Unilateral rNurr1-V5 transgene expression in nigral dopaminergic neurons mitigates bilateral neuropathology and behavioral deficits in parkinsonian rats with α-synucleinopathy
2024-01-10BismarkGaticaGarciaMichaelBannonIrmaAliciaMartnezvilaLuisSotoRojasDavidReyesCoronaLourdesEscobedoMinervaMaldonadoBernyMEGutierrezCastilloArmandoEspadasAlvarezManuelFernandezParrillaJuanMascotteCruzCPRodrguezOviedoIrais
Bismark Gatica-Garcia, Michael J.Bannon, Irma Alicia Martínez-Dávila, Luis O.Soto-Rojas, David Reyes-Corona,Lourdes Escobedo, Minerva Maldonado-Berny, ME Gutierrez-Castillo, Armando J.Espadas-Alvarez,Manuel A.Fernandez-Parrilla, Juan U.Mascotte-Cruz, CP Rodríguez-Oviedo, Irais E.Valenzuela-Arzeta,Claudia Luna-Herrera, Francisco E.Lopez-Salas, Jaime Santoyo-Salazar0, Daniel Martinez-Fong,,,*,#
Abstract Parkinsonism by unilateral, intranigral β-sitosterol β-D-glucoside administration in rats is distinguished in that the α-synuclein insult begins unilaterally but spreads bilaterally and increases in severity over time, thus replicating several clinical features of Parkinson’s disease, a typical α-synucleinopathy.As Nurr1 represses α-synuclein, we evaluated whether unilateral transfected of rNurr1-V5 transgene via neurotensin-polyplex to the substantia nigra on day 30 after unilateral β-sitosterol β-D-glucoside lesion could affect bilateral neuropathology and sensorimotor deficits on day 30 post-transfection.This study found that rNurr1-V5 expression but not that of the green fluorescent protein (the negative control) reduced β-sitosterol β-D-glucoside-induced neuropathology.Accordingly, a bilateral increase in tyrosine hydroxylase-positive cells and arborization occurred in the substantia nigra and increased tyrosine hydroxylasepositive ramifications in the striatum.In addition, tyrosine hydroxylase-positive cells displayed less senescence marker β-galactosidase and more neuroncytoskeleton marker βIII-tubulin and brain-derived neurotrophic factor.A significant decrease in activated microglia (positive to ionized calcium-binding adaptor molecule 1) and neurotoxic astrocytes (positive to glial fibrillary acidic protein and complement component 3) and increased neurotrophic astrocytes (positive to glial fibrillary acidic protein and S100 calcium-binding protein A10) also occurred in the substantia nigra.These effects followed the bilateral reduction in α-synuclein aggregates in the nigrostriatal system, improving sensorimotor behavior.Our results show that unilateral rNurr1-V5 transgene expression in nigral dopaminergic neurons mitigates bilateral neurodegeneration (senescence and loss of neuron-cytoskeleton and tyrosine hydroxylase-positive cells),neuroinflammation (activated microglia, neurotoxic astrocytes), α-synuclein aggregation, and sensorimotor deficits.Increased neurotrophic astrocytes and brain-derived neurotrophic factor can mediate the rNurr1-V5 effect, supporting its potential clinical use in the treatment of Parkinson’s disease.
Key Words: A1 astrocytes; A2 astrocytes; gene therapy; microglia; motor deficits; nanoparticles; neurodegeneration; neuroinflammation; senescence;α-synuclein aggregates
Introduction
Nurr1 (also referred to as NR4A2) is a ligand-independent transcription factor of the orphan nuclear receptor family that activates different responsive elements for genes not only critical for the differentiation and maturity of midbrain dopaminergic neurons during brain ontogeny but also necessary for their maintenance in the adult brain (Kim et al., 2020; Pulcrano et al.,2022).In adulthood, Nurr1 participates in dopamine neurotransmission by regulating dopamine synthesis enzymes, dopamine transporter, and vesicular monoamine transporter 2 (Kim et al., 2020).Furthermore, Nurr1 plays a double neurotrophic function by activating the expression of brain-derived neurotrophic factor (BDNF; Volpicelli et al., 2007), an intrinsic neurotrophic factor of midbrain dopaminergic neurons (Hernandez-Chan et al., 2015;Razgado-Hernandez et al., 2015; Fernandez-Parrilla et al., 2022), and the transmembrane co-receptor RET (REarranged during Transfection), which mediates the intracellular signaling of glial cell line-derived neurotrophic factor (GDNF) receptor alpha (GFRα) activated by GDNF and neurturin(Conway et al., 2020).Therefore, through BDNF and RET expression, Nurr1 differentiates and preserves dopaminergic neurons (Kim et al., 2021).
