Construction of a Highly Active Rh/CeO2-ZrO2-Al2O3 Catalyst Based on Rh Micro-Chemical State Regulation and Its Three-Way Catalytic Activity
2024-01-22JialinMouLiulingChenJunFanLuZengXueJiangYiJiaoJianliWangYaoqiangChen
Jialin Mou ,Liuling Chen ,Jun Fan ,Lu Zeng ,Xue Jiang ,Yi Jiao ,*,Jianli Wang ,Yaoqiang Chen
1 Institute of New Energy and Low-Carbon Technology,Sichuan University,Chengdu 610064,China.
2 College of Chemistry,Sichuan University,Chengdu 610064,China.
Abstract: With the increasing number of automobile vehicles,the exhaust emitted poses a severe menace to the environment and human health,necessitating the purification of exhaust pollutants.Meanwhile,the high price of noble metals and their limited supply require a decrease in noble metal loading to reduce the costs of three-way catalysts (TWCs).Therefore,improving the utilization efficiency of noble metals and their catalytic behavior is critical for the development of next-generation TWCs with low noble metal loading.Herein,the Rh micro-chemical state was modulated using the liquid-phase reduction method combined with atmospheric heat treatment to enhance the catalytic behavior of Rh-based catalysts with low Rh loading.The catalyst was characterized using X-ray diffraction (XRD),hydrogen temperature programmed reduction (H2-TPR),CO chemisorption,X-ray photoelectron spectroscopy (XPS),the FTIR spectroscopy of chemisorbed CO (CO-FTIR),transmission electron microscopy (TEM),and in situ diffuse reflectance IR (in-situ DRIFTS) to illustrate the relationship between Rh micro-chemical state (including valence state ratio and dispersion) and catalytic activity.The asprepared catalyst re-Rh/CeO2-ZrO2-Al2O3-H2 (re-Rh/CZA-H2) exhibited better catalytic activity and a wider air/fuel ratio (λ)operating window with T90 values 30–73 °C and 51–86 °C lower than those of the catalysts synthesized by liquid-phase reduction and traditional impregnation method,respectively.In addition,aged samples prepared by the combined scheme also exhibited excellent activity and stability,where the T50 and T90 values were lower than the fresh catalyst.Structureactivity relationship results demonstrated that the better catalytic activity of re-Rh/CZA-H2 could be attributed to the optimal valence state ratio and highly dispersed Rh species,which increased the number of effective active sites.The considerable stability was attributed to the stable structure of the CeO2-ZrO2-Al2O3 (CZA) support,improved dispersion,and the high contents of active Rh species,which exposed more active sites to promote reactant conversion.In addition,the synergistic effect between the metallic Rh and oxygen vacancies could facilitate the anchoring of Rh nanoparticles to inhibit Rh sintering.Therefore,adjusting the micro-chemical state of noble metals by the combinatorial scheme developed herein provides a novel route for improving the catalytic activity,high-temperature stability,and air/fuel operating window of these catalysts.
Key Words: Micro-chemical state; Three-way catalyst; Liquid-phase reduction; Valence state ratio;Atmospheric heat treatment
1 Introduction
Environmental pollution caused by vehicle exhaust has become one of the primary sources of urban air pollution environment with the increase of vehicle ownership year by year1.Three-way catalysts (TWCs),as the most satisfactory and efficient catalytic converter system,have been widely used over the last decades.They can synchronously transform carbon oxide (CO),hydrocarbons (HCs),and nitrogen oxides (NOx) into harmless CO2,H2O,and N2near the stoichiometric air-fuel ratio2–4.Modern commercial TWCs are constituted of active components,supports,and cordierite honeycomb ceramics.Noble metals,such as Pt,Pd,and Rh are regarded as the primary active components of TWCs5,in which Rh is the most scarce and expensive.However,Rh,as an efficient active component for dissociating NOxinto N2,cannot be completely substituted by Pt or Pd so far6,7.CeO2-ZrO2-Al2O3(CZA) composite oxide,which combines the excellent oxygen storage and release property of CeO2-ZrO2(CZ) with the high specific surface area of Al2O3,has been regarded as the third-generation oxygen storage materials and new generation support of TWCs8,9.Although the technology of TWCs for gasoline vehicles has been relatively mature,the emission limit of pollutants is getting lower and lower with the continuous tightening of automobile exhaust emission regulations,and the cost of the catalyst is also getting higher,which require the catalyst to maintain higher activity,durability,and wider air/fuel ratio (λ) operating window even at lower content of noble metals.Therefore,to improve the utilization efficiency of noble metals has become a trend in the development of TWCs in the future.
Micro-chemical state of noble metals,such as valence state,particle size,coordination environment,and interaction between noble metals and supports are the decisive factors affecting the utilization efficiency of noble metals and catalytic activity.Actually,many studies have indicated that the preparation method,calcining condition and so on could strongly affect the micro-chemical state of noble metals,which further influenced the adsorption and activation of reactants as well as the redox performance of catalysts.Kimetal.10had researched how the valence state and dispersion of Pt affected CO oxidation activity and found that the content of metallic Pt increased as the reducing temperature,in which the Pt/TiO2catalyst calcined at 600 °C exhibited the best CO oxidation activity and high turnover frequency (TOF) value due to its optimal valence state ratio and higher dispersion.Jeongetal.11also investigated the effect of Pt valence state ratio on catalytic activity by changing the reduction temperature during the preparation process.It was found that the proportion of active Pt0species increased with the increasing reduction temperature and the relationship between the catalytic activity and valence state ratio showed a volcanic curve.The catalyst reduced at 300 °C showed the best activity due to the optimal valence state ratio of Pt species.Ohyamaet al.12synthesized Rh/CeO2catalysts using various methods to study the effect of valence state ratio on catalytic behavior.The results indicated that the catalysts prepared by different methods exhibited different Rh valence state ratio and the TOF value increased drastically with the fraction of Rh0species,which suggested metallic Rh species was favorable for NO reduction reaction.Gonzalez-Velascoetal.13studied the influence of Pt dispersion on the catalytic performance,and the results indicated that the different catalytic behavior was attributed to the differences in Pt dispersion,in which the catalyst with higher dispersion exhibited better catalytic activity.
Noble metal-support interaction (MSI) also has a substantial influence on catalytic activity and stability of the catalysts14,15.Caoetal.16investigated the effect of Rh-based catalysts with different supports and atmosphere treatments on the catalytic activity.It was found that Rh micro-chemical state was strongly influenced by MSI,and the interaction between Rh and ZrO2was more effective in inhibiting the sintering of Rh particles as well as preserving more active Rh species.Leeetal.17investigated the effect of different interaction between Pt and CeO2viavarying treated atmosphere on high-temperature stability of Pt/CeO2catalysts.During the oxidation treatment,Pt was anchored with the surface oxygen of CeO2to form Pt―O―Ce bonds which not only resulted in high dispersion of Pt nanoparticles but also improved the high-temperature stability of the catalyst.Wangetal.18examined the influence of Pd structural properties on CO,HC and NOxconversion performance under various pretreatment atmosphere.The Pd/CZ catalyst pretreated under air flow showed higher HC oxidation activity due to higher Pd dispersion and stronger metal-support interaction,while the Pd/CZ catalyst pretreated under hydrogen atmosphere had better NOxconversion activity due to its more metallic Pd species and oxygen vacancies.Based on the literature review,most of the related studies were focused on studying the effect of a single factor (such as valence state,particle size,MSI,etc.) on the performance of a specific catalytic reaction.However,three-way catalytic reaction is a complex system of reactions involving oxidation,reduction,steam reforming,etc.,and each reaction is influenced by different factors,few researchers have systematically investigated the effect of the micro-chemical state of noble metal to the complex catalytic reaction system.
