Efficacy of exercise rehabilitation for managing patients with Alzheimer’s disease
2024-01-24DanLiJinningJiaHaiboZengXiaoyanZhongHuiChenChenjuYi
Dan Li ,Jinning Jia ,Haibo Zeng ,Xiaoyan Zhong ,Hui Chen ,Chenju Yi
Abstract Alzheimer’s disease (AD) is a progressive and degenerative neurological disease characterized by the deterioration of cognitive functions.While a definitive cure and optimal medication to impede disease progression are currently unavailable,a plethora of studies have highlighted the potential advantages of exercise rehabilitation for managing this condition.Those studies show that exercise rehabilitation can enhance cognitive function and improve the quality of life for individuals affected by AD.Therefore,exercise rehabilitation has been regarded as one of the most important strategies for managing patients with AD.Herein,we provide a comprehensive analysis of the currently available findings on exercise rehabilitation in patients with AD,with a focus on the exercise types which have shown efficacy when implemented alone or combined with other treatment methods,as well as the potential mechanisms underlying these positive effects.Specifically,we explain how exercise may improve the brain microenvironment and neuronal plasticity.In conclusion,exercise is a cost-effective intervention to enhance cognitive performance and improve quality of life in patients with mild to moderate cognitive dysfunction.Therefore,it can potentially become both a physical activity and a tailored intervention.This review may aid the development of more effective and individualized treatment strategies to address the challenges imposed by this debilitating disease,especially in low-and middle-income countries.
Key Words:Alzheimer’s disease;cerebrovascular function;cognitive function;epigenetic regulation;exercise;hippocampal neurogenesis;mitochondria;neuroinflammation;neuronal plasticity
Introduction
Alzheimer’s disease (AD) is a chronic and progressive neurodegenerative disorder that significantly impairs neurocognitive and behavioral functions,especially among the aging population.It is the leading cause of dementia (Lane et al.,2018).The World Alzheimer Report 2022 projected the number of people living with dementia will continue to increase worldwide in the next 30 years,more in low-and middle-income countries,with costly medicare expenses(Alzheimer’s Disease International,2022).
While specific pharmacotherapies may slow cognitive decline in selected cohorts of patients with early-stage AD,these are often of low efficacy and unable to halt irreversible neuronal loss due to ongoing neurodegeneration.Consequently,a decline in patient’s independent living capabilities persists despite these interventions (Burns et al.,2009).Additionally,adverse reactions restrict the use of these drugs among the aging population.For example,Donepezil can cause extrapyramidal symptoms,bradycardia,gastrointestinal bleeding,nausea,and vomiting;Rivastigmine is linked to an increased risk of all-cause mortality,especially among patients who are critically ill;and Memantine,which has relatively mild side effects,may still cause dizziness,headache,hypertension,somnolence,and constipation (Shi et al.,2016).In contrast,exercise programs can effectively enhance cognitive function,daily living abilities,and life quality,while alleviating depressive symptoms in patients with AD (Hoffmann et al.,2016;Jia et al.,2019).Exercise has thus emerged as an indispensable complementary strategy to pharmacotherapy in patients with AD,to preserve their cognitive functions and independent living abilities,especially in low-and middle-income countries where drugs to improve AD symptoms are too expensive for most patients.As such,the goal of this review was to provide an overview of how exercise rehabilitation can improve neurocognitive functions in patients with AD,and the possible molecular mechanisms involved.
Search Strategy
Searches of PubMed,Ovid Medline,and Web of Science were performed using “AD”,“cognitive function”,“aerobic exercise”,“resistance exercise”,“multimodal exercise”,“Aβ”,and “tau”.The search for mechanistic studies used the keywords “AD”,“exercise”,and “cognitive function”,in combination with one of the following keywords: “cerebrovascular dysfunction”,“synaptic plasticity”,“hippocampal neurogenesis”,“microglia”,“astrocyte”,“BDNF”,“IGF-1”,“irisin”,“mitochondrial integrity”,“epigenetics regulation”,or “sex difference”.We included peer-reviewed original research papers published in English between January 2000 and June 2023,in either animal models (to compare different exercise types) or humans with AD (randomized controlled trials,meta-analyses,and observational studies) which reported positive results.The effects of various forms of exercise,in combination with other treatments for cognitive function and their proposed mechanisms,are summarized herein.We did not include publications with insufficient statistical power (e.g.,case studies).
Pathophysiology of Alzheimer’s Disease
The primary neuropathological features of AD are amyloid-β(Aβ) plaque formation and neurofibrillary tangles,leading to the gradual loss of functional neurons (also called‘neurodegeneration’) in the hippocampal,neocortical,and basal ganglia regions.Several of these brain regions are involved in memory formation and retrieval.The hippocampus is closely related to the formation of learning and memory,and plays a key role in spatial navigation during memory formation (Voss et al.,2017).The prefrontal cortex is responsible for executive functions,such as consolidating long-term memory and decision-making,and regulates hippocampal activity (Weilbächer and Gluth,2016).
Aβ peptides are cleaved from amyloid precursor protein(APP),an essential membrane protein for synapse formation and repair.In the AD brain,APP is processed by β-secretase,instead of α-secretase,to generate a soluble APP beta.The remaining transmembrane portion of APP is then recognized and cleaved by γ-secretase to generate an Aβ monomer fragment,Aβ40or Aβ42.Then,several Aβ monomer fragments,especially Aβ42,assemble to form insoluble oligomers or senile plaques (LaFerla et al.,2007;Sha et al.,2022;An and Hulme,2023).Excessive Aβ deposition and abnormal nervous structural changes activate microglia for clearance,which in turn initiates proinflammatory responses that promote oxidative stress and further neuronal damage(Hong et al.,2016).Synaptic connections and plasticity are critical for memory formation,storage,and retrieval,thus enabling learning from experiences.Aβ oligomers can lead to overstimulation of N-methyl-D-aspartate (NMDA) receptors(Fani and Chiti,2022),the glutamate receptors responsible for regulating synaptic plasticity.This overstimulation can cause dysfunction of hippocampal neuronal activation,memory coding,and storage,ultimately resulting in decreased memory retrieval abilities (Tu et al.,2014).