The NURR1-mediated neurophysiological and neurotrophic functions decrease in Parkinson’s disease (PD).As association and experimental studies suggest,NURR1 alterations can lead to PD.In PD patients, mutations in theNURR1gene (Jacobsen et al., 2008), variousNURR1genetic variants (Wu et al., 2008),and decreasedNURR1gene expression (Le et al., 2008) have been shown,suggesting its involvement in this disease.Accordingly, studies on genetically modified mice have demonstrated that embryonic ablation of theNurr1gene causes agenesis of midbrain dopaminergic neurons in homozygousNurr1-null mice (Le et al., 1999) and fewer dopaminergic neurons in aged heterozygousNurr1-null mice (Jiang et al., 2005).Furthermore, the selective disruption of Nurr1 expression in mature dopaminergic neurons in conditionalNurr1genetargeted mice results in progressive striatal dopamine fall, dopaminergic neuron loss, dystrophic ramifications, and locomotor deficits (Kadkhodaei et al., 2013; Gamit et al., 2023).In addition, α-synuclein (ASYN)(d)/Nurr1+/–(2-hit) double transgenic mice, which were hemizygous for Nurr1, display Nurr1 down-regulation and α-synuclein overexpression that are associated with dopaminergic neurodegeneration and neuroinflammation (Argyrofthalmidou et al., 2021).Neuroinflammation could be triggered by the secreted α-synuclein activating microglia via Toll-like receptor-2 and NFκB, thus leading to the overexpression of proinflammatory cytokines such as TNFα and Il -1β(Dutta et al., 2021).
Interestingly, transcriptional and functional impairment of Nurr1 has been induced by overexpression of either mutant or wildtype α-synuclein in cultured cells (Meng et al., 2020) and rats (Jia et al., 2020).Similarly,abnormal α-synuclein accumulation resulting from an α-synuclein preformed fibrils injection inducesNurr1downregulation and parkinsonism in nonhuman primates (Chu et al., 2019).As Nurr1 and α-synuclein transcriptionally repress each other by still unknown mechanisms (Jia et al., 2020; Gamit et al.,2023), regulating the expression of either gene could control dopaminergic neurodegeneration in PD.On this basis, pioneer studies have demonstrated that adeno-associated virus (AAV)-mediated overexpression of Nurr1 alone(Decressac et al., 2012) or combined with Foxa2 induces neuroprotection (Oh et al., 2015) by reducing α-synuclein neurotoxicity (Decressac et al., 2012)and neuroinflammation (Liu et al., 2017).Furthermore, Nurr1 activators (Li et al., 2019) can alleviate α-synuclein disrupted gene expression, possibly stimulating Nurr1/Nor1 through direct binding to the activation function (AF)-1 N-terminal domain (Volakakis et al., 2015).New potent Nurr1 activators can produce neuroprotective and anti-inflammatory effects (Montarolo et al., 2014; De Miranda et al., 2015).However, none of these compounds have shown evidence of Nurr1 activation by direct physical interaction with its ligand-binding domain (Dong et al., 2016).
