLRP3 deficiency reduced methamphetamine-induced seeking behavior,attenuated the impaired synaptic plasticity,and prevented neuronal damage.Finally,NLRP3 activation reversed the effect of 3′-deoxyadenosin on behavior and synaptic plasticity,suggesting that the anti-neuroinflammatory mechanism of 3′-deoxyadenosin on aberrant synaptic plasticity reduces methamphetamine-induced seeking behavior.Taken together,3′-deoxyadenosin alleviates methamphetamine-induced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome.
Key Words: 3′-deoxyadenosin;hippocampus;long-term potentiation;methamphetamine;NOD-like receptor family pyrin domain containing-3(NLRP3) inflammasome;synaptic plasticity
Introduction
Methamphetamine (METH) is a recreational drug that is associated with serious addiction and dependency (Zhao et al.,2021).Although repeated METH-induced neuroinflammation and aberrant synaptic plasticity are associated with the formation of drug-seeking behavior (Du et al.,2017;Everett et al.,2021),the connection between these elements is unclear.Understanding the relationship between this neuroinflammation and aberrant synaptic plasticity could lead to new therapeutic strategies to treat drug-seeking behavior in individuals experiencing METH addiction.
Synaptic plasticity is an important component of learning and memory (Magee and Grienberger,2020;Cornell et al.,2022;Wu et al.,2022;Gutiérrez et al.,2023).Addictive drugs have been found to elicit or modify synaptic plasticity,and these changes may be associated with drug-induced alterations in memory and behavior.For example,repeated drug exposure,such as METH and cocaine,increased the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)/N-methyl-D-aspartate receptor (NMDAR)ratio in neurons in the ventral tegmental area (VTA) (Kauer and Malenka,2007),and increased the number of synaptic Ca2+-permeable AMPARs at postsynapses (Loweth et al.,2014;Martinez-Rivera et al.,2017).Although the addiction memory center includes the connection from the VTA to the nucleus accumbens via the mesolimbic pathway,no direct relationship has been elucidated between addiction and activity in the hippocampus,which is a well-known center for learning and memory.However,previous studies have demonstrated the direct involvement of the hippocampus in mediating behaviors associated with drug reward (Ricoy and Martinez,2009;Luscher and Malenka,2011).METH-mediated overactivation of AMPARs interferes with the normal production of longterm potentiation (LTP) in the hippocampus,impairing normal synaptic plasticity (Minichiello,2009;Avchalumov et al.,2020).Taken together,these data indicate that aberrant synaptic plasticity may contribute to drug-induced memory.However,the neural mechanisms underlying the memory loss and cognitive decline associated with METH addiction have not fully understood.The neuroinflammatory effect of METH is an important factor in the associated neuronal damage (Wisor et al.,2011;Huang et al.,2022).The NODlike receptor family pyrin domain containing-3 (NLRP3)inflammasome is a molecular complex that plays an important role in coordinating the innate immune responses (Pang et al.,2022).The neuroinflammation mechanism is involved in the upregulation of the NLRP3 inflammasome and its signaling pathway (Du et al.,2017;Xu et al.,2018;Ding et al.,2022).The NLRP3 inflammasome has been found to affect the formation of normal cognitive memory (Guo et al.,2020),suggesting that neuroinflammation may interact with aberrant synaptic plasticity (Snider et al.,2013).Given that NLRP3-mediated neuroinflammation contributes to this synaptic plasticity,the NLRP3 inflammasome might be a suitable target for the treatment of METH-induced seeking behavior (Snider et al.,2013).
3′-Deoxyadenosine (3′-dA),also known as cordycepin,is a major bioactive component of Cordyceps militaris,which is an herb that has been used for hundreds of years in traditional Chinese medicine (Serpi et al.,2022).Because of its homologous transportation mechanism,3′-dA can effectively cross the blood-brain barrier (Yang et al.,2017).Notably,3′-dA is known to modulate the expression of proinflammatory factors such as IL-1β,TNF-α,and IL-2 (Xu et al.,2018).An increasing number of studies have demonstrated that 3′-dA has a wide range of pharmacological effects,including immune regulation,antidepressant,anticancer,antiviral,and antifungal activities (Yang et al.,2017,2020;Gao et al.,2019).However,whether 3′-dA can prevent aberrant synaptic plasticity and decrease METH-induced seeking behavior through its anti-inflammatory properties remains unclear.In this study,we aimed to explore the relationship between neuroinflammation and aberrant synaptic plasticity in mice following repeated METH administration.Our findings revealed that activation of the NLRP3 inflammasome enhanced aberrant glutamatergic transmissions.We investigated the effects of 3′-dA on the expression of the NLRP3 inflammasome in the hippocampus and evaluated its role in attenuating aberrant synaptic plasticity and METHinduced seeking behavior.
