Status of biomarker development for frontotemporal dementia and amyotrophic lateral sclerosis
2024-01-24YueYangQiChengJianqunGaoWoojinScottKim
Yue Yang,Qi Cheng,Jianqun Gao,Woojin Scott Kim
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are neurodegenerative diseases that belong to the same disease spectrum,with overlapping of genetic and pathological features.Genetic mutations inTARDBP,C9ORF72,MAPT,andGRNhave been identified in these diseases.TheTARDBPgene encodes transactive response DNA binding protein 43 kDa (TDP-43),and abnormal deposition of TDP-43 is present in approximately 50% of FTD and 95% of ALS (Neumann et al.,2006).It is present as native TDP-43,phosphorylated TDP-43 (pTDP-43),and other truncated forms.C9ORF72is the most common genetic abnormality implicated in behavioral-variant FTD (bvFTD),and in approximately 40% of familial ALS.The number ofC9ORF72G4C2hexanucleotide repeats in healthy individuals is approximately 2–20,whereas in bvFTD and ALS hundreds or thousands.The second most common pathological protein aggregation in FTD is theMAPTgene product tau.Over 50MAPTmutations have been identified in FTD.Abnormal accumulation of SOD1 is the second most common pathology in ALS,and a number ofSOD1mutations have been identified in ALS.Heterozygous mutations inGRNlead to autosomal-dominant FTD,which is associated with TDP-43 deposits,as well as other pathologies.Similar pathologies resulting from these mutations,as well as similar or overlapping clinical features between the two diseases and their subtypes,underscore the importance of developing biomarkers for FTD and ALS.Peripheral biomarkers would flag cases at the pre-symptomatic stage,facilitate more accurate diagnosis and differential diagnosis of diseases or disease subtypes,predict the progression and prognosis of diseases,and monitor the effects of therapeutic interventions (Figure 1).At present,there are no definitive biomarkers that serve these purposes in FTD and ALS.However,recent progress in biomarker development has identified candidates with improved biomarker potential.
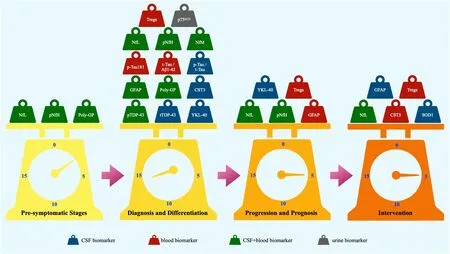
Figure 1 |Potential biomarkers for FTD and ALS under investigation.
Development of biomarkers for pre-symptomatic stage:In FTD,few proteins have been shown to change at the pre-symptomatic stage.Cerebrospinal fluid (CSF) and blood level of neurofilament light chain (NfL) is one of the earliest markers that change during the transition from pre-symptomatic to symptomatic in FTD patients.CSF and serum levels of NfL were shown to be elevated at 3.5 years before symptom onset,and plasma levels of NfL elevated before symptom onset in those with genetic mutations.Though less explored,phosphorylated neurofilament heavy chain (pNfH) has also been shown to increase in CSF and serum at the pre-symptomatic stage of the disease.Other than NfL and pNfH,little is known on other proteins,with ongoing research.
Development of biomarkers for disease diagnosis and differentiation:Since FTD and ALS,and their subtypes,have similar or overlapping clinical and pathological features,which are also present in other neurodegenerative diseases,much research has been carried out to develop biomarkers to differentiate different diseases and different disease subtypes.To date,several candidates have shown limited biomarker potential to differentiate mutation carriers from non-carriers,different diseases,and different disease subtypes.For example,CSF and plasma pTDP-43 levels were found to be higher in FTD patients withC9ORF72mutations compared to bvFTD subtypes withoutC9ORF72or GRN mutations.Likewise,serum levels of NfL were higher in FTD with those mutations.In addition,C9ORF72mutation carriers also had higher levels of poly-GP in both CSF and peripheral blood than non-carriers.Patients withGRNmutations had higher levels of pTDP-43 in CSF and plasma compared to other bvFTD subtypes withoutGRNorC9ORF72mutations,and altered serum levels of NfL in FTD withGRNmutations.Moreover,FTD patients withGRNmutations had higher CSF levels of YKL-40 compared to those withC9ORF72mutations,together with elevated plasma levels of GFAP.Furthermore,FTD patients withGRNmutations had lower levels of CST3 compared to those withC9ORF72mutations.In contrast,CSF and plasma levels of pTDP-43 could not differentiateGRNandC9ORF72mutations.Although CSF levels of TREM2 were not different betweenGRNmutation carriers and noncarriers,higher levels were observed in a subset of GRN mutation carriers (van der Ende et al.,2021).The complement proteins C1q and C3b in CSF and C2 and C3 in plasma were elevated in symptomatic mutation carriers compared to presymptomatic carriers and non-carriers (van der Ende et al.,2022).The p-tau/t-tau ratio was significantly correlated with the right lateral orbital frontal cortex (Fenu et al.,2022).Despite these findings,further work is required to improve the potentiality of the markers.