In the present study,a series of Rh/CZA catalysts were synthesizedviaimpregnation method,liquid-phase reduction method and atmospheric heat treatment technology to investigate the significant influence of Rh micro-chemical state on the catalytic performance.Catalytic conversions of CO,HCs,and NOxat stoichiometric conditions were carried out to evaluate the catalytic performance of the Rh/CZA catalysts.Moreover,the evaluation of air/fuel operating window was performed by regulating the feed stream to lean/rich conditions at 400 °C.Concurrently,to investigate the relationship between Rh microchemical states and catalysts performance,the results were correlated to characterization by XRD,XPS,H2-TPR,CO-FTIR,andinsituDRIFTS.The purpose of this work is to regulate Rh micro-chemical state by using the synergistic effect of liquidphase reduction and atmospheric heat treatment method,thereby improving Rh utilization efficiency and catalytic activity of Rh/CZA catalysts.Analysis on the correlation between Rh micro-chemical state and catalytic activity provided technical basis for the catalysts with higher catalytic performance.
2 Experimental section
2.1 Catalyst preparation
Rh(NO3)3aqueous (Heraeus Metals Co.,Ltd.,Shanghai,China) served as the active component precursor,and Rh loading amount was 0.4% (mass fraction).CeO2-ZrO2-Al2O3support(Solvay,Belgium,pretreated at 900 °C for 5 h,SBET= 79 m2·g-1)prepared by co-precipitation method was a typical tetragonal phase with the mass ratio of CeO2: ZrO2: Al2O3= 10 : 20 : 70,marked as CZA.
The reference Rh/CZA catalyst was preparedviaincipientwetness impregnation method.The CZA support powder was poured into Rh(NO3)3aqueous solution with continuous stirring at room-temperature,and then the mixture was dried at 90 °C for a night and pre-calcined at 550 °C in air for 3 h to decompose the nitrate and surfactant.After that,the powder calcined at 900 °C in nitrogen for 5 h as fresh samples,denoted as im-Rh/CZA,and aged one was treated at 1000 °C in nitrogen for 5 h,denoted as im-Rh/CZA-a.
The re-Rh/CZA catalyst was preparedvialiquid-phase reduction method,and the experimental procedures are similar to those in our previous work reported19–21.Rh(NO3)3aqueous solution was quantitatively added into ethylene glycol(analytical reagent,Kelong Chemical Reagent Factory,Chengdu,China),while the temperature was maintained at 80 °C for 1 h to reduce Rh3+,and then the CZA powders were poured into the colloidal solution during continuous stirring.Next,the obtained slurries were evaporated at 90 °C for a night.All dried samples were calcined under the same condition as the im-Rh/CZA catalysts,and the obtained catalysts were denoted as re-Rh/CZA and re-Rh/CZA-a,respectively.
The re-Rh/CZA-H2samples were prepared by synergistic treatment which combined liquid-phase reduction method with atmospheric heat treatment technology.The preliminary preparation method of re-Rh/CZA-H2was same with that of re-Rh/CZA.Firstly,Rh(NO3)3aqueous solution was quantitatively added into ethylene glycol (analytical reagent,Kelong Chemical Reagent Factory,Chengdu,China) and the temperature was maintained at 80 °C for 1 h to obtain Rh nanoparticles colloidal solution.Secondly,the CZA powders were poured into the colloidal solution during continuous stirring.And then,the obtained slurries were evaporated at 90 °C for a night and precalcined at 550 °C for 3 h to decompose the nitrate and surfactant.Thirdly,the obtained catalyst powders were calcined at 900 and 1000 °C under hydrogen atmosphere (2%,volume fraction,H2/N2) for 5 h,which was denoted as re-Rh/CZA-H2,re-Rh/CZA-H2-a,respectively.
According to the experimental procedures of our laboratory22,the monolithic honeycomb catalysts were prepared by applying a slurry to a monolithic honeycomb.The slurry consisted of calcined catalyst powders,deionized water and inorganic binder.After that,the prepared monolithic honeycomb catalysts were calcined at 550 °C under static air flow for 3 h.The volume of monolithic honeycomb was 2.5 mL and the mass of catalyst loaded on the monolithic honeycomb was 0.4 g.Therefore,the loading of Rh over the catalyst was calculated to be 0.64 g·L-1.
2.2 Catalyst characterizations
The catalysts were characterized by XRD,H2-TPR,pulse CO chemisorption,CO-FTIR,TEM,XPS andinsituDRIFTS according to the procedure described in our previous work19,20,23.
2.3 Catalytic activity tests
The catalytic activity experiments on monolithic honeycomb catalysts were performed using the self-assembled fixed-bed continuous gas flow reactor according to the process described in our previous work20.The composition of simulated exhaust gas was referenced to automobile enterprise such as Ford,Hyundai and so on24–27.The simulated exhaust gas included 4500 ppm (1 ppm = 10-6) CO,1250 ppm NO,120 ppm C3H8,230 ppm C3H6,4010 ppm O2,1500 ppm H2,11% CO2,10%H2O,and N2,corresponding to the stoichiometric air-fuel ratio,and the gas hourly space velocity (GHSV) was calculated to be 50000 h-1.The variations of reactants/products were monitored by Thermo Scientific Antaris IGS Gas Analyzer (Thermo Fisher Scientific,USA).Prior to the measurement,the catalysts were pretreated at 550 °C under the simulated exhaust gas flow for 1 h,and then cool down below 100 °C.The concentration of pollutants was recorded in the process of gradually increasing the temperature to obtain the transformation curve with the change of temperature.Catalytic activity was evaluated by usingT50andT90(the temperature at which the pollutants conversion value was 50% and 90%) which was recorded from the conversion date.In addition,the air/fuel ratio (λ) experiment was performed at 400 °C by adjusting the concentration of O2,and the conversion rate of all four pollutants should reach 80% and above within the air/fuel ratio operating window (as listed in formula (1)).The calculation of TOF was calculated by formula(2),in whichXis the conversion of CO or NO species (%),Cindicates the concentration of CO or NO species (mol∙L-1),vis the flow rate of CO or NO species (L∙s-1),WRhdenotes the mass of Rh in the catalyst (g),MRhis the atomic weight of Rh (102.91 g∙mol-1),andDRhrepresents the dispersion of Rh calculated by pulse CO-chemisorption measurements.