Methods
Animals
Male wild-type (WT) C57BL/6 mice aged 7–8 weeks (22–25 g,n=80) were acquired from the Animal Core Facility of Nanjing Medical University (Jangsu,Nanjing,license No.SCXK(Su) 2021-0001).Male NLRP3 gene knockout C57BL/6 mice(NLRP3 KO) aged 7–8 weeks (22–25 g,n=8) were provided by Nanjing Medical University (Nanjing,China).All mice were specific-pathogen-free (SPF),and were housed at 24 ± 2°C with a relative humidity of 55 ± 5%.The animals were housed individually in a room with a 12-hour light-dark cycle (light from 7 a.m.to 7 p.m.) and hadad libitumaccess to food and water.All aspects of animal care and the experimental protocols were approved by the Nanjing Medical University Institutional Animal Care Committee on May 6,2022(IACUC-2204041) and conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thed.,National Research Council,2011).All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE)guidelines (Percie du Sert et al.,2020).
Eighty C57BL/6 mice were used in our investigation.These 80 mice were randomly divided into 4 groups,comprising the saline group,METH group,METH+3′-dA group,and METH+3′-dA+Nigericin (Nig) group.The mice in the saline group received injections of saline only (0.1 mL/10g).In the METH group,mice were administered an intraperitoneal injection of 2 mg/kg METH.In the METH+3′-dA group,3′-dA (12.5 mg/kg)injection was administered 1 hour prior to the intraperitoneal injection of METH.For the METH+3′-dA+Nig group,both 3′-dA (12.5 mg/kg) and Nig (10 mg/kg) injections were given 1 hour before the intraperitoneal injection of METH.Following a conditioned place preference behavioral assessment,12 mice were used for whole-cell electrophysiological inquiries and 16 mice were used forin vitrofield recording.Meanwhile,16 mice were used for morphological investigations,24 mice were used for western blotting experiments,and 12 mice were used for an enzyme-linked immunosorbent assay.In the METH+3′-dA+Nig group,1 mouse died and was not included in the analysis.Furthermore,we administered METH to 8 NLRP3 KO mice,4 of which were designated for electrophysiological evaluation and 4 for morphological investigation.
Conditioned place preference
The conditioned place preference (CPP) apparatus contained two chambers.The bottom of the box had a distinct floor texture,and the walls were either black and white or entirely black.The CPP apparatus was 18 cm in width,38 cm in length,and 30 cm in height.A middle box divided the two sides,effectively isolating them,and there was a top door to introduce and remove the mice from the enclosure.Following an intraperitoneal (i.p.) METH (National Institutes for Food and Drug Control,Beijing,China) injection at a dose of 2 mg/kg,WT C57BL/6 mice and NLRP3 KO mice were confined to the black and white box,while a normal saline injection led to their confinement in the black box (Werner et al.,2021;Zhu et al.,2022).Each training session lasted 45 minutes.On day 0,a 15-minute baseline pre-test was conducted for each subject.On days 1,3,5,and 7,METH was administered to the mice,and they were subsequently confined to the black and white boxes for 45 minutes.Conversely,on days 2,4,6,and 8,equivalent quantities of saline (0.1 mL/10 g) were injected into the mice,followed by confinement in the black box for 45 minutes.On day 9,the mice were granted unrestricted access to the entire apparatus for 15 minutes;this post-training test is referred to as the CPP-test.The time spent by the mice in both boxes was recorded for the training sessions and during the CPP test.The CPP scores indicated that the mice established a connection between the METH box and METH preference,and spent more time in the METH box.The CPP score was calculated as the difference in the amount of time spent in the METH box versus the saline box (time in the METH box minus time in the saline box).A higher CPP score indicated a more pronounced METH addiction.As a treatment for this addiction,3′-dA (Aladdin,Shanghai,China,Cat# 73-3-0) at a dose of 12.5 mg/kg was administered via i.p.injection to the mice 1 hour before the condition phase.The CPP behavior test was scored using Trackermaster software (Zhongshi,Beijing,China).
Nissl staining
After the behavioral test,the entire brains from each group were fixed in 4% paraformaldehyde for preservation after mice were anaesthetized with 1.4% isoflurane (RWD Life Science Cat# R510-22,Shenzhen,China) with oxygen (O2) at 1 L/min and sacrificed.Serial coronal 30-µm slices containing the hippocampus were cut using a freezing microtome (Leica,Wetzlar,Hessen,Germany) and then mounted on plexiglass and subjected to single-immunostaining using cresyl fast violet(Solarbio,Beijing,China),which enabled the histochemical demonstration of Nissl substances.The images were collected by 40× brightfield microscope (Pannoramic SCAN,3DHISTECH Ltd.,Budapest,Hungary).For subsequent analysis,the minimum pixel threshold was set at 50 pixels,and the average relative optical density (OD) values of the Nissl bodies were examined.To compare the differences among the 3 groups,the OD in the saline group was used as a control reference.