In early-stage AD,site-specific phosphorylation of tau protein can inhibit Aβ toxicity (Ittner et al.,2016).However,tau hyperphosphorylation makes it unable to bind to tubulin in the AD brain,and its accumulation results in the formation of neurofibrillary tangles,which block the production and function of several proteins.For example,hyperphosphorylated tau can interact with c-Jun N-terminal kinase-interacting protein 1,impairing the formation of the kinesin complex and affecting axonal transport (Ittner et al.,2009).The synergistic effect of extracellular Aβ plaques and intracellular neurofibrillary tangles precipitates neurodegeneration (Busche and Hyman,2020).Depletion of the tau gene in APP transgenic J20 mice can reduce hippocampal hyperactivation and thus improve motor function and spatial memory recall (Yoshikawa et al.,2018).This suggests that tau dysfunction may be more critical than Aβ toxicity in early-stage AD pathogenesis.
The buildup of Aβ plaques can also increase redox-mediated oxidative stress and cytoplasmic Ca2+levels (Tu et al.,2014).Subsequently,downstream signaling (e.g.,serine/threonine protein phosphatase 2A and 2B) is activated to inhibit calcium/calmodulin-dependent protein kinase II and induce endocytosis of ionotropic glutamate receptors (e.g.,α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors).Similarly,oligomeric Aβ can induce endocytosis of NMDA receptors,mediated by dephosphorylation of NMDA receptor subunit NR2B,leading to synaptic dysfunction(Tu et al.,2014;Figure 1).Accumulation of damaged mitochondria is a distinctive hallmark of both aging and agerelated neurodegenerative conditions,which are closely intertwined with impaired Aβ clearance mechanisms (Fang et al.,2019).Mitophagy,the mitochondrial self-renewing mechanism,can restore functional neuronal mitochondrial populations in neurons in AD models,resulting in increased microglial phagocytosis of extracellular Aβ plaques and insoluble Aβ1–42and Aβ1–40,reduced neuroinflammation,and improved cognitive function (Fang et al.,2019).In AD models,mitochondrial damage within neurons also contributes to an increased complement-mediated synapse tag,which activates adjacent microglia to initiate presynaptic elimination (Györffy et al.,2020).This further impairs neuroplasticity and cognitive function,particularly learning and memory functions.
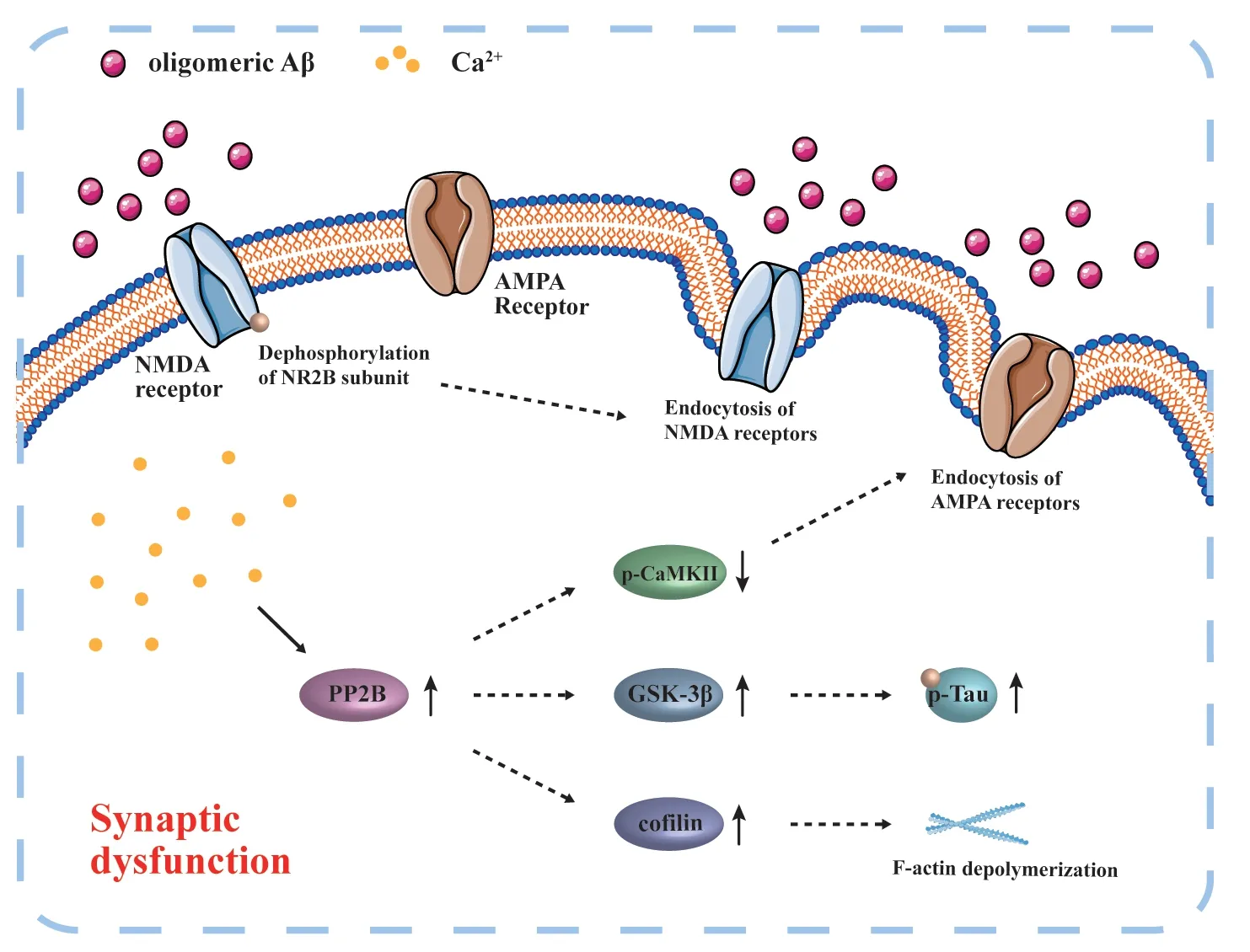
Figure 1 |Schematic diagram of oligomeric Aβ-induced synaptic damage.