3 Results and discussion
3.1 Catalytic activity
The light-off curves of CO,NOx,and HCs at stoichiometric air-fuel ratio over the fresh and aged catalysts were shown in Fig.1,and the corresponding conversion values ofT50andT90were listed in Table 1.It could be concluded from Fig.1 that the four types of pollutants showed similar conversion tendency in all catalysts and were completely transformed within 450 °C.However,the catalysts after various treatment protocols presented different catalytic activities.In which of them,re-Rh/CZA-H2prepared by synergistic treatment exhibited the best catalytic activity,where theT90values of CO,NOx,and HCs over the re-Rh/CZA-H2were 30–73 and 51–86 °C lower than those of re-Rh/CZA and im-Rh/CZA,respectively.Interestingly,the aged re-Rh/CZA-H2-a catalyst exhibited enhancement of active performance with a decrease of 11 °C for NO,19 °C for C3H8,and 15 °C for C3H6onT90compared with the fresh one.However,a significant decrease in activity could be observed over im-Rh/CZA-a catalyst and itsT90shifted to higher temperature with an increase of 25 °C for CO,43 °C for C3H8,and 5 °C for C3H6compared with im-Rh/CZA catalysts.In addition,we further investigated the influence of aging time and temperature on catalytic activity and the results are concluded in Fig.S1 (Supporting Information).The re-Rh/CZA-H2catalyst showed outstanding high-temperature durability and the increase of aging time and aging temperature had little promotion on the catalytic performance.To evaluate the sensitivity of the catalysts to the fluctuation of air-to-fuel ratio (λ),frequency sweep experiments were carried out at 400 °C with constant feed.The left and right sides of the stoichiometric air/fuel ratio (λ= 1)represented rich-burn condition (λ< 1) and lean-burn condition(λ> 1) respectively.From the conversion curves of CO,NOx,and HCs shown in Fig.2,it was abundantly clear that a widerλoperating window over re-Rh/CZA-H2was found compared with re-Rh/CZA and im-Rh/CZA,which is better accommodate the fluctuation of air-fuel ratio.
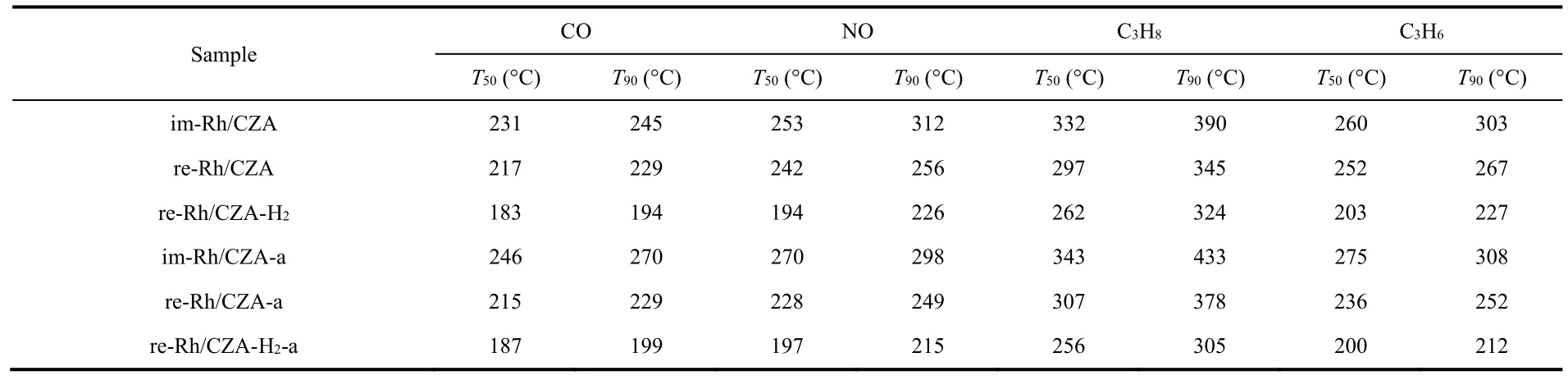
Table 1 Values of T50 and T90 of CO,NO,and HCs over im-Rh/CZA,re-Rh/CZA,re-Rh/CZA-H2 fresh and aged catalysts.
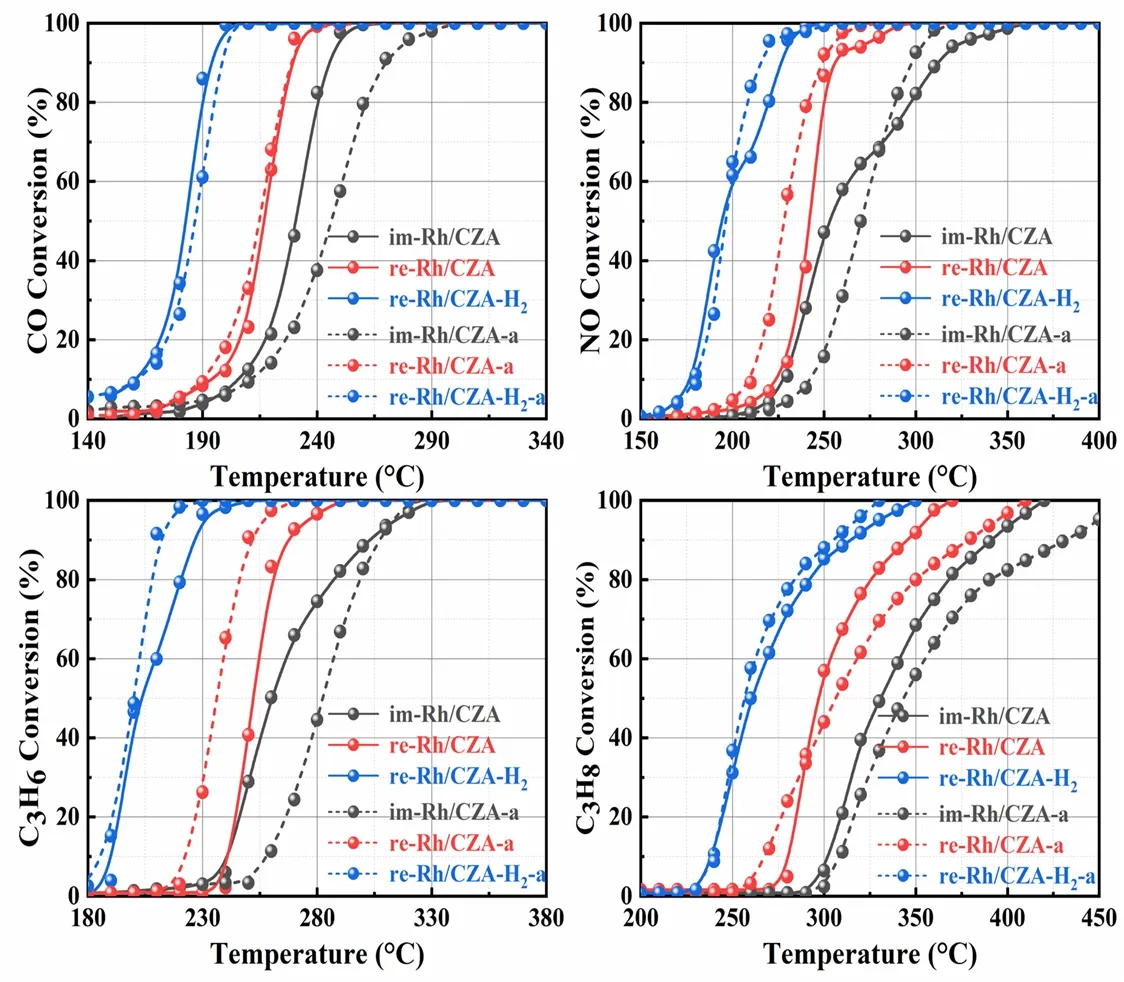
Fig.1 Light-off curves of CO,NOx,and HCs over im-Rh/CZA,re-Rh/CZA,re-Rh/CZA-H2 fresh and aged catalysts.