Enzyme-linked immunosorbent assay
Mice were anaesthetized with 1.4% isoflurane with oxygen (O2)at 1 L/min and sacrificed for enzyme-linked immunosorbent assay (ELISA).We carefully extracted the mice hippocampi after the behavioral test.Then,the hippocampus was centrifuged at 12,000 ×gat 4°C for 15 minutes.We collected the clear supernatant and measured the levels of IL-1β and TNF-α using ELISA kits (Meimian,Yancheng,Jiangsu,China).
Western blotting
After mice were anaesthetized with 1.4% isoflurane with oxygen (O2) at 1 L/min and sacrificed,the hippocampal tissues were homogenized in radio immunoprecipitation assay (RIPA) buffer,ultrasonically ground,and supplemented with the protease inhibitor phenylmethanesulfonyl fluoride(PMSF,Beyotime,Shanghai,China,Cat# ST506) for western blotting assays.Following a 30-minute incubation period,the supernatant was separated via centrifugation at 12,000×gfor 15 minutes at 4°C.Subsequently,the supernatants were mixed with sodium dodecyl sulfate (SDS) sample buffer,subjected to SDS-polyacrylamide gel electrophoresis (PAGE)electrophoresis,and transferred to a 0.45 µm polyvinylidene fluoride (PVDF) membrane (Merck Milliore,Darmstadt,Germany).The proteins were then detected by adding the following primary antibodies: polyclonal mouse NLRP3 (1:1000 dilution;Adipogen,South Korea,Cat# AG-20B-0014,RRID:AB_2490202),polyclonal rabbit postsynaptic density protein 95 (PSD-95;1:1000 dilution;Proteintech,Wuhan,China,Cat#20665-1-AP,RRID: AB_2687961),polyclonal rabbit spinophilin(1:1000 dilution;Proteintech Wuhan,China,Cat# 55129-1-AP,RRID: AB_10837358),and monoclonal mouse GAPDH(1:10,000 dilution;Proteintech Wuhan,China,Cat# 60004-1-lg).We adjusted the brightness and darkness in western blotting to account for the high level of background staining caused by the protein exposure time.The protein bands were quantitatively analyzed using ImageJ (v1.52a,National Institutes of Health,Bethesda,MD,USA).
Immunofluorescence
Mice were anaesthetized with 1.4% isoflurane with oxygen(O2) at 1 L/min and subjected to intracardial perfusion with 4%paraformaldehyde in phosphate-buffered saline (PBS).Whole brains were immersed in 4% paraformaldehyde at 4°C for overnight postfixation.This was replaced with a 30% sucrose solution to dyhydrate the brain tissue.After freeze-embedding the tissue in optimal cutting temperature compound (O.C.T,SAKURA,Torrance,CA,USA,Cat# 4583),coronal brain slices(30-µm) were prepared (Leica Biosystems,Barrington,IL,USA,Cat# CM1950) and imaged using a fluorescence microscope(Nikon,Japan).We used the following primary antibodies:monoclonal mouse c-fos (1:1000 dilution;Abcam,Cambridge,UK,Cat# ab208942,RRID: AB_2747772),anti-mouse IgG(1:10,000 dilution,CST,Danvers,Massachusetts,USA,Cat#7076S),and anti-rabbit IgG (1:10,000 dilution,CST,Danvers,MA,USA,Cat# 7074S).All images were processed using Image J.For c-fos+cells in the CA1 area,we counted the positive cells per mm2.
Electrophysiology
The mice were anaesthetized with 1.4% isoflurane with O2at 1 L/min and sacrificed for electrophysiological experiments after the CPP test.Coronal brain sections (250 µm) were prepared in an ice-cold cutting solution comprising the following(in mM): 148.5 sucrose,40 NaCl,4 KCl,25 NaHCO3,1.25 NaH2PO4,0.5 CaCl2,7 MgCl2,10 glucose,1 sodium ascorbate,3 sodium pyruvate,and 3 myoinositol.The sections were then incubated in a mixture of cutting solution and external solution (in a 1:1 ratio) at 32°C for 45 minutes.The external solution contained the following (in mM): 125 NaCl,2.5 CaCl2,4.5 KCl,1.25 NaH2PO4,1.3 MgCl2,25 NaHCO3,15 glucose,and 15 sucrose.Following incubation,the slices were maintained in the external solution (saturated with 95% O2and 5% CO2) at room temperature until further use.