Types of Exercise Rehabilitation in Patients with Alzheimer’s Disease
Regular exercise facilitates balanced coordination of the body’s response to unexpected situations and supports the preservation of normal brain functions,effectively preventing significant cognitive decline.The risk of cognitive decline is 35–38% lower in physically active individuals compared with their sedentary counterparts (Sofi et al.,2011).Evidence continues to mount regarding the positive roles of exercise in managing and preventing neurodegenerative disorders like AD.In APPswe/PS1ΔE9 mice,voluntary exercise has been shown to prevent memory loss and reverse neuropathological changes related to AD progression (Tapia-Rojas et al.,2016).
Aerobic exercise
Aerobic exercise is the predominant approach to slowing cognitive decline in older adults (De la Rosa et al.,2020).Several clinical trials have demonstrated that regular moderate-intensity exercise (40–60 minutes duration,3 days/week) can significantly increase brain volume,which is indicative of neurogenesis,and is associated with improved memory function in healthy older adults (Colcombe et al.,2006;Erickson et al.,2011;De la Rosa et al.,2020).In patients with mild AD,moderate-intensity aerobic exercise also effectively improves cognitive function (Yang et al.,2015).Aerobic exercise has the capacity to enhance brain energy metabolic homeostasis by increasing ketone uptake and metabolism,a phenomenon associated with cognitive improvement (Castellano et al.,2017).Moreover,engaging in moderate-to-high-intensity aerobic exercise has favorable effects on cardiopulmonary function,physical performance in single and dual tasks,and exercise self-efficacy in patients with mild AD (Sobol et al.,2016).Exercise also alleviates neuropsychiatric symptoms in patients with mild AD (Sobol et al.,2018).Even acute aerobic exercise of moderate intensity(20 minutes of cycling) can benefit thinking abilities in patients with mild AD,and is even more effective when combined with cognitive mental training games (Ben Ayed et al.,2021).An animal model study showed that aerobic exercise improves cognitive performance by reducing neuronal apoptosis by activating the phosphoinositide 3-kinase (PI3K)/serinethreonine kinase (Akt)/glycogen synthase kinase 3 beta (GSK-3β) signaling pathway in D-galactose-and aluminum chlorideinduced AD model mice (Peng et al.,2022),which may be a mechanism underlying the cognitive benefits in humans described above.Therefore,engaging in aerobic exercise may be an option for older adults,to delay age-related dementia,and for patients with mild AD,to mitigate the rapid neurocognitive decline.
Resistance exercise
Resistance exercise,also called strength training,has been suggested to reverse the loss of muscle mass,muscle function,and brain structural deterioration in patients with AD.In a rat AD model,a single injection of Aβ1–42into the cornu ammonis (CA) 1 region of the hippocampus was sufficient to cause muscle atrophy from loss of myonuclear number and satellite cell content,whereas resistance training successfully restored muscle mass by significantly enhancing the level of myosin heavy chain IIb fiber in myofibers (Rahmati et al.,2023).Resistance exercise is also beneficial for increasing muscle strength in trained individuals and alleviating depressive symptoms in older adults affected by AD (Chang et al.,2020).In 3×Tg AD model mice (a transgenic mouse AD model),short-term resistance exercise reduces Aβ load,tau hyperphosphorylation,reactive astrogliosis,and inflammatory responses in the frontal cortex and hippocampus;this effect also correlated with improved synaptic plasticity and cognitive functions,including short-term memory and working memory functions (Liu et al.,2020).Resistance exercise can also reverse cognitive dysfunction from neuroinflammation via insulin-like growth factor 1 (IGF-1) signaling in the hippocampal dentate gyrus region (Kelty et al.,2019).Long-term resistance training in APP/PS1 mice can also activate microglia recruitment,without enhancing inflammatory responses,but increasing elimination of Aβ deposition (Hashiguchi et al.,2020).Consequently,locomotor hyperactivity was ameliorated in those AD model mice (Hashiguchi et al.,2020).
In humans,it has been shown that with every unit increase in muscle strength,there is a 43% reduction in the chance of developing AD at the onset of cognitive impairment (Balsamo et al.,2013).This suggests that there may be a direct interaction between muscle function and brain well-being,possibly involving chemokines or non-coding RNAs released from exercised muscles.These molecules reach the central nervous system to promote synaptic plasticity and improve neurological functions.The ‘exerkine’ concept was introduced when the skeletal muscle was considered an endocrine organ,in the context of physical activity-induced secretions,along with liver and adipose tissues.Exerkines may act as messengers during muscle-brain crosstalk.Exercise-mediated myokines (e.g.,lactate,irisin,interleukin [IL]-6) are released into circulation,cross the blood-brain barrier (BBB),and positively affect synaptic plasticity and memory by enhancing brain mitochondrial function (Heo et al.,2023).
It is important to note that strength training is often highintensity and can be challenging to maintain as a regular,longterm activity,especially by middle-aged and older patients with movement impairments.In these scenarios,it may be appropriate to consider assisted strength training,which involves facilitative support by trained carers or personal trainers.
Flexible exercise regimen
Older adults can benefit from a multimodal exercise regimen that combines aerobic exercise,postural balance,muscular strength training,and flexibility training.A combination of different exercise types may be easier to follow compared with a single type.Both aerobic and resistance exercises are important for improving the cognitive status of patients with AD (Tsai et al.,2018).It has been shown that 12 weeks of multimodal exercise can decrease the fall risk in older women with moderate cognitive impairment,and enhance their focus and ability to perform dual tasks (Thaiyanto et al.,2021).It can also significantly increase frontal lobe function,contributing to better cognitive function,postural balance,and physical capacity (de Andrade et al.,2013).Therefore,in individuals with dementia,multimodal exercise may help improve cognitive and physical functionality in daily living activities.
Repeated transcranial magnetic stimulation (rTMS) has been found to significantly improve cognitive function in patients with mild-to-moderate AD (Xie et al.,2021),and alleviates cognitive deficits in 3×Tg AD model rodents by activating the PI3K/Akt/glutamate transporter-1 pathway (Cao et al.,2022).High-frequency rTMS may improve executive functions and behaviors in patients with AD,while moderate-intensity aerobic exercise may enhance balance and mobility (Budak et al.,2023).The combination of rTMS and physical exercise may better ameliorate neurological impairment in patients with AD.Exercise rehabilitation combined with music therapy is also more effective in ameliorating neuropsychiatric symptoms and boosting the positive effects of exercise rehabilitation in individuals with mild-to-moderate AD (Li et al.,2022).Exercise holds promise for reducing fall risk among individuals with AD who use antihypertensives and psychotropics (Perttila et al.,2018).Engaging in exercise with functional tasks can have considerable benefits for those with mild cognitive impairment,including general cognitive function,memory,executive function,and everyday problem-solving abilities(Law et al.,2014).