In terms of disease differentiation,CSF levels of NfL were 20-fold higher in ALS and 3-fold higher in FTD compared to healthy controls (Gaetani et al.,2019),much stronger differential compared to other neurodegenerative diseases,indicating its value in screening FTD and ALS patients from other neurodegenerative diseases,although validated cut-off values need to be defined.Other neurofilament chains were also investigated as markers to differentiate diseases.For example,neurofilament medium chain levels have been shown to be higher in FTD CSF (Remnestal et al.,2020) and ALS plasma (Haggmark et al.,2014) compared to controls.In addition,CSF levels of pNfH were elevated in FTD compared to early-onset AD.Also,CSF levels of pNFH were significantly higher in patients with ALS compared to those with other forms of motor neuron disease (Behzadi et al.,2021).Several studies have shown decreases in T regulatory cells (Tregs) and increases in urinary neurotrophin receptor p75 extracellular domain in ALS.Several other markers have shown some potential to differentiate FTD from other types of dementia.For example,the t-tau/amyloid-β42 ratio was shown to be lower in FTD compared to AD,and p-tau/t-tau combined with YKL-40 was able to differentiate FTD from AD and dementia with Lewy bodies,with a sensitivity of 90% and a specificity of 78%.However,it should be noted that these markers could not differentiate FTD from controls.Some markers are being explored to differentiate FTD from ALS.For example,FTD patients with 4-repeat tau inclusions had higher levels of p-tau181 as well as p-tau/t-tau compared to ALS patients,and bvFTD patients had higher CSF levels of total TDP-43 compared to ALS.For differentiation of clinical subtypes,ALS with bulbar onset had higher plasma levels of NfL compared to ALS with spinal onset,suggesting its potential to differentiate clinical subtypes of ALS.In terms of pathological subtypes,FTD with TDP-43 pathology had a lower p-tau181/t-tau ratio compared to FTD with tau pathology,and a combination of measurement of NfL and p-tau181/t-tau in CSF was capable of differentiating FTDtau and FTD-TDP,with a sensitivity of 80% and a specificity of 81%.
Development of biomarkers for progression and prognosis evaluation:NfL is of great interest in the development of biomarkers for monitoring of disease progression and prognosis.The robust correlation of NfL levels between serum and CSF further strengthens NfL as a prognostic biomarker.Moreover,NfL levels correlated significantly with functions and disease severity in FTD,as well as disease progression in ALS (Sun et al.,2020).In addition,NfL levels correlated inversely with survival time in both FTD and ALS.Furthermore,changes in NfL levels in blood have been proposed to participate in disease progression in a prediction model (Witzel et al.,2021).Additionally,pNfH levels were positively correlated with disease progression in serum,plasma,and CSF in ALS,and have been tested in a prognostic biomarker panel to reflect neuronal integrity in ALS (Devos et al.,2019).Other biomarker candidates include GFAP,the serum of which was shown to correlate with cognition in FTD (Oeckl et al.,2022),and Tregs,which was shown to correlate with progression rate and survival in ALS.Furthermore,CSF levels of YKL-40 have also been reported to serve as a prognostic marker in ALS (Andres-Benito et al.,2018;Gille et al.,2019).
Development of biomarkers for monitoring therapeutic interventions:Some markers have been trialed for monitoring therapeutic interventions.SOD1,which showed little value as a disease biomarker for ALS,has shown promise as a CSF marker for monitoring antisense oligonucleotide treatment.SOD1 has also been used as an outcome measure to monitor AROSOD1 and inhibitor pyrimethamine clinical trials in ALS.Utilization of NfL has been shown to increase the power of monitoring accuracy in clinical trials(Witzel et al.,2021).For example,NfL has been used as a biomarker for the pharmacokinetic study of monoclonal antibody IC14 in a phase 1b trial (Henderson et al.,2021),and to monitor intervention in a clinical trial of Baricitinib in ALS.Other markers utilized for monitoring intervention in clinical trials in ALS include CST3 for evaluating the effectiveness of GM604 and Arimoclomol.Measurement of Tregs in blood has also been trialed as a marker for monitoring therapeutic effects in clinical trials.
Conclusion and future remarks:Although the development of biomarkers for FTD and ALS is still at an early or ongoing stage,several proteins have come to the fore that could potentially be developed as biomarkers for disease diagnosis in asymptomatic patients,for aiding in the diagnosis of disease subtypes,for monitoring and predicting disease progression and prognosis,and for evaluating therapeutic interventions.Factors,such as protein form,as is the case with TDP-43 and tau,with various forms in different biofluids,need to be taken into consideration when developing biomarkers.Another aspect that needs to be considered is the existence of multiple subtypes in each disease that renders challenges in biomarker specificity.A panel consisting of multiple biomarkers would increase the power and accuracy of disease subtype identification.In addition,choosing the right sample source is also important,as the expression or presence of biomarker candidates may not be consistent across all biofluids,e.g.,serum,plasma,and CSF.Furthermore,the development of improved assay techniques and equipment would also facilitate advancing biomarker development.
Yue Yang,Qi Cheng,Jianqun Gao,Woojin Scott Kim*
Brain and Mind Centre &School of Medical Sciences,The University of Sydney,Sydney,NSW,Australia (Yang Y,Kim WS)
Department of Neurology and Neurophysiology,Liverpool Hospital,Sydney,NSW,Australia(Cheng Q)
Stroke and Neurology Research Group,Ingham Institute for Applied Medical Research,Sydney,NSW,Australia (Cheng Q)
Shanghai Key Laboratory of Anesthesiology and Brain Functional Modulation,Shanghai,China(Gao J)
Clinical Research Center for Anesthesiology and Perioperative Medicine,Shanghai,China (Gao J)
Translational Research Institute of Brain and Brain-Like Intelligence,Shanghai Fourth People’s
Hospital,School of Medicine,Tongji University,Shanghai,China (Gao J)
*Correspondence to:Woojin Scott Kim,PhD,woojin.kim@sydney.edu.au.
https://orcid.org/0000-0002-4707-933X(Woojin Scott Kim)
Date of submission:October 15,2023
Date of decision:November 25,2023
Date of acceptance:December 10,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392883 How to cite this article:Yang Y,Cheng Q,Gao J,Kim WS(2024)Status of biomarker development for frontotemporal dementia and amyotrophic lateral sclerosis.Neural Regen Res 19(10):2117-2118.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Annibale Antonioni,University of Ferrara,Italy.
杂志排行
中国神经再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