During the experiment,the slices were perfused with the external solution at a flow rate of 3–4 mL/min at 32°C.Hippocampal pyramidal neurons in CA1 were identified and patched for data recording.An IPA-2 integrated patch amplifier controlled with SutterPatch software (Sutter Instrument,Novato,CA,USA) was used to record the data.For whole-cell recordings,a Cs-based solution was used,comprising the following (in mM): 119 CsMeSO4,8 TEA.Cl,15 HEPES,0.6 EGTA,0.3 Na3GTP,4 MgATP,5 QX-314.Cl,and 7 phosphocreatine (Sigma-Aldrich,Darmstadt,Germany).The pH was adjusted to 7.3 with CsOH,with an osmolarity of 270–280 mOsm.
To induce glutamatergic transmission in pyramidal neurons via electrical stimulation,bipolar stimulating electrodes were positioned 100–150 µm away from the recording neurons.To determine the input-output relationships for AMPAR-mediated excitatory postsynaptic currents (EPSCs),measurements were taken at five different stimulating intensities.The AMPAR/NMDAR ratio was measured by recording the peak currents of AMPAR-mediated EPSCs at a holding potential of–70 mV,and NMDAR-mediated EPSCs were estimated at +40 mV,50 ms after the peak of AMPAR-EPSCs.The AMPAR/NMDAR ratio was calculated by dividing the NMDAR-EPSC by the AMPAR-EPSC.The paired-pulse ratios (PPRs) of the AMPAR-mediated EPSCs were obtained using two electrical stimuli at an interval of 50 ms.
Forin vitrofield recording,field excitatory postsynaptic potentials (fEPSPs) in the CA1 region were recorded using glass electrodes filled with NaCl (1 M) solution.A stimulating electrode placed in the Schaffer collateral pathway delivered a constant voltage stimulation that was 50-µs in duration and repeated at 20-second intervals.LTP was induced by three trains of tetanus stimuli,also known as high-frequency stimulation (HFS),at 100 Hz for 1 second,and the trains were repeated after a 30-second interval.The slopes of the evoked fEPSPs were measured and expressed relative to the normalized preconditioning baseline,providing a quantification of the LTP magnitude.
Nigericin treatment
Nigericin (Selleck,Houston,Texas,USA,Cat# S6653) was adminstered to METH+3′-dA mice (i.p.injection) at 10 mg/kg on days 1,3,5,and 7 after the CPP test.A vehicle including 5% DMSO,5% Tween 80,and 40% PEG 300 was used for dissolving drugs.
Statistical analysis
Although no statistical methods were used to predetermine the sample sizes,our sample sizes were similar to those reported in previous publications (Li et al.,2022;Ma et al.,2022).The assessors were blinded to group identity for both the biochemical and histological analyses to minimize potential bias.All of the samples were coded and interpreted by a reviewer who had no knowledge of the experimental groups during the analysis.All data are presented as the mean± standard error of the mean (SEM).To determine statistical significance,a one-way analysis of variance and two-way repeated-measures analysis of variance were used,followed by the Student-Newman-Keuls (SNK)post hoctest.The significance threshold was set atP<0.05.
Results
3′-dA reduces METH-mediated CPP scores in mice and decreases hippocampal neuron activity
To determine whether 3′-dA plays a role in METH-acquired behavior,we treated 8-week-old C57BL/6 mice with 3′-dA prior to the CPP test (Figure 1A).The CPP scores of the METH group were significantly increased compared with those of the Saline group (P<0.001).Meanwhile,the administration of 3′-dA decreased CPP scores in the post-test period (Figure 1B–D).Although the main region implicated in addictive memory is the nucleus accumbens,which receives dopamine inputs from the VTA via the mesolimbic pathway,we wanted to investigate associated changes in hippocampal neurons following METHinduced seeking behavior.Accordingly,we examined the expression of c-fos,a marker of neuronal activity,in the three groups (Chen et al.,2022).We found that the METH+3′-dA group had fewer c-fos+cells than the METH-treated group (P<0.05;Figure 1EandF).These results demonstrate that 3′-dA improves METH-acquired behavior and decreases the activity of hippocampal neurons in mice.