Voluntary physical activities are also beneficial to cognitive performance compared with a sedentary lifestyle.In a transgenic mouse model expressing the human mutant APP(APPSw,Ind),environmental enrichment with a running wheel for voluntary exercise restored adult animal neurogenesis and memory function after seven weeks of exposure (Valero et al.,2011).This model may represent engagement with outdoor activities and experiencing diverse surroundings,which are considered important for sculpting the brain for memory consolidation (Bonaccorsi et al.,2013).The results of the animal study also support the theory that context may significantly improve hippocampal-dependent spatial learning and memory deficits at early-stage AD development in humans (Valero et al.,2011).The associated adult hippocampal neurogenesis is reflected by increased synaptic number,dendritic length,and neural projections to the CA3 region.However,Aβ levels and the number of neurons in the dentate gyrus are unchanged (Valero et al.,2011).This cumulative evidence suggests that promoting neurogenesis is a key treatment strategy for improving cognitive function in patients with AD,rather than focusing only on Aβ clearance.
Potential Mechanisms of Exercise
Various forms of exercise likely benefit cognitive performance in patients with AD via different biological mechanisms.Studies investigating the mechanisms of AD pathophysiology often use genetically modified murine models,such as transgenic APP/PS1 mice (also called TgAPP/PS1 mice),3×Tg AD model mice,the senescence-accelerated mouse prone 8(SAMP8) mice,and 5×FAD model mice.Other studies have used wildtype rodents by introducing exogenous neurotoxins,such as Aβ analogs,D-galactose with aluminum chloride,and streptozotocin.The proposed mechanisms by which exercise alters AD pathology based on animal model research are listed inTable 1andFigure 2.Clinical trials with patients with AD are listed inTable 2.
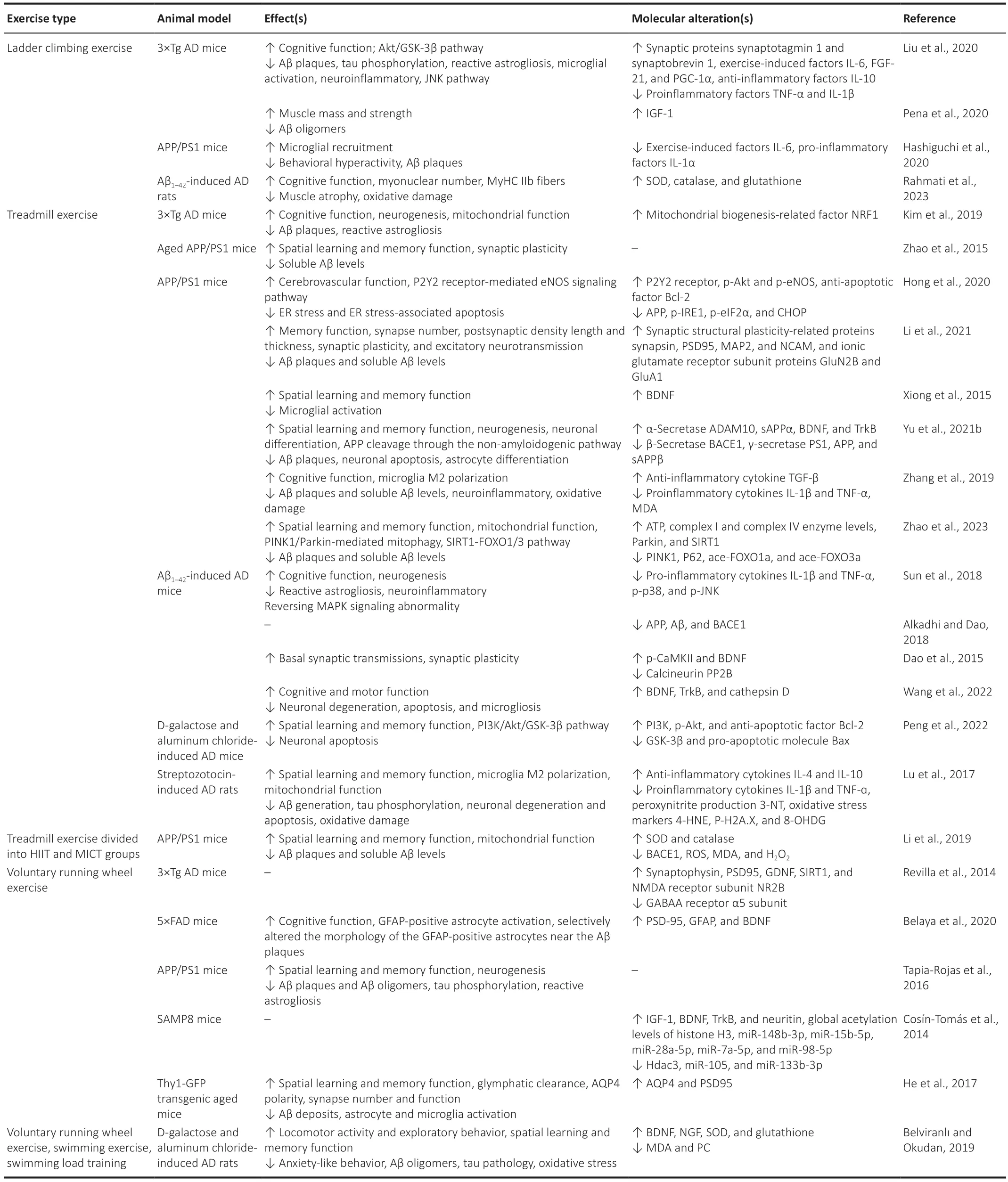
Table 1 |Effects of exercise training on rodent AD models
Exercise improves cerebrovascular dysfunction
Coordination between neuronal activity and cerebral blood flow is maintained by a mechanism called neurovascular coupling (Kisler et al.,2017a).The neurovascular unit,consisting of endothelial cells,vascular smooth muscle cells,pericytes,neurons,and glia,controls this coupling (Kugler et al.,2021).The glutamate-NMDA receptor-neuronal nitric oxide (NO) synthase axis is critical for cerebrovascular function,because it triggers soluble guanylate cyclase in nearby arteriolar smooth muscle cells to promote vasodilation(Lourenço and Laranjinha,2021).In patients with AD,a progressive reduction in cerebral blood flow in affected brain regions is closely linked to their cognitive decline (Weijs et al.,2023).Exercise training can improve peripheral vascular function in patients with AD,by increasing NO and vascular endothelial growth factor to cause vasodilation and increase arterial blood flow and shear rate (Pedrinolla et al.,2020).