Fig.2 Conversion curves of CO,NOx,and HCs at 400 °C for im-Rh/CZA (a),re-Rh/CZA (b),and re-Rh/CZA-H2 (c) as a function of λ.
Under normal circumstances,the commercial TWCs are mainly Pt-Rh bimetallic system or Pt-Pd-Rh trimetallic system for different technical and emission requirements.Therefore,we synthesized Pt-Rh bimetallic catalysts to investigate the effect of combining scheme in actual application.The results of catalytic activity were shown in Figs.S2 and S3,and the corresponding conversionT50andT90values for all catalysts were listed in Table S1.The Pt-Rh bimetallic catalyst exhibited better activity of NOxreduction and HCs oxidation compared with Pt-based catalyst,which implied that combining scheme was of great significance in commercial application.
3.2 Structural property
The physical phase structures and compositions of fresh and aged CZA supports and the corresponding catalysts were measured by XRD,and the results were shown in Fig.3.As deduced from the XRD pattern in Fig.3a,the main peaks of all the fresh and aged samples were the feature of tetragonal Ce0.16Zr0.84O2(CZ) phase withP42/nmc space group,and the diffraction peaks of CZ became sharper and more intense after aging.It can be seen from Fig.3b,c,the separation of peaks around 35° and 58° had taken place over fresh and aged CZA supports,im-Rh/CZA and re-Rh/CZA catalysts,which indicated the occurrences of severe sintering28.However,it should be noteworthy that the diffraction peak intensity over re-Rh/CZAH2-a catalyst was weakened when comparing with the fresh one and the separation of peak was only observed around 58°.Meanwhile,the crystal size listed in Table S2 was calculated by the Debye-Scherrer equation which used the half maximum of main reflection peak (101) of CZ and the crystallite size of re-Rh/CZA-H2-a decreased compared with the fresh one.Moreover,the characteristic peak of CZ over re-Rh/CZA-H2-a catalyst shifted to a higher angle compared with the fresh one.According to Bragg equation (2dsinθ=nλ),the shift of characteristic peak to a higher angle was associated with the contraction of the CZ lattice,which generally originated from the doping of ions with smaller radius.Considering in particular that the ionic radius of Zr4+(0.084 nm) and Al3+(0.057 nm) were smaller than Ce4+(0.097 nm) and Al3+hardly enter into the CZ lattice,it could be inferred that a part of Zr4+on the surface had moved into the crystal lattice of CeO2to take place of Cex+over re-Rh/CZA-H2-a to improve the solid solubility of CZ lattice,resulting in higher stability of CZA supports9,28–32.It has been reported in the literature that the micro-chemical state of noble metals (valence state and particle size) could influence the structural stability of the support,but this phenomenon only occurred when the noble metals were atomically dispersed33,34.Moreover,the structural property was closely related to the calcined atmosphere16,35,36.Considering the low content and non-atomic level dispersion of Rh in the present study,the excellent stability of CZA support over fresh and aged re-Rh/CZA-H2catalysts was attributed to atmospheric heat treatment,which could promote the migration of Zr4+from the surface into the lattice of CeO2to enhance structural stability of CZA support.In addition,no diffraction peaks of Rh species were detected and this phenomenon was attributed to Rh content below the detection limit or/and highly dispersed on the CZA support,which was consistent with the literatures published by Chen19and so on33,36,37.

Fig.3 (a) XRD patterns of im-Rh/CZA,re-Rh/CZA,and re-Rh/CZA-H2 fresh and aged catalysts,(b) XRD patterns of catalysts in the range of 32° < 2θ < 64°,and (c) XRD patterns of catalysts in the range of 28° < 2θ < 52°.
3.3 Rh micro-chemical state
3.3.1 XPS results
The surface elemental states over the fresh and aged catalysts were analyzed by X-ray photoelectron spectroscopy measurements.The obtained spectra of Rh 3d,Ce 3d,and O 1swere illustrated in Fig.4,and the relative amounts of Rh,Ce and O were listed in Table 2.The spectra of Ce 3dwere displayed in Fig.4a,b,which could be divided into eight peaks,corresponding to four pairs of spin-orbit doublets.The six peaks marked as u,u’’,u’’’ and v,v’’,v’’’ were attributed to Ce4+,respectively,and the other doublet marked as u’ and v’ were assigned to Ce3+38,39.The existence of Ce3+correlated with the formation of oxygen vacancy.Oxygen vacancy could adsorb and activate O2to promote CO oxidation and anchor Rh nanoparticles which could inhibit the sintering of Rh at high temperature34,40,41.As the results listed in Table 2,there were no apparent differences in Ce3+/Ce content between im-Rh/CZA and re-Rh/CZA catalysts.However,the proportion of Ce3+/Ce over re-Rh/CZA-H2was significantly higher than those over im-Rh/CZA and re-Rh/CZA,which indicated that there was a large amount of oxygen vacancies in re-Rh/CZA-H2.The same pattern was also observed over the aged catalysts,and interestingly,the Ce3+content increased on the surface of re-Rh/CZA-H2-a catalyst compared with the fresh one,probably due to the atmospheric heat treatment method improved the uniformity of CZ solid solution and promoted the formation of more oxygen vacancies.

Table 2 Surface elemental information of catalysts derived from the XPS analysis.
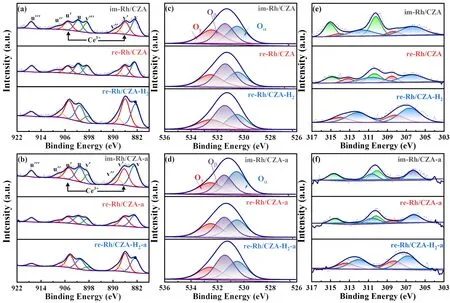
Fig.4 XPS spectra of Ce 3d (a,b),O 1s (c,d),and Rh 3d (e,f) for fresh (a,c,e) and aged (b,d,f) samples.
In addition,the O 1sspectrum could be divided into three peaks,where the corresponding binding energy (BE) values were 530.5,531.4,and 532.5 eV,attributed to lattice oxygen species (Oα),defective oxygen species within the matrix of CeO2(Oβ) and surface chemical absorbed oxygen species (Oγ),respectively42,43.According to literature,it was generally recognized that Oβwas more active to promote the catalytic activity due to its higher mobility when compared with Oαand Oγ,that is,higher Oβ/O ratio over the catalysts implied that more oxygen vacancies existed which were more conducive to redox reactions.As displayed in Table 2,the Oβconcentrations of the catalysts followed the sequence of re-Rh/CZA-H2> re-Rh/CZA > im-Rh/CZA.Moreover,although the Oβ/O ratio on the surface of the catalysts decreased after aging treatment,re-Rh/CZA-H2-a still maintained a high Oβ/O ratio,which demonstrated that atmospheric heat treatment could significantly stabilize the defective oxygen species.Therefore,it was reasonable to speculate that adjusting Rh micro-chemical state by synergistic treatment was beneficial to enhance the activity and stability of the catalysts.