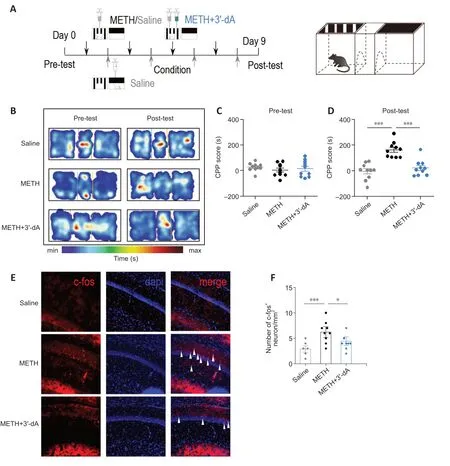
Figure 1 |3′-Deoxyadenosine (3′-dA) reduces methamphetamine (METH)-mediated conditioned place preference (CPP) scores in mice and decreases hippocampal neuron activity.
3′-dA decreases METH-induced abnormal synaptic transmission
Previous studies have shown that METH elicits abnormal increases in glutamatergic transmission (Tokunaga et al.,2009;Luscher and Malenka,2011).Accordingly,we investigated whether 3′-dA affects METH-induced alterations in glutamatergic synaptic transmission.Following the behavioral test (Figure 2A),we performed whole-cell recordings on neurons in CA1 coronal slices and placed bipolar stimulating electrodes on the Schaffer collateral fibers(Figure 2B).We found that the AMPAR/NMDAR ratio and AMPAR-mediated input output were increased compared with those in the Saline group.The group treated with 3′-dA showed a reduced AMPAR/NMDAR ratio (P<0.01;Figure 2CandD) and reduced AMPAR-mediated responses (P <0.001;Figure 2EandF) compared with the METH group,suggesting that 3′-dA prevents METH-induced excessive glutamatergic transmission in hippocampal pyramidal neurons in CA1.We also measured the PPR of hippocampal neurons in the three groups.The PPR is correlated with the presynaptic release probability,which determines the short-term computational properties of the synapse (Hanse and Gustafsson,2001).The results showed that there were no obvious changes in PPR in the three groups (P>0.05;Figure 2GandH),suggesting that no presynaptic mechanisms contributed to the effect of 3′-dA on glutamatergic transmission.Taken together,these data suggest that 3′-dA decreases METH-induced abnormal synaptic transmission via postsynaptic mechanisms.
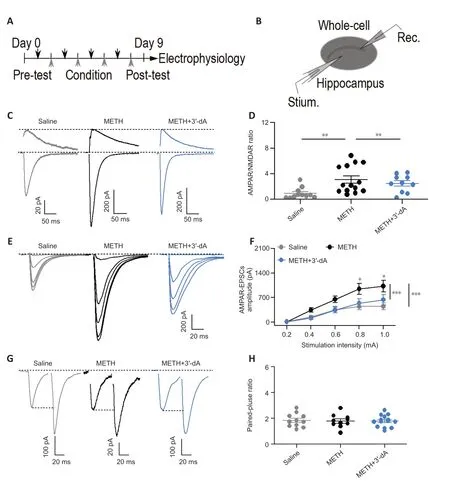
Figure 2 |3′-Deoxyadenosine (3′-dA) decreases methamphetamine (METH)-induced aberrant synaptic transmission.
Abnormal synaptic plasticity is prevented by 3′-dA in METHaddicted mice
Given that repeated METH administration impairs cognitive memory (Reichel et al.,2012),we next determined whether 3′-dA treatments could alleviate METH-induced damage to functional memory.We used a field recording method to test HFS-induced LTP in hippocampal CA1 neurons.We found that the HFS-induced fEPSP of LTP was impaired by METH,and that treatment with 3′-dA prevented this impairment (Figure 3A–C).Spinophilin (SPN) is a postsynaptic protein involved in synaptic plasticity,and PSD-95 is postsynaptic scaffolding protein associated with changes in synaptic plasticity (Zeng et al.,2016;Liu et al.,2018).We next examined the expression of SPN and PSD-95 in the hippocampus.We found that both SPN (P<0.05) and PSD-95 (P<0.01) were reduced in the METH group compared with the findings in the Saline group.The group treated with 3′-dA showed an increased expression of SPN (P=0.145) and PSD-95 (P<0.05) compared with the METH group (Figure 3D–F).These results suggest that 3′-dA prevents the impairments in synaptic plasticity caused by METH administration.
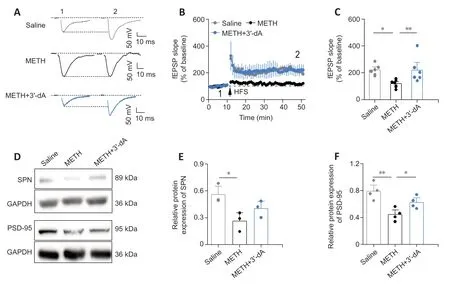
Figure 3 |The impairment of synaptic plasticity is prevented by 3′-deoxyadenosine (3′-dA) in methamphetamine (METH)administered mice.