NO,a major vasodilator,is generated by the activation of endothelial NO synthase.The decrease in NO in the cerebrovascular endothelium is associated with increased levels of APP and β-site APP-cleaving enzyme 1,resulting in increased production of cytotoxic Aβ1–40and Aβ1–42(Austin et al.,2010,2013a).In addition,endothelial NO plays important roles in regulating synaptic plasticity,mitochondrial biogenesis,and function of neuronal progenitor cells (Katusic and Austin,2014),linking cerebrovascular function with cognition.Indeed,endothelial NO synthase-deficient mice exhibit impaired cognitive performance (Austin et al.,2013b).In APP/PS1 mice,exercise can ameliorate cerebrovascular dysfunction by enhancing P2Y2 receptor-mediated endothelial NO synthase signaling and NO release (Hong et al.,2020).Exercise can also alleviate endoplasmic reticulum stress and associated apoptosis by reducing phosphorylated inositolrequiring enzyme 1,phosphorylated eukaryotic initiation factor 2 and CCAAT-enhancer-binding protein homologous protein,which are all significantly elevated in the brain of AD model mice (Hong et al.,2020).
Pericytes play a critical role in the stabilization of the capillary wall,maintenance of the BBB,and regulation of capillary diameter and cerebral blood flow.Pericyte degeneration leads to neurovascular uncoupling,reduced brain oxygen supply,and metabolic stress (Kisler et al.,2017b).Reduced pericyte number may disrupt BBB properties and result in neuronal dysfunction during AD pathogenesis (Sengillo et al.,2013).Aβ can induce pericyte-mediated cerebral capillary blood vessel constriction,resulting in reduced cerebral blood flow during early-stage AD (Nortley et al.,2019).Therefore,managing dysfunctional neurovascular units may help slow neurodegeneration and improve cognitive function in patients with AD.
Indeed,12 months of aerobic exercise by patients with mild cognitive impairment has been shown to improve memory function and blood flow in the hippocampus and anterior cingulate cortex,without changes in brain volume (Thomas et al.,2020).Restoring blood supply by exercise can increase brain oxygenation and nutritional supply and benefit cognitive functions.However,another study showed that 16 weeks of moderate-to-high intensity aerobic exercise was insufficient to produce a sustained increase in cerebral blood flow,possibly due to the short intervention time or small sample size (van der Kleij et al.,2018).In some cases,there may be improved blood vessel oxygen and nutrition delivery function,rather than an increase in absolute blood volume.This speculation needs to be confirmed in future studies.Nevertheless,sustained exercise,especially at the early stage of AD,may prevent vascular lesions and dysfunction by maintaining sufficient cerebral perfusion.
Exercise enhances synaptic plasticity and hippocampal neurogenesis
Synaptic plasticity is fundamental to learning and memory.At the early stage of AD,synaptic dysfunction and loss of dendritic spines are associated with cognitive decline and other neurological impairments (Selkoe,2002).Aβ can interact with ionic glutamate receptors to reduce synaptic integrity and plasticity,resulting in synaptic loss and neuronal death(Shankar et al.,2007).There is also a significant increase in synaptic markers in cerebrospinal fluids,such as postsynaptic density protein 95,presynaptically localized synaptosomalassociated protein 25,and neurogranin,which may be used as early diagnostic biomarkers (Kivisäkk et al.,2022).Exercise can reduce Aβ40,Aβ42,and Aβ deposition,resulting in significantly increased synaptic number,as well as length and thickness of postsynaptic structures in hippocampal CA1 (Li et al.,2021).In a rat AD model,impaired basal synaptic transmission and long-term potentiation in the dentate gyrus can be rescued by 4 weeks of moderate treadmill exercise,as well as normalized basal levels of phosphorylated calcium/calmodulindependent protein kinase II and protein phosphatase 2B(Dao et al.,2015).In 3×Tg mice,resistance and running wheel exercises can increase synaptic density and plasticity,resulting in improved cognitive performance (Revilla et al.,2014;Liu et al.,2020) via brain fibronectin type III domain-containing protein 5 (FNDC5)-irisin signaling (Lourenco et al.,2019).Therefore,improving synaptic density and plasticity may be key to restoring cognitive function in patients with AD by exercise.
Adult hippocampal neurogenesis is important in maintaining learning and memory functions throughout life.Alterations in hippocampal neurogenesis occur at the early stage of AD,even before neurofibrillary tangles or Aβ plaques appear in the dentate gyrus (Moreno-Jiménez et al.,2019).Interestingly,inducing hippocampal neurogenesis alone by drugs or genetic modification yields marginal cognitive benefits in 5×FAD mice;however,additional exercise can improve cognition,along with reduced Aβ deposition and increased brain derived neurotrophic factor (BDNF),FNDC5,and synapses.It is likely that exercise improves the local environment and thus enables hippocampal neurogenesis benefits (Choi et al.,2018).In fact,exercise alone,whether via running wheel or treadmill exercise,increases hippocampal neurogenesis and ameliorates cognitive dysfunction in several mouse models of AD,including Aβ1–42-induced AD,3×Tg,and APP/PS1 mice(Tapia-Rojas et al.,2016;Sun et al.,2018;Kim et al.,2019).Exercise can also increase brain BDNF levels,promote APP proteolysis,and reduce toxic Aβ peptides (Nigam et al.,2017),which contribute to a healthy hippocampal microenvironment for neurogenesis (Yu et al.,2021b).Therefore,hippocampal neurogenesis may be the goal for developing effective therapeutic strategies for patients with AD.