We recently developed a local unilateral BSSG (β-sitosterol β-D-glucoside)rat parkinsonism model in which the α-synucleinopathy starts unilaterally but spreads bilaterally and increases in severity over time, thus better modeling the clinical condition of PD compared to many other neurotoxin models (Soto-Rojas et al., 2020b, c).The BSSG neuropathology consists of progressive bilateral dopaminergic nigrostriatal neurodegeneration,aggregation and spreading of α-synuclein, neuroinflammation with the participation of neurotoxic reactive A1 astrocytes, and motor and non-motor behavior alterations (Luna-Herrera et al., 2020; Soto-Rojas et al., 2020a, b,c).So, the question addressed by this study is whether unilateral transfection of the rNurr1-V5 construct can mitigate bilateral dopaminergic neuron degeneration, neuropathology, and behavioral deficits in the unilateral BSSG model.This issue was addressed by transfecting therNurr1-V5gene on day 30 after the BSSG lesion when neuropathology and locomotor deficits were still progressive, resembling a stage with early parkinsonism (Soto-Rojas et al., 2020c).We used the neurotensin (NTS)-polyplex nanoparticle system forrNurr1-V5gene transfection because of its previously shown high effectiveness in reducing neuroinflammation (Nadella et al., 2014) and restoration of dopaminergic neurons in the 6-hydroxydopamine (6-OHDA)parkinsonian model (Gonzalez-Barrios et al., 2006; Hernandez-Chan et al.,2015; Razgado-Hernandez et al., 2015; Reyes-Corona et al., 2017; Fernandez-Parrilla et al., 2022).Evaluations with cell biology techniques were performed on day 60 after BSSG administration when the parkinsonism symptomatology becomes maximum without corrective treatment (Soto-Rojas et al., 2020c).
Methods
Plasmids
pTracer-rNurr1-V5 (7774 bp) expresses V5 epitope-tagged rat Nurr1 (rNurr1)driven by the human elongation factor α1 promoter and green fluorescent protein (GFP) by the cytomegalovirus (CMV) promoter.TheNurr1sequence(1796 bp; GenBank, NM_019328; https://www.ncbi.nlm.nih.gov/search/all/?term=NM_019328) was obtained by aNheI-SpeIdigestion from the prNurr1-DsRed2-N1 plasmid and subcloned between the SpeI restriction sites of the pTracer TM-EF/V5-His B plasmid (Invitrogen, Carlsbad, CA, USA).Correct construction was confirmed by restriction enzyme analysis and sequencing.
pScript-rNurr1 (6231 bp) expresses rNurr1 under the control of the CMV promoter (Espadas-Alvarez et al., 2017).
pEGFP-N1 (4733 bp) expresses enhanced green fluorescent protein (EGFP)driven by the CMV promoter (Clontech, Mountain View, CA, USA, RRID:Addgene_32454).
pNBRE3x-hCDNF (4486 bp) codes for the human cerebral dopamine neurotrophic factor (hCDNF) under the transcriptional control of the synthetic NBRE3x promoter, which contains three repeats of the nurr1-responsive element and is specific for dopaminergic neurons (Nadella et al., 2014;Espadas-Alvarez et al., 2017).
Assembly of the neurotensin-polyplex nanoparticles
The synthesis of the NTS carrier, a poly-L-lysine conjugate with NTS and HA-2 fusogenic peptide (FP), the formation of the plasmid (pDNA)-karyophilic peptide (KP) complex, and the assembling of NTS-polyplex nanoparticles(NPs) have been described in detail elsewhere (Lopez-Salas et al., 2020).We assembled NTS-polyplex NPs for pTracer-rNurr1-V5 with 18 nM pDNA, 18µM KP, and 540 nM NTS carrier, and those for pEGFP-N1 with 18 nM pDNA,18 µM KP, and 450 nM NTS carrier.For expression assays, the total plasmid injected was 184.71 ng for pTracer-rNurr1-V5 and 112.46 ng for pEGFP-N1 dissolved in 2 µL of Dulbecco’s Modified Eagle Medium (DMEM; Thermo Scientific, Rockford, IL, USA).For internalization assays, NTS-polyplex NPs were assembled with propidium iodide-labeled pDNA (pTracer-rNurr1-V5 or pEGFP-N1) and fluorescein-amidite (FAM)-labeled KPRa (Lopez-Salas et al.,2020).