3′-dA reduces METH-induced NLRP3 activation and hippocampal neuronal damage
To investigate whether 3′-dA could attenuate neuroinflammation and neuronal damage in METH-addicted mice,we examined NLRP3 inflammasome expression and histological neuronal data in the three groups.We found that NLRP3 expression was higher in the METH group than in the Saline group,while treatment with 3′-dA reduced this elevation (P<0.05;Figure 4A).Next,we extracted tissue supernatant to determine the relative expression of TNF-α and IL-1β.ELISA results showed that the relative expression levels of TNF-α and IL-1β were significantly higher in the METH group compared with the levels in the Saline group,while treatment with 3′-dA suppressed the elevation of these inflammatory factors (P<0.05;Figure 4BandC).According to the Nissl staining data,3′-dA attenuated the damage to neurons caused by METH administration (Figure 4DandE).Taken together,these results suggest that 3′-dA reduces METH-induced NLRP3 activation and IL-1β-and TNF-α-mediated inflammatory responses,thereby decreasing hippocampal neuronal damage.

Figure 4 |3′-Deoxyadenosine (3′-dA) reduces methamphetamine (METH)-induced NOD-like receptor family pyrin domain containing-3 (NLRP3) activation and hippocampal neuronal damage.
NLPR3 deficiency decreases METH-induced seeking behavior,abnormal synaptic plasticity,and neuronal damage
We have shown that 3′-dA decreased the expression of the NLRP3 inflammasome and aberrant synaptic plasticity in METH-exposed mice.Next,we asked whether the NLRP3 inflammasome contributes to aberrant synaptic plasticity,thereby affecting seeking behavior.We used NLRP3 knockout mice to verify the link between neuroinflammation and synaptic plasticity (Figure 5A).The three groups of mice were wild-type mice treated with saline (WT+Saline),wildtype mice treated with METH (WT+METH),and NLPR3 KO mice treated with METH (NLRP3+METH).We found that CPP scores were higher in the WT+METH group compared with those in the WT+Saline group (P<0.001).The CPP scores in the NLRP3 KO+METH group were lower than those in the WT+METH group,suggesting that a NLPR3 deficiency decreases METH-induced seeking behavior (P<0.001;Figure 5B–D).Furthermore,we examined hippocampal synaptic plasticity in the NLRP3 KO+METH mice after METH administration.The results showed an increase fEPSP slope in the NLRP3 KO mice,suggesting that the impaired synaptic plasticity was restored in mice with a NLPR3 deficiency (P<0.01;Figure 5E–G).Moreover,we collected brain tissue from the three groups of mice and conducted Nissl staining.We found that the damage to hippocampal CA1 neurons was attenuated in the NLRP3 KO mice compared with that in the WT+METH mice (P<0.05;Figure 5HandI).Taken together,these data indicate that a NLPR3 deficiency reduces METH-related addictive behavior,neuronal damage,and altered synaptic plasticity.

Figure 5 |NOD-like receptor family pyrin domain containing-3 (NLRP3) deficiency decreases methamphetamine (METH)-induced seeking behavior,reduces impaired synaptic plasticity,and prevents hippocampal neuronal damage.
Application of an NLRP3 agonist reverses the therapeutic effects of 3′-dA in METH-administered mice
To further determine whether 3′-dA exerts its therapeutic effect through the NLRP3 inflammasome,we applied nigericin(Nig),which is an NLRP3 agonist.We measured the CPP scores of mice given both Nig and 3′-dA (Figure 6A).There were no significant differences in CPP scores among the three groups during the pre-test (P>0.05;Figure 6BandC).The CPP scores in the 3′-dA group were lower than those in the METH group (P<0.001).The CPP scores in the METH+3′-dA+Nig group were significantly increased compared with those in the METH+3′-dA group (P<0.001;Figure 6D),suggesting that Nig reversed the effects of 3′-dA on METH-induced preference.The hippocampal field potential data showed that simultaneous administration of Nig,3′-dA,and METH failed to induce LTP,suggesting that Nig reversed the recovery effect of 3′-dA on METH-induced synaptic plasticity (P<0.01;Figure 6E–G).The Nissl staining data also demonstrated that Nig reversed the neuroprotective effect of 3′-dA in METH-addicted mice (P<0.01;Figure 6HandI).Taken together,these data indicate that NLRP3 agonists can reverse the therapeutic effect of 3′-dA on METH administered mice,suggesting that the NLRP3 inflammasome mediates the role of 3′-dA in seeking behavior.