Rh 3dspectra were showed in Fig.4,where the spectra could divide into four peaks for re-Rh/CZA-H2,and six peaks for im-Rh/CZA and re-Rh/CZA,respectively.The peak at the range of 306.2–306.8 eV corresponded to metallic Rh,the peak at the range of 308.2–308.5 eV was assigned to Rh2O3,and the peak at range of 310.1–310.4 eV was ascribed to irreducible RhO2species36,44–46.According to the literature19,47–50,the catalytic activity of Rh species increased as the valence state decreased,that is,Rh0and Rh3+were catalytic active species because of their excellent redox property compared with Rh4+.From the results summarized in Table 2,the active Rh0and Rh3+species were co-existed in the re-Rh/CZA-H2catalyst,in which Rh0species were predominated.However,for the im-Rh/CZA and re-Rh/CZA catalysts,the coexistence of Rh0,Rh3+,and Rh4+species was observed,and a relatively large number of irreducible Rh4+species would have a negative influence on catalytic activity.According to the literature16,36,44,45,the interaction between Rh species and CZA material was highly attributed to the treating atmosphere and properties of the support.Most of the surface Rh species existed as oxidized species over im-Rh/CZA and re-Rh/CZA samples due to the oxygen supply capacity of CZ16,while re-Rh/CZA-H2still contained a significant amount of metallic Rh species due to atmospheric heat treatment,meanwhile,the adverse effect of valence state generated by MSI could also be attenuated by atmospheric heat treatment51.For the aged catalysts,an increase in the binding strength between Rh species and their surrounding chemicals led to the generation of more oxidative Rh species,but re-Rh/CZA-H2-a catalyst still maintained high content of metallic Rh species.Thus,it could be concluded that synergistic treatment not only increased the content of metallic Rh species,but also generated suitable interaction to reduce adverse effects40,52,53.
3.3.2 CO-FTIR results
The dispersion and electronic state of Rh species in each sample were analyzed by CO-FTIR,and the results were illustrated in Fig.5.According to the CO-FTIR spectra,all the bands were corresponded to the adsorption of CO on Rh species.For the fresh catalyst,the presence of at least three distinct corresponding doublets at (2090; 2020),(2078; 2010),(2086;2014) cm-1,which were all attributed to the symmetrical and asymmetrical vibration modes of germinal dicarbonyl species adsorbed on Rh48,was related to Rh species dispersion.Obviously,the peak intensities over re-Rh/CZA-H2were larger than those over im-Rh/CZA and re-Rh/CZA,indicating that Rh species were better dispersed on re-Rh/CZA-H2catalyst.Although all samples showed varying degrees of decrease in peak intensity after aging 1000 °C,which indicated that Rh dispersion decreased with different degrees,the peak intensity of re-Rh/CZA-H2-a catalyst was still higher than that of the others.Meanwhile,no obvious CO-adsorption peak was observed around 1850 cm-1,indicating that there were no bridge-absorbed species (Rh2(CO)) over re-Rh/CZA-H2catalyst.It could be further indicated that Rh species had a higher degree of dispersion on the surface of re-Rh/CZA-H2catalyst54.In addition,the peaks located at 2060 cm-1over re-Rh/CZA-H2catalyst was attributed to bridging CO adsorbed on Rh0,indicating that the proportion of Rh0in re-Rh/CZA-H2was larger than those in im-Rh/CZA and re-Rh/CZA,which was contributed to improving catalytic activity55.

Fig.5 CO-FTIR spectra of (a) im-Rh/CZA,re-Rh/CZA,re-Rh/CZA-H2 catalysts,and (b) im-Rh/CZA-a,re-Rh/CZA-a,re-Rh/CZA-H2-a catalysts
Furthermore,the results of Rh dispersion measured by pulse CO chemisorption followed the sequence of re-Rh/CZA-H2(80%) > re-Rh/CZA (31.7%) > im-Rh/CZA (28.7%).Upon aged treatment,Rh dispersion declined over all samples owing to the aggregation of Rh nanoparticles,which also followed the same sequence of re-Rh/CZA-H2-a (32.6%) > re-Rh/CZA-a (28.5%) >im-Rh/CZA-a (25.0%).To provide more direct evidence for the influence of Rh nanoparticles size on the catalytic performance,TEM was measured and the results are displayed in Fig.S4.Rh nanoparticles were not observed over all samples,which could be attributed to the low content of Rh species and be consistent with the XRD results.As described above,synergistic treatment could enhance Rh dispersion,which played important roles in catalytic activity.
3.4 Redox property
The redox property of the catalysts was measured by H2-TPR,and the corresponding profiles were displayed in Fig.6.It could be observed that the temperatures of reduction peaks for all catalysts were below 400 °C,which were corresponded to the co-reduction of RhOxspecies and CZA support7,47.There were three types of substance reduction can be seen from the fresh catalysts50,56,57,the reduction peaks with a temperature of <135 °C were associated with the reduction of well-dispersed Rh oxides on the surface of CZA support and the reduction of interfacial Ce4+species,while the reduction peaks located at 265 °C and 289 °C were attributed with the reduction of CZA support.According to the literatures57–60,the well-dispersed surface Rh oxides are considered to be reactive oxidation species for catalytic reactions,in which the maximum peak area over re-Rh/CZA-H2indicated that it contained more reducible active Rh species to facilitate the reaction.Meanwhile,the Rh species could accelerate the reduction of interfacial Ce species which is caused by the H2spill-over from Rh species to CZA support,and the valence state and particle size of Rh played a decisive role in the reduction of Ce species,in other terms,reduced Rh nanoparticles represented a crucial prerequisite.It was obvious that the peak intensity over re-Rh/CZA-H2was higher than those over im-Rh/CZA and re-Rh/CZA,meaning that there were more metallic Rh species over re-Rh/CZA-H2to facilitate the ceria reduction at lower temperatures,which was highly consistent with the result of CO-FTIR and XPS.