Field emission scanning electron microscopy
Five µL of NTS-polyplex NPs harboring either the pTracer-rNurr1-V5 or the pEGFP-N1 was smeared over a glass slide.These preparations were then dried in a vacuum chamber (Secador PD3 Desiccator cabinet; EDWARDS, West Sussex, UK) for 24 hours at room temperature (RT).Samples were examined using a JEOL JSM-7401F field emission scanning electron microscope (FESEM;JEOL; Akishima, Tokyo, Japan) at 1 kV accelerating voltage (Fernandez-Parrilla et al., 2022).
Dynamic light scattering
The Litesizer DLS 500 (Anton Paar, Graz, Austria) was used to measure the size and charge of NTS-polyplex NPs harboring either the pTracer-rNurr1-V5 or the pEGFP-N1 prepared in DMEM without phenol red.First, the diameter distribution was determined by dynamic light scattering in 1 mL of NTSpolyplex NPs at RT (25°C, angle 90°) using a disposable cuvette (Sarstedt,Nümbrecht, Germany).Then, the zeta potential was measured in 50 µL of NTS-polyplex NPs using a Univette Low Volume and 4 V.The dataset was processed using the KalliopeTM Software (Anton Paar).
Culture conditions
Neuroblastoma N1E-115 cells (ATCC Cat# CRL-2263, RRID: CVCL_0451) were purchased from American Tissue Culture Collection (ATCC, Manassas, VA,USA) and cultured in DMEM supplemented with 10% fetal bovine serum(FBS), 1% Glutamax, penicillin (100 U/mL), and 100 µg/mL of streptomycin,all reagents obtained from Thermo Fisher Scientific (Waltham, MA, USA).Cell cultures were kept at 37°C under a 5% CO2atmosphere (Espadas-Alvarez et al., 2017; Lopez-Salas et al., 2020).
Transient transfections in vitro
N1E-115 cells lacking Nurr1 (Espadas-Alvarez et al., 2017) were used to demonstrate the ability of pTracer-rNurr1-V5 plasmid to express a functional rNurr1 compared with the positive control pScript-rNurr1 plasmid.N1E-115 cells were seeded in 24-well dishes at a density of 70,000 cells/cm2 and transfected after a 24-hour incubation using lipofectamine 2000 as recommended by the manufacturer (Life Technologies, San Diego, CA,USA).Transfections of pScript-rNurr1 or pTracer-rNurr1-V5 plasmids were performed to demonstrate rNurr1 expression.In contrast, cotransfections of the plasmids pScript-rNurr1 or pTracer-rNurr1-V5 with pNBRE3xhCDNF were made to show rNurr1 activity.After a 48-hour incubation, the medium was removed.Then, the cells were washed with PBS once, fixed with 4% paraformaldehyde (PFA), and mounted on glass slides.The indirect immunofluorescence of rNurr1 and hCDNF and the native fluorescence of EGFP were detected with an SP8 confocal microscope (Leica TCS SPE,Heidelberg, Germany) and a DMIRE2 epifluorescence microscope (Leica Microsystems, Nussloch, Germany) as described in the immunostaining section.
Animals
All the animal work was conducted according to protocol #162-15 protocol(authorization No.162-15; approval date: June 9, 2019), authorized and supervised by the Institutional Animal Care and Use Committee (IACUC) of The Center for Research and Advanced Studies (CINVESTAV) as the regulatory office for the approval of research protocols involving the use of laboratory animals and in accomplishment of the Mexican Official Norm (NOM-062-ZOO-1999) “Technical Specifications for the Care and Use of Laboratory Animals” based on the Guide for the Care and Use of Laboratory Animals “The Guide,” 2011, NRC, USA with the Federal Register Number # 800.02.01.01.01.0576/2019, Code AUT-B-C-0419-051, awarded by the Secretaría de Agricultura y Desarrollo Rural (SADER), (Federal Agriculture Department)who is the federal authority that verifies the compliance of the NOM-062-ZOO-1999 in Mexico.A total of 96 male Wistar rats were used, which was the minimum number according to the experimental design and statistical validity in compliance with the Guide for the Care and Use of Laboratory Animals(The National Academies Collection: Reports funded by National Institutes of Health, 2011) and considering the three R’s (Reduction, Refinement, and Replacement) for animal experimentation (Hampshire and Gilbert, 2019).Moreover, all efforts were made to minimize animal suffering.