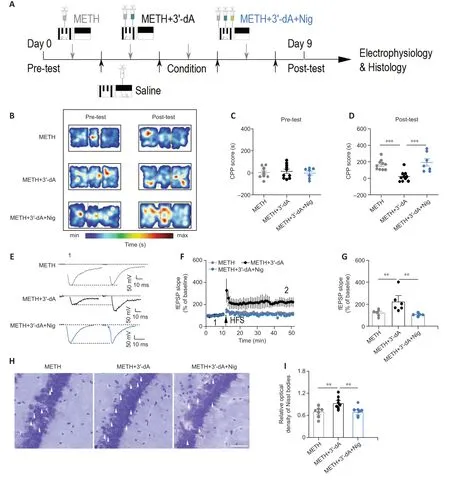
Figure 6 |An NOD-like receptor family pyrin domain containing-3 (NLRP3)agonist reverses the therapeutic effects of 3′-deoxyadenosine (3′-dA) in methamphetamine (METH)-treated mice.
Discussion
The present study demonstrated that 3′-dA attenuated CPP scores in METH-treated mice and decreased the activity of hippocampal neurons.Furthermore,3′-dA reduced the aberrant potentiation of glutamatergic transmission caused by METH administration.While METH impaired synaptic plasticity,postsynaptic protein function was restored via treatment with 3′-dA.Moreover,we found that 3′-dA decreased METH-induced NLRP3 expression and neuronal injury.Importantly,a NLRP3 deficiency directly reduced METH-induced seeking behavior,restored the impaired synaptic plasticity,and prevented neuronal damage.Finally,NLRP3 activation reversed the effect of 3′-dA on behavior and synaptic plasticity,suggesting that the anti-neuroinflammatory effect of 3′-dA prevents aberrant synaptic plasticity,thereby reducing METH-induced seeking behavior.Taken together,these findings indicate that 3′-dA application could be a promising novel strategy for treating METH addiction.
Repeated METH use can lead to addictive memory,which may trigger drug-seeking behavior.3′-dA acts as a neurotransmitter and is easily transported across the blood-brain barrier,thereby influencing neurons in the brain (Pardridge,2012;Li et al.,2016).Although 3′-dA has a positive effect on abnormal memory in individuals with depression and Parkinson’s disease(Cox et al.,2017;Zhang et al.,2021),whether and how 3′-dA attenuates METH-induced seeking behavior remains unclear.Our results showed that 3′-dA reduced seeking behavior in METH-addicted mice,suggesting that 3′-dA plays an important role in the attenuation of abnormal memory.Indeed,studies have demonstrated that METH-induced changes in memory affect seeking behavior (Gass et al.,2009;Nazari-Serenjeh et al.,2023).Therefore,it is not surprising that 3′-dA prevented METH-induced seeking behavior in our mouse model.C-fos,an immediate early gene,is used to measure memory activity in the hippocampus.3′-dA reduced METH-induced changes in the expression of c-fos,suggesting that the activity of the hippocampus contributes to the attenuation of METH-induced seeking behavior by 3′-dA.Given that the seeking behavior was accompanied by changes in hippocampal activity,3′-dA may function via a postsynaptic mechanism.The abundance of AMPARs in the postsynaptic region has been closely linked with LTP,long-term depression,and homeostatic scaling(Menon et al.,2013;Ma et al.,2017,2018,2022).Notably,pathological increases in AMPAR levels have been observed in hippocampal CA1 neurons in mice following METH addiction (Kauer and Malenka,2007;Pascoli et al.,2018).We found that METH induced an increase in the AMPARmediated current and AMPAR/NMDAR ratio,which is in line with previous findings indicating that AMPAR is the primary target affected by METH (Luscher and Bellone,2008;Luscher and Malenka,2011;Gipson et al.,2014;Sanderson et al.,2021).Furthermore,3′-dA decreased the activity of AMPARmediated responses,together with the PPR,suggesting that the action of 3′-dA is related to postsynaptic mechanisms that influence abnormal glutamatergic transmissions (Jiang et al.,2022).These observed effects of 3′-dA on AMPAR function are consistent with a report indicating that 3′-dA promotes the phosphorylation of GluR1 s845 in AMPARs (Frey et al.,2009;Iino et al.,2020).Indeed,abnormal synaptic plasticity may be mediated by calcium-permeable AMPARs (Frey et al.,2009;Yagishita et al.,2014;Azarnia Tehran et al.,2022).Specifically,when there is an increase in aberrant glutamatergic transmissions,this could impair normal synaptic plasticity(McCutcheon et al.,2011).We found that METH exposure induced a decrease in synaptic plasticity and that 3′-dA recovered this depression.Additionally,treatment with 3′-dA resulted in elevated expression of PSD-95 and SPN,indicating that 3′-dA restores normal synaptic function (Sanderson et al.,2016).