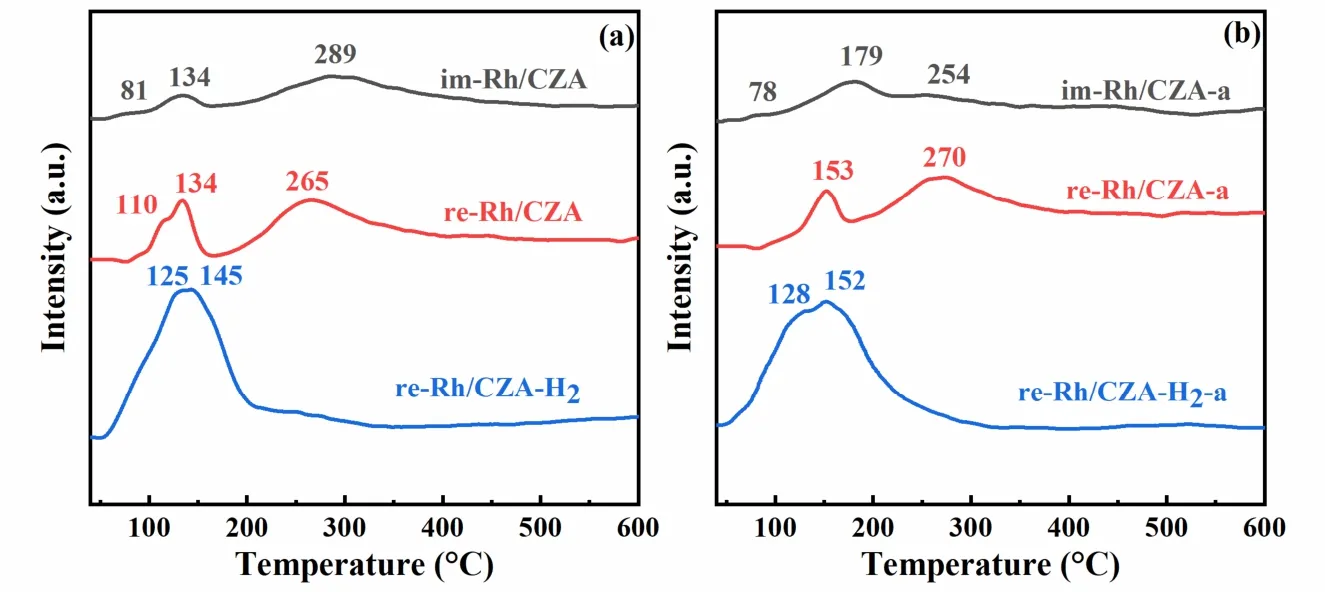
Fig.6 H2-TPR profiles of (a) im-Rh/CZA,re-Rh/CZA,and re-Rh/CZA-H2,and (b) im-Rh/CZA-a,re-Rh/CZA-a,and re-Rh/CZA-H2-a.
As shown in Fig.6b,for the im-Rh/CZA-a catalyst,the peak located at 179 °C was corresponded with the reduction of relatively more stable bulk-like crystalline Rh oxide on the sample with larger particle sizes56,61,62.The intensity of reduction peaks associated with Rh2O3species was weakened due to the aggregation of the Rh species and the formation of a large amount of irreducible RhO2species.That is,the aging treatment at 1000 °C resulted in severe deterioration of reduction capacity for im-Rh/CZA-a catalyst.However,the re-Rh/CZAH2-a and re-Rh/CZA-a catalysts still maintained higher content of active Rh2O3species when compared with im-Rh/CZA-a catalyst,and there were also some reduced Rh nanoparticles promoting the reduction of Ce species on the surface over aged catalysts,which were favorable for the catalytic reaction.In addition,the shape of the reduction profiles for re-Rh/CZA-a and re-Rh/CZA-H2-a was quite similar to the corresponding fresh ones,demonstrating the excellent high-temperature stability of Rh-based catalysts prepared by liquid-phase reduction method46.Therefore,the combining scheme of atmospheric heat treatment and liquid-phase reduction not only increased the active species that facilitate the reaction but also enhanced the ceria reduction at low temperature.
3.5 In situ DRIFTS results
InsituDRIFTS experiments were performed under the stoichiometric air-fuel ratio conditions of CO-NO-C3H6-C3H8with temperature ranging from 50 to 400 °C to investigate the effect of the micro-chemical state of fresh catalysts on the formation and elimination of reaction intermediates.InsituDRIFTS spectra were shown in Fig.7.At the beginning of the reaction,the bands at 1230,1275,and 1300 cm-1were ascribed to the bidentate/monodentate nitrates species adsorbed on Cex+/Zr4+sites63.The three catalysts showed difference on these bands,in which re-Rh/CZA-H2catalyst had more characteristic peaks than im-Rh/CZA and re-Rh/CZA catalysts,indicating the NO adsorption was enhanced over re-Rh/CZA-H2,which is more beneficial to promote the reduction of NO species at lower temperatures.The adsorption peaks of Rh0-NO at 1845 and 1902 cm-1observed over all catalysts were weakened64,65and disappeared when the temperature increased to 300 °C.Among these catalysts,the re-Rh/CZA-H2showed a higher peak intensity,indicating that the amount of Rh0on the surface was higher to facilitate the conversion of NO at lower temperature.Weak but distinct bands at 1870 and 2175 cm-1appeared in the spectra for all catalysts were corresponded to symmetric vibrations of a gem-dinitrosyl complex (Rh-(NO)2) and CN species adsorbed on Rh species,respectively66,67,in which Rh-(NO)2was solely observed on Rh species and Rh-CN was considered as the reactive intermediate of CO + NO reaction.As can be seen from Fig.7,stronger adsorption intensity of Rh-CN species was observed on re-Rh/CZA-H2catalyst,indicating that re-Rh/CZA-H2was more favorable for the transformation of CO and NO.Another adsorbed peak located at 2238 cm-1was associated with N2O which was intermediate of NO transformation and its band intensity also followed the sequence of re-Rh/CZA-H2> re-Rh/CZA > im-Rh/CZA,which further proved that re-Rh/CZA-H2was more favorable for the conversion of NO species66.