Exercise modulates glial functions
Microglia are the innate immune cells in the brain and the first responders to pathological changes (Nguyen and Chauhan,2023).Early microglial activation promotes Aβ clearance and is neuroprotective.With disease progression,these activated microglia produce large numbers of the proinflammatory cytokines IL-1β and tumor necrosis factor alpha (TNF-α),which inhibit microglial Aβ-binding receptors(e.g.,scavenger receptor A,CD36) and Aβ-degradation enzymes (e.g.,insulysin,neprilysin),decreasing its phagocytic capacity and exacerbating Aβ accumulation (Hickman et al.,2008).Oligomeric Aβ also induces endoplasmic reticulum stress and Ca2+release,leading to GSK-3β-mediated tau phosphorylation and neurofibrillary tangles and,subsequently,neurodegeneration (Resende et al.,2008).
Growing evidence suggests that exercise exerts neuroprotective effects by inhibiting microglia activities and related neuroinflammation in the AD brain.In APP/PS1 mice,12 weeks of treadmill exercise can preserve hippocampal cognitive functioning and suppress Aβ deposits at an early stage of AD,possibly by modulating microgliamediated neuroinflammation and oxidative stress (Zhang et al.,2019).Treadmill exercise promotes the transition of microglia from the proinflammatory (i.e.,neurotoxic) to the anti-inflammatory (i.e.,neuroprotective) phenotype,with increased anti-inflammatory cytokine transforming growth factor beta and decreased proinflammatory cytokines IL-1β and TNF-ɑ (Zhang et al.,2019).Similar effects of treadmill exercise on microglial phenotype change were also observed in streptozotocin-induced AD model rats,with increased anti-inflammatory cytokines IL-4 and IL-10 (Lu et al.,2017).In Aβ1–42-induced AD model mice,treadmill exercise attenuates proinflammatory responses in the hippocampus by modulating mitogen-activated protein kinase signaling (Sun et al.,2018).Resistance exercise also inhibits neuroinflammation in the frontal cortex of 3×Tg AD model mice (Liu et al.,2020).In older adults with mild cognitive impairment,both aerobic and resistance exercises can reduce serum TNF-α (Tsai et al.,2019).This cumulative evidence indicates that exercise can mitigate neuroinflammation by inhibiting microglial activities and promoting microglia polarization to an anti-inflammatory phenotype.
Astrocytes,which are supporting cells,assist in neuronal metabolism and synaptic transmission,maintain BBB integrity,and finely tune neuroinflammation with microglia.Astrocytes may also be neuroprotective by phagocytosis of Aβ deposits and dystrophic neurites (Wyss-Coray et al.,2003;Gomez-Arboledas et al.,2018).Astrocyte dysfunction due to the deletion of glial fibrillary acid protein and vimentin genes leads to increased Aβ plaques and related dystrophic neurites in APP/PS1 mice (Kraft et al.,2013).Similar to microglia,astrocytes have proinflammatory and anti-inflammatory phenotypes (Kwon and Koh,2020).Microglia-derived IL-1α,TNF-α,and complement component 1 subcomponent q can convert astrocytes to a neurotoxic phenotype,in which they lose their primary supporting functions (Liddelow et al.,2017).Astrocytes may represent a significant source of Aβ during neuroinflammation in AD,as amyloidogenic APP synthesis in astrocytes can be increased by the presence of proinflammatory cytokines (TNF-α and interferon-γ),as well as Aβ oligomers and fibrils (Zhao et al.,2011).
Both aerobic and resistance exercises can reduce astrocyte activation in the brain of AD model mice (Tapia-Rojas et al.,2016;Sun et al.,2018;Kim et al.,2019;Liu et al.,2020).In 5×FAD mice,6 months of voluntary exercise can remodel astrocytes,reversing cognitive impairment.Morphological analysis indicates that voluntary exercise induces significant increases in the primary branch number,branch length,and soma size of plaque-associated astrocytes,and increases BDNF and postsynaptic density protein 95 levels in all glial fibrillary acid protein-positive astrocytes(Belaya et al.,2020).Astrocytic water channel aquaporin 4 (AQP4) is normally located in the perivascular astrocytic end-feet ensheathing the brain vasculature,which facilitates the clearance of Aβ;loss of perivascular AQP4,also known as AQP4 depolarization,promotes Aβ plaque formation(Zeppenfeld et al.,2017;Simon et al.,2022).In aged mice,6 weeks of voluntary exercise promotes glymphatic clearance of Aβ and attenuates neuroinflammation by increasing AQP4 expression and polarization and restoring its perivascular localization (He et al.,2017).
Overall,this evidence indicates that exercise can mitigate neuroinflammation by regulating both microglia and astrocyte functions,improving cognitive function in AD models and patients.Exercise thus represents a promising option in the early management of AD.
Exercise induces neurotrophic factors
Brain BDNF is one of the most important growth factors,playing critical roles in neuronal survival,neurite outgrowth,and synaptic plasticity.It is highly expressed in the hippocampus,cerebral cortex,and basal forebrain,all of which are involved in learning,memory,and cognition.BDNF exerts its effects by interacting with tropomyosin-related kinase B receptors and subsequently activating various signaling pathways,including mitogen-activated protein kinase,PI3K,and phospholipase C-γ pathways (Huang and Reichardt,2003).BDNF can also reduce Aβ levels by enhancing α-secretase activity and shifting APP towards the non-amyloidogenic pathway (Nigam et al.,2017;Baranowski et al.,2023).Decreased BDNF levels may lead to synapse loss and cognitive dysfunction (Budni et al.,2015).Reduced brain BDNF occurs in early-stage AD and is associated with cognitive impairment (Peng et al.,2005).As the condition progresses,patients with AD also show decreased serum BDNF (Ng et al.,2019).Exercise can improve cognitive function by enhancing BDNF expression.In APP/PS1 transgenic mice,treadmill exercise can enhance BDNF expression in the hippocampus,which is associated with hippocampal neurogenesis and spatial memory improvement (Xiong et al.,2015;Yu et al.,2021b).In D-galactose and aluminum chloride-induced AD model rats,voluntary,involuntary,and forced exercises can equally reverse behavioral impairment by increasing hippocampal neurotrophic factors,including nerve growth factor and BDNF (Belviranlı and Okudan,2019).A metaanalysis showed that acute and chronic exercises in patients with AD can ameliorate cognitive impairment by increased blood BDNF levels,which may be used as a biomarker for evaluating the effect of exercise in patients with AD (Huang et al.,2021).