After demonstrating that 3′-dA restores synaptic plasticity,we investigated the mechanisms of 3′-dA in METH-induced seeking behavior.METH activates NOD-like receptors,triggering the innate immune response and leading to the release of TNF-α and IL-1β (Buchanan et al.,2010).We found that the NLRP3 was activated in METH-treated mice and that 3′-dA attenuated the expression of NLRP3.This result is consistent with findings regarding the prevention of the NLRP3 inflammasome by 3′-dA in other diseases (Yang et al.,2017).Simultaneously,3′-dA reduced hippocampal neuronal damage,indicating a neuroprotective effect on METHinduced neuronal damage.Given that NLRP3 is involved in the attenuation of METH-induced plasticity by 3′-dA,there may be a direct link between NLRP3 and synaptic plasticity.NLRP3-deficient NLRP3 KO mice showed a decrease in METH-induced CPP behavior.Indeed,several studies have demonstrated that NLRP3 is significantly increased in various brain regions during METH addiction (Li et al.,2022).With the changed synaptic plasticity in METH-exposed NLRP3 KO mice,we demonstrated a link between NLPR3 and synaptic plasticity in METH-induced seeking behavior.NLRP3-deficiency induces hippocampal dysfunction and reduces the amplitude of synaptic plasticity(Komleva et al.,2021).In our study,NLRP3 KO mice exhibited a recovery of synaptic plasticity after METH administration.Although this seems like a contradiction,increasing evidence has shown that repeated METH administration activates NLRP3 and increases IL-1β and TNF-α (Guo et al.,2023),which may aggravate hippocampal neuronal injury and impair synaptic plasticity.The reason for NLRP3 knockout-mediated recovery of synaptic plasticity is that NLRP3 deficiency prevents METHinduced neuronal damage and restores hippocampal function.This idea is in agreement with previous studies indicating that neuroinflammation drives glutamatergic transmission,thereby affecting behavior (Merkel et al.,2017;Mirabella et al.,2021).Importantly,nigericin,an NLRP3 agonist,was able to inhibit the therapeutic effect of 3′-dA on METH-addicted mice,further demonstrating that 3′-dA exerted its role through the NLRP3 inflammasome (Han et al.,2019).Thus,our findings demonstrate that 3′-dA ameliorates METH-induced aberrant synaptic plasticity via NLRP3 activation,thereby attenuating METH-induced seeking behavior.
This study has some limitations that should be noted.The mice in the saline group were not treated with a solvent vehicle like that used to dissolve the nigericin.Although prior investigations have shown that solvents exert a negligible influence on mouse behavior and inflammation (Colucci et al.,2008),subsequent studies are needed to confirm the role of solvents on METH-induced seeking behavior.Further work is needed to examine the expression of signaling cascade of the NLPR3 inflammasome,including caspase-1 and an apoptosis-associated speck-like protein containing a caspase recruitment domain.This would provide further evidence regarding the effects of the NLRP3 inflammasome on METHinduced seeking behavior.Furthermore,although we obtained some data regarding the mechanisms of action by which the NLRP3 inflammasome attenuates the effect of 3′-dA on METHinduced aberrant synaptic plasticity,the other underlying mechanisms of neuroinflammation remain unclear.More studies to determine the mechanisms of 3′-dA in this context are warranted.
In summary,we demonstrated that 3′-dA reduces METHinduced seeking behavior in mice.The mechanisms involved the prevention of aberrant synaptic plasticity and the attenuation of impaired synaptic plasticity.3′-dA also inhibited NLPR3 and decreased the neuronal damage.Importantly,a direct NLRP3 deficiency could reduce METH-induced impairments in synaptic plasticity.Our results demonstrate that 3′-dA reduces expression of the NLRP3 inflammasome and attenuates aberrant synaptic plasticity,providing possible strategies for the treatment of METH addiction.
Acknowledgments:We acknowledge Qiulun Lv’s lab for kindly providing NLRP3 KO mice on related experiments.
Author contributions:Study design and supervision:TM,SF and LZ;animal model and behavioral test:FZ,YW,MY,and CW;immunofluorescence staining and western blotting:YQ,YZ,JL,and GG;electrophysiology experiments:YQ,YZ,and JL;data collection and analysis:YQ,YZ,and JL;manuscript draft:TM,SF,LZ and YQ;project administration and manuscript revision:TM,SF and LZ.All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- Small extracellular vesicles from hypoxiapreconditioned bone marrow mesenchymal stem cells attenuate spinal cord injury via miR-146a-5p-mediated regulation of macrophage polarization