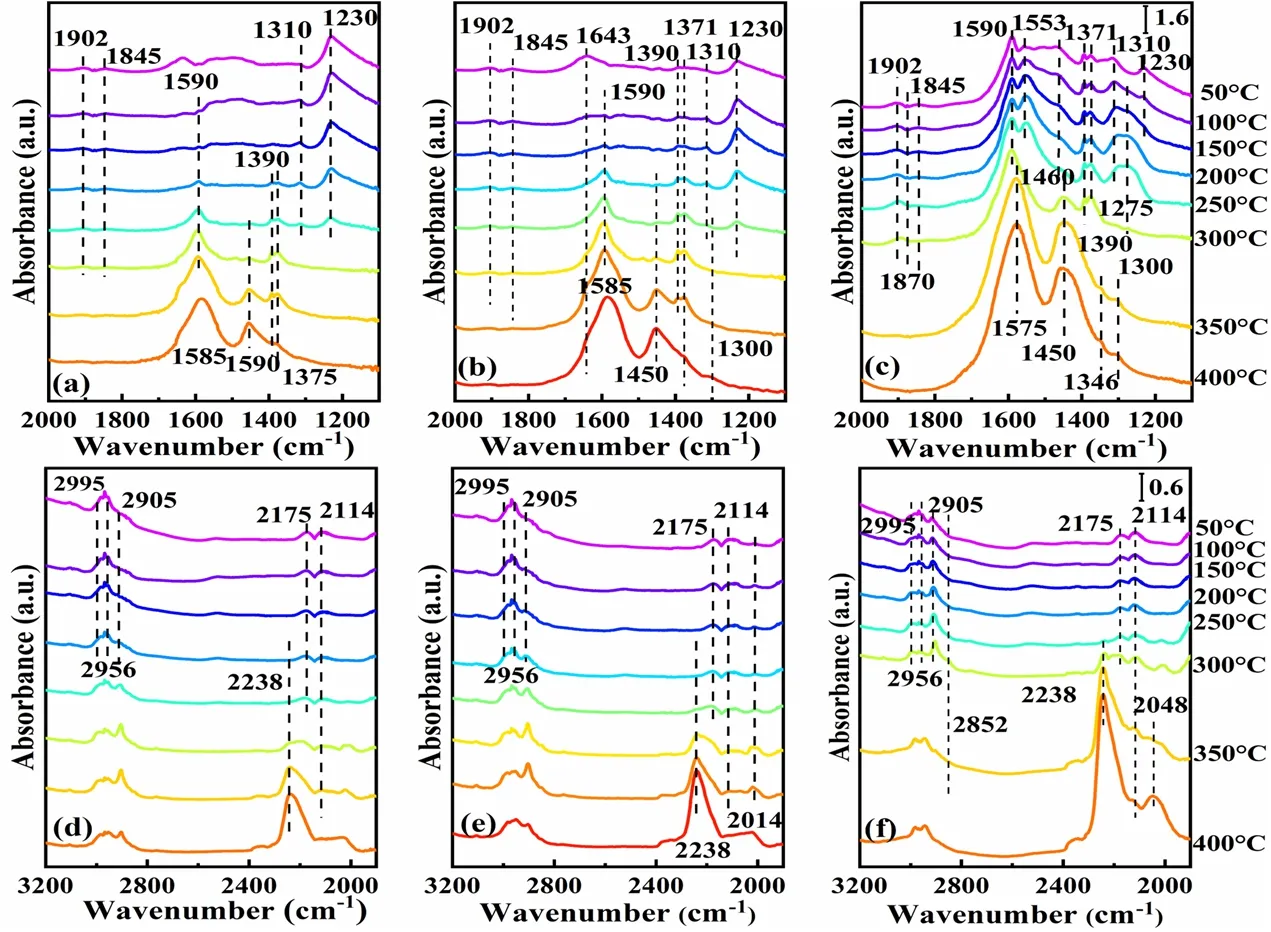
Fig.7 In situ DRIFTS spectra over im-Rh/CZA (a,d),re-Rh/CZA (b,e),re-Rh/CZA-H2 (c,f) under stoichiometric NO-CO-C3H6-C3H8 condition.
Furthermore,the formate,acetate,and carboxylate are the main intermediates in the HCs conversion process6,68–71.The bands corresponding with formate species at around 1371,1390,and 1590 cm-1were detected,and the band intensities over re-Rh/CZA-H2were higher than those for re-Rh/CZA and im-Rh/CZA.Meanwhile,in the high frequency region,the remaining bands at 2905 and 2995 cm-1were related to adsorbed hydrocarbon fragments originating from propene or carboxylate species63.The carboxylates species were observed on all catalysts and the peaks for re-Rh/CZA-H2appeared at lower temperature than others.In addition,the carboxylates species were disappeared over re-Rh/CZA-H2catalyst at 300 °C,while the characteristic peak of carboxylates species still presented over re-Rh/CZA and im-Rh/CZA catalysts at 350 and 400 °C,respectively.The above results showed that carboxylates species were appeared and disappeared at lower temperature over re-Rh/CZA-H2,which were basically consistent with HCs conversion temperature in catalytic activity results.By the comprehensive analysis above,re-Rh/CZA-H2could quickly form and eliminate the reaction intermediates,so it drew the conclusion that re-Rh/CZA-H2could effectively promote catalytic conversion of pollutions more effectively than im-Rh/CZA and re-Rh/CZA.
3.6 Kinetics analysis
Kinetic investigations were carried out to explore the differences of catalytic activity over these fresh and aged catalysts,kinetic investigations were conducted.It was well known that three-way catalytic reaction mainly included the oxidation of CO and HCs as well as the reduction of NO.Considering the reaction characteristics of Rh-based catalysts,that is,Rh-based catalysts were favorable for CO-NO reaction.Therefore,we calculated the TOF values for CO and NO conversions as a representative of oxidation and reduction reactions,respectively.The TOF values for CO and NO conversions in the CO-NO reaction over fresh and aged catalysts were shown in Fig.8.The conversion temperatures of pollutants for re-Rh/CZA-H2catalyst were much lower than the other two catalysts at the same conversion.From the viewpoint of TOF,it should be noted that the TOF values of CO at 180 °C followed the sequence of re-Rh/CZA-H2(1.30 s-1) > re-Rh/CZA (0.47 s-1) > im-Rh/CZA (0.19 s-1),which also confirmed the excellent catalytic behavior of re-Rh/CZA-H2,and the aged catalysts showed a similar trend.Meanwhile,the TOF values of NO conversion over fresh and aged catalysts also followed the same trends as the TOF of CO conversion,in which the TOF values of NO at 190 °C followed the sequence of re-Rh/CZA-H2(1.98 s-1) > re-Rh/CZA (0.23 s-1) > im-Rh/CZA (0.03 s-1).Fig.9 presented the relationship between TOF and the metallic Rh species.The TOF increased sharply with the proportion of metallic Rh species,which suggested that the fraction of metallic Rh species contributed significantly to the catalytic performance.The strong relevance of the TOF on the metallic Rh species might indicate that Rh0species enhanced the catalytic behavior for the CO oxidation and NO reduction.
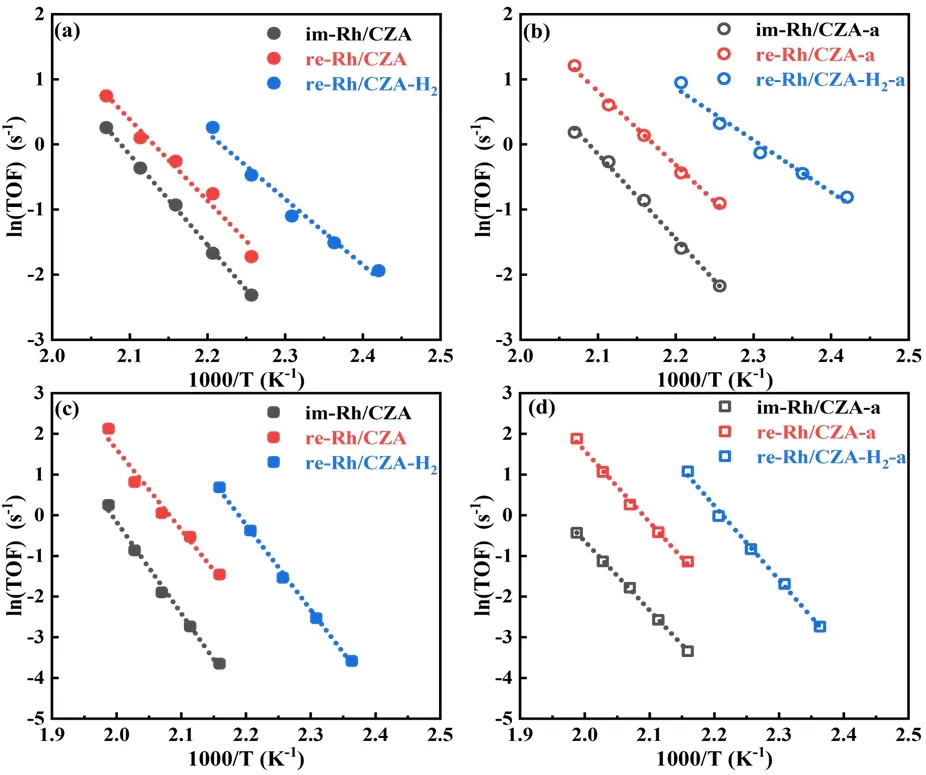
Fig.8 Arrhenius plots for CO (a,b) and NO (c,d) reduction rate in the CO-NO reactions over fresh and aged im-Rh/CZA,re-Rh/CZA,and re-Rh/CZA-H2 catalysts.