IGF-1 is an important neurotrophic factor that modulates neuronal excitability,metabolism,growth,and differentiation(Arsenijevic and Weiss,1998;Bassil et al.,2014).During AD progression,IGF-1 levels in the blood and cerebrospinal fluid are reduced,which may serve as a potential biomarker for predicting cognitive deterioration (Xu et al.,2021).Moreover,low baseline serum IGF-1 levels are associated with more rapid cognitive decline in patients with AD (Vidal et al.,2016).Exercise can significantly increase IGF-1 levels in the blood and boost brain uptake of circulating IGF-1 (Carro et al.,2000).The study found that intracarotid injection of IGF-1 can mimic the effect of exercise for increasing BDNF in the hippocampus,indicating that IGF-1 may be an upstream regulator of BDNF(Carro et al.,2000).Further research using experimental neurodegenerative mice confirmed that subcutaneous administration of anti-IGF-1 antibodies can block circulating IGF-1 from entering the brain,diminishing exercise-induced neuronal protection.This suggests that circulating IGF-1 is indispensable to exercise-induced neuroprotection (Carro et al.,2001).In individuals with mild cognitive impairment,acute aerobic exercise increases serum levels of both IGF-1 and BDNF,while acute resistance exercise only increases serum IGF-1 levels,indicating different working mechanisms of these two exercise types (Tsai et al.,2018).
Irisin,a myokine released by the proteolysis of FNDC5 in skeletal muscle after exercise (Madhu et al.,2022),can cross the BBB and induce BDNF expression in the hippocampus,improving neuronal function (Pedersen,2019) by activating the peroxisome proliferator-activated receptor-γ coactivator-1α/FNDC5 pathway (Wrann et al.,2013).Irisin can also promote hippocampal cell proliferation via signal transducer and activator of transcription 3 (STAT3) signaling pathway(Moon et al.,2013),and can reduce oxidative stressinduced neuronal damage by activating Akt and extracellular signal-regulated kinase 1/2 signaling pathways (Li et al.,2017).FNDC5 is also expressed in the hippocampus,and its levels both there and in cerebrospinal fluid are reduced in patients with AD and a rat AD model (Lourenco et al.,2019).Knockdown of brain FNDC5/irisin can weaken the neuroprotective effect of exercise on synaptic plasticity and memory retention in AD model mice (Lourenco et al.,2019).Furthermore,the administration of exogenous irisin effectively ameliorates both cognitive deficit and neuropathology in APP/PS1 and 5×FAD mice (Islam et al.,2021).Thus,irisin may represent a novel treatment option for managing cognitive decline in patients with AD.
Exercise improves mitochondrial integrity
Maintaining mitochondrial structural and functional integrity is critical to upholding cellular energy and metabolic equilibrium.Mitochondria damage leads to energy supply deficiency,intracellular calcium imbalance,and oxidative stress,all of which aggravate tau hyperphosphorylation and Aβ accumulation,resulting in synaptic dysfunction,cognitive decline,and memory loss (Chakravorty et al.,2019).Exercise-induced lactate can increase brain mitochondrial biogenesis-associated factors (e.g.,peroxisome proliferatoractivated receptor-γ coactivator-1α,nuclear respiratory factor 1 and 2,mitochondrial transcription factor A) and mitochondrial DNA copy numbers,and can improve mitochondrial dynamics in hippocampal neurons (Kim et al.,2019;Heo et al.,2023).In 6-month-old APP/PS1 mice,exercise has also been shown to improve the mitophagy machinery,which can promote mitochondrial renewal and mitochondrial function through the silent information regulator factor-1/forkhead transcription factors 1/3-phosphatase and tensin homolog-induced putative kinase 1/Parkin pathway (Liang et al.,2021;Zhao et al.,2023).High-intensity interval exercise and moderateintensity continuous exercise can also improve hippocampal mitochondrial morphology and reduce mitochondrial fragmentation and hippocampal Aβ burden in APP/PS1 mice(Li et al.,2019).Exercise can also increase the repair capacity of oxidative stress-induced damage to mitochondrial DNA and mitochondrial ATP production,leading to increased synaptic plasticity and synaptic density in the hippocampus and cerebral cortex of APP/PS1 mice (Pang et al.,2019).This suggests that mitochondrial integrity and function play key roles in synaptic plasticity in the AD brain.
Exercise affects epigenetic regulation
Epigenetic regulation is a key mechanism of neural response and adaptation to external environmental stimuli.The three main epigenetic mechanisms are DNA methylation,histone modification,and non-coding RNAs (e.g.,microRNAs).Aβ can increase DNA methylation of neprilysin (an enzyme responsible for Aβ degradation) and further suppress its mRNA expression and protein levels (Chen et al.,2009).Moreover,the frontal lobe of the AD brain has lower DNA methylation levels at the apolipoprotein E CpG island and exhibits increased mRNA expression of total apolipoprotein E,which is the most significant hereditary risk factor for late-onset AD (Lee et al.,2020).It has been confirmed that methyl-CpG binding protein 2-mediated dysregulation of the epigenome in the striatum is linked to impaired cognitive functions and abnormal neuronal activity in AD model mice,which can be rescued by knocking down striatal methyl-CpG binding protein 2 (Lee et al.,2022).A possible protective role of mild cognitive impairment by exercise is supported by changes in genome-wide DNA methylation patterns (Ngwa et al.,2021),suggesting that exercise can alter epigenetic regulations associated with cognitive function.
Histone acetylation also plays a significant role in regulating synaptic plasticity and memory processes (Chatterjee et al.,2018).Histone acetylation is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs).Some HATs,such as CREB-binding protein and its homolog p300,are significantly decreased in the frontal cortex and hippocampus of the AD brain,associated with learning and memory deficits (Bartolotti et al.,2016;Schueller et al.,2020).However,treadmill exercise can increase global HAT activity in the cortex and hippocampus of rodent models (Elsner et al.,2011;Spindler et al.,2014).HDACs play an important role in memory formation and synaptic plasticity.HDAC2,which is increased in the AD brain,is associated with memory impairments via reducing the histone acetylation of genes important for learning and memory.Inhibiting HDAC2 can restore neuroplasticity-related gene expression (e.g.,BDNF,synaptophysin),reinstate synaptic plasticity,and diminish neurodegeneration-associated cognitive decline (Gräff et al.,2012).Exercise can reduce several HDACs,including HDAC2,HDAC3,and HDAC5,increasing histone acetylation,consistent with improved memory performance (de Meireles et al.,2016;Fernandes et al.,2017).
MicroRNAs are increasingly recognized as playing a key role in neural development and synaptic plasticity,and their dysregulation has been linked to the development and progression of AD (Tregub et al.,2023).For example,miR-155 is over-expressed in the brain of 3×Tg AD model mice,which is associated with the activation of astrocytes and microglia,and increased expression of inflammatory factors like IL-6 and interferon-β (Guedes et al.,2014).Several studies have confirmed that exercise can modulate the effect of miRNA expression on cognitive function (Cosín-Tomás et al.,2014;Dong et al.,2018;Jessop and Toledo-Rodriguez,2018).Voluntary running wheel exercise suppresses overexpressed miR-132 in the hippocampus of SAMP8 mice,improving cognitive function (Dong et al.,2018).MiR-137 is downregulated in both the hippocampus and cerebral cortex of APP/PS1 mice,resulting in tau hyperphosphorylation (Jiang et al.,2018),while voluntary wheel running can upregulate miR-137 expression and improve memory function in mice(Jessop and Toledo-Rodriguez,2018).miR-15b,reduced in brain tissue samples from AD model animals and patients,is associated with increased expression of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) (Gong et al.,2017).Chronic aerobic exercise can upregulate miR-15b expression in the hippocampus of SAMP8 mice and reduce BACE1 level,decreasing brain Aβ accumulation (Cosín-Tomás et al.,2014).Exercise can also regulate other miRNAs,including miR-124,miR-146a,and miR-148b (Improta-Caria et al.,2020);their mechanisms of action in neurodegeneration and cognitive function will require future studies.
Sex differences in exercise-induced cognitive changes
There are sex differences in the effects of exercise on cognitive functional outcomes.In streptozocin-induced AD model rats,treadmill exercise decreases depression-related behaviors in females and reduces anhedonia-like behaviors in males (Naghibi et al.,2021).However,treadmill exercise only increases BDNF levels in the hippocampus,and IL-10 levels in the prefrontal cortex in female rats,suggesting sex differences in working mechanisms (Naghibi et al.,2021).Another study found the total white matter volume and myelinated fibers were significantly lower in the female AD model mice compared with the male counterpart (Zhou et al.,2018).However,running exercise was more effective in delaying the decline in spatial learning and memory functions,and attenuating changes in myelinated fibers,in female AD model mice than it was in male AD model mice (Zhou et al.,2018).Sex differences in neuroplasticity and neurotrophic factors may mediate differences in the efficacy of exercise on improving cognition in AD models (Barha and Liu-Ambrose,2018),suggesting that individualized exercise protocols may be needed for male and female patients with AD.
Conclusions and Future Perspectives
Exercise has been shown to have a significant capacity to enhance cognitive performance across various life stages,encompassing both youth and later years,as well as within specific populations with cognitive impairments.This phenomenon is supported by a body of evidence ranging from moderate-to-robust (Erickson et al.,2019).Both solitary aerobic and resistance training,as well as a flexible training regimen,are effective in enhancing executive function among older adults with mild dementia (Karssemeijer et al.,2017).
Exercise rehabilitation stands out as an exceptionally promising and multifaceted domain that merits comprehensive investigation in future research initiatives.Its potential implications may extend well beyond the confines of research,holding significant promise for integration into routine care for individuals with AD.By harnessing exercise as a therapeutic tool,healthcare practitioners can potentially enhance the quality of life and cognitive function in their patients with AD(Figure 3).

Figure 3 | Proposed therapeutic regimens for patients with AD at different stages.
These findings open new avenues for personalized care plans,in which exercise becomes both a physical activity and a tailored intervention.It has the potential to serve as a predictive biomarker,offering insights into an individual’s potential response to exercise-based interventions.These biomarkers might guide healthcare professionals in designing exercise regimens that are precisely aligned with a patient’s unique needs and capabilities.This approach holds the potential to optimize the therapeutic benefits of exercise,promoting both physical and cognitive well-being in patients with AD.
Further research into the intricate mechanisms underlying the cognitive benefits of exercise will be needed to unveil novel biomarkers.These biomarkers,which may encompass neurochemical,neuroimaging,or even epigenetic markers,could offer crucial insights into the underlying molecular and physiological changes brought about by exercise.Importantly,these mechanistic biomarkers might also serve as viable targets for the development of new drugs aimed at slowing AD progression or even preventing its onset.Incorporating exercise rehabilitation into routine care for patients with AD necessitates a collaborative effort among healthcare providers,researchers,and policymakers.Such integration would require tailored exercise protocols that consider the varying degrees of cognitive impairment and physical abilities present among patients with AD.Additionally,establishing standardized guidelines and protocols for assessing exercise-induced biomarkers can facilitate their consistent use across clinical settings,aiding in treatment planning and decision-making.
This review was not without limitations.We only searched for and reviewed studies published in English using selected platforms.There may be other studies published in journals that are not listed on these platforms,especially newer journals.We may also have missed non-English language studies that provide valuable findings on exercise and the management of AD.The review may suffer from publication bias,where negative findings are unlikely to be published by the researchers.This could lead to an overestimation of the positive effects of exercise rehabilitation in AD.In the studies included in this review,ethical background,sex,and lifestyle factors (e.g.,diet,alcohol use,smoking) were not considered,as this information may not have been provided in the original studies.Such heterogeneity can make it challenging to draw definitive conclusions and generalize findings across all patients with AD.It is also important to acknowledge that the effectiveness of exercise rehabilitation interventions may vary significantly in different socioeconomic settings;this should be further assessed in future studies.
Taken together,the evidence reviewed herein indicates that exercise is a cost-effective intervention for improving physical and cognitive fitness among patients with AD.Different forms of exercise exert positive effects through different mechanisms of action.The prospects of exercise rehabilitation in the context of AD research and clinical practice are highly promising.
Author contributions:Conceptualization:DL,CY;methodology:JJ;writing—original draft preparation:JJ,HC;writing—review and editing:DL,CY,HC,HZ,XZ;supervision:DL,CY,HC;funding acquisition:DL,CY.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors have no competing interests to declare.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Zhixiong Sun,New York State Psychiatric Institute,USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