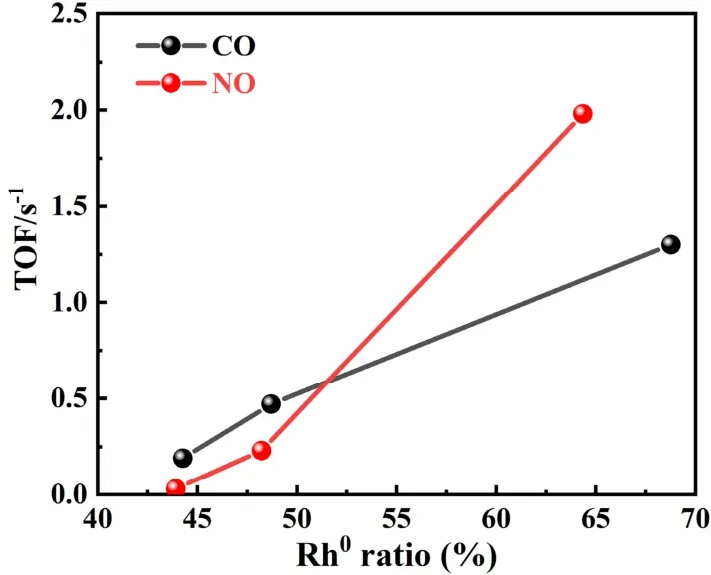
Fig.9 Plot of the TOF against the Rh metal fraction.
3.7 Discussion of the structure-activity relationship
Three-way catalytic system is a complex multicomponent redox reaction system,and the micro-chemical state of noble metal is a major factor to determine the catalytic performance and high-temperature durability of the catalyst.In this work,re-Rh/CZA-H2catalyst prepared by synergistic treatment showed the best catalytic activity and stability among these catalysts.On the one hand,theT90values of re-Rh/CZA-H2were significantly lower than those of re-Rh/CZA and im-Rh/CZA.Associated with the characterization results,we can obtain the following three aspects from the H2-TPR,CO-FTIR,XPS,insituDRIFTS analysis: (1) re-Rh/CZA-H2had more active species,such as higher contents of metallic Rh species and defective oxygen species,which could promote the adsorption and decomposition of reactants on the catalyst surface; (2) more metallic Rh nanoparticles promoted the reduction of Ce4+during low temperatures,which resulted the generation of more oxygen vacancy to improve the redox properties; (3) more active sites resulted from higher dispersion over re-Rh/CZA-H2were in favor of the reactants oxidation.On the other hand,re-Rh/CZAH2-a exhibited better catalytic activity than the fresh catalyst.The high-temperature stability could be attributed to the following factors: (1) the support structure remained stable to inhibit the sintering of CZ at high temperatures and improve the high-temperature durability of CZA support,which could inhibit the embedding of noble metals.Meanwhile,Rh species over the catalyst still maintained high dispersion after aging; (2) the synergistic effect between metallic Rh and oxygen vacancy could anchor Rh nanoparticles to inhibit the sintering of Rh so that the catalyst exposed more metallic Rh species to promote the reaction.In summary,the better catalytic activity and hightemperature stability were owing to the modulation of microchemical state of Rh to provide optimal valence state ratio,high dispersion and suitable interaction.Accordingly,the evolution of micro-chemical state of Rh under diverse treatment protocols is schematically illustrated in Fig.10 based on all the results discussed above.

Fig.10 Schematic diagram of the structure active relationship between Rh micro chemical state and catalytic performance
4 Conclusions
In our present work,a series of Rh/CZA catalysts were prepared by impregnation,liquid-phase reduction method,and synergistic treatment to modulate Rh micro-chemical state.It was obvious that the re-Rh/CZA-H2catalysts prepared by synergistic treatment effectively improved catalytic performance and high-temperature durability of TWC.The results indicated that synergistic treatment greatly increased dispersion of Rh species and structure stability of support.In addition,it was clear that the synergistic treatment increased the content of active species,such as metallic Rh species and reactive oxygen species,and promoted the reduction of Ce4+at lower temperature,which were beneficial to enhance the adsorption and activation of the reactants.Even after aging,the valence state ratio of Rh remained essentially stable and a large number of Rh species were stabilized at metallic state,determining its superior hightemperature stability.TOF results further demonstrated that synergistic treatment was beneficial to promote the reaction at lower temperature,meanwhile,the relationship between TOF and metallic Rh content indicated that active Rh0species enhanced the catalytic behavior of the CO oxidation and NO reduction.The present work provided an efficient method to modulate the micro-chemical state of noble metals,thus improving noble metals utilization efficiency.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
Author Contribution:Conceptualization,Jialin Mou,Liulin Chen,Jun Fan,Lu Zeng and Xue Jiang; Methodology,Jialin Mou; Software,Jialin Mou; Validation,Jialin Mou; Formal Analysis,Jialin Mou; Investigation,Jialin Mou; Resources,Jialin Mou; Data Curation,Jialin Mou; Writing-Original Draft Preparation,Jialin Mou; Writing-Review & Editing,Liulin Chen,Jun Fan,Lu Zeng,Xue Jiang and Yi Jiao; Visualization,Jialin Mou and Yi Jiao; Supervision,Yi Jiao,Jianli Wang and Yaoqiang Chen; Project Administration,Yaoqiang Chen;Funding Acquisition,Yi Jiao,Jianli Wang and Yaoqiang Chen.
杂志排行
物理化学学报的其它文章
- 用于高灵敏快速核酸检测的荧光碳点
- Performance Improvement and Antibacterial Mechanism of BiOI/ZnO Nanocomposites as Antibacterial Agent under Visible Light
- 2D/3D S-Scheme Heterojunction Interface of CeO2-Cu2O Promotes Ordered Charge Transfer for Efficient Photocatalytic Hydrogen Evolution
- Pickering Emulsion Templated Proteinaceous Microsphere with Bio-Stimuli Responsiveness
- Electronic Modulation of Ni-Mo-O Porous Nanorods by Co Doping for Selective Oxidation of 5-Hydroxymethylfurfural Coupled with Hydrogen Evolution
- RuP Nanoparticles Anchored on N-doped Graphene Aerogels for Hydrazine Oxidation-Boosted Hydrogen Production