Adult male Wistar rats were used to avoid fluctuation of dopamine activity in midbrain neurons known to occur across the estrous cycle of female rodents (Shanley et al., 2023).Rats weighing 210 to 230 g at the beginning of experiments were provided by CINVESTAV animal facilities and housed together three to four per jumbo cage (34 × 44 × 20 cm3) with water and food available ad libitum, under inverted light-dark cycle (12-hour dark/12-hour light), constant temperature (23 ± 2°C), and humidity (55 ± 5%).Animals(n= 96) were randomly divided into the following four groups: 1) UT =untransfected (UT) rats with BSSG lesion (n= 24); 2) pEGFP = rats with BSSG lesion and transfected with pEGFP-N1 (n= 24); 3) prNurr1 = rats with BSSG lesion and transfected with pTracer-rNurr1-V5 (n= 24); 4) healthy = rats without lesion (n= 24; Additional Figure 1).All 96 rats were subjected to behavioral tests as follows: 24 rats (n= 6 rats per experimental group) were assessed with four tests (vibrissae, beam, corridor, and cylinder), whereas 72 animals (n= 18 rats per experimental group) evaluated only with the vibrissae and beam tests because of their high sensitivity to detect locomotor deficits in BBSG-induced early parkinsonism (Soto-Rojas et al., 2020a, b, c).For immunostaining assays, the brains of the same 96 rats evaluated by behavioral tests were processed with immunohistochemistry and immunofluorescence as follows: 60 rats were used for the five immunohistochemistry assays (n= 3 rats per experimental group per assays), which were for tyrosine hydroxylase (TH),α-synuclein, ionized calcium-binding adaptor molecule 1 (Iba1), glial fibrillary acidic protein (GFAP), and double TH with senescence-β-galactosidase (Senβ-Gal), whereas 36 rats were used for double immunofluorescence assays,which were TH + V5 and TH + βIII-tubulin, α-synuclein + thioflavin T and TH +BDNF, and GFAP + complement component 3 (C3), and GFAP + S100 calciumbinding protein A10 (S100A10) (n= 3 rats per experimental group per assays).Additional Figure 1 displays the experimental design.
Stereotaxic injection
All surgical procedures were performed under general anesthesia with ketamine (120 mg/kg) mixed with xylazine (9 mg/kg), both anesthetics obtained from PiSA Agropecuaria, Guadalajara, Mexico, and injected intraperitoneally.Parkinsonian rats were generated by a single injection of BSSG (10 µg/1 µL of DMSO; MedChemExpress; Monmouth Junction, NJ, USA)in the left substantia nigra (SN) as reported previously (Luna-Herrera et al.,2020; Soto-Rojas et al., 2020b).The coordinates were confirmed according to the Paxinos and Watson stereotaxic rat brain atlas (Paxinos and Watson,2004) and were: anteroposterior, +2.5 mm from the interaural midpoint;mediolateral, +2.0 mm from the intraparietal suture; dorsoventral, –6.7 mm from the dura mater.Four weeks after BSSG injection, rats weighing 380 ± 10 g received an infusion of NTS-polyplex NPs (2 µL) into the left SN at the coordinates: anteroposterior, +2.8 mm from the interaural midpoint;mediolateral, +2.2 mm from the intraparietal suture; dorsoventral, –6.8 mm from the dura mater.After total recuperation under an adequate heat source,the rats were individually housed in the animal facilities until their use.
Behavioral testing
All experimental groups were evaluated with four independent sensorimotor tests 3 days before the BSSG injection to determine basal values and four weeks after the BSSG lesion to select the groups to be transfected with the plasmids pTracer-rNurr1-V5 or pEGFP-N1 and the lesion control group.Then,4 weeks after transfection (corresponding to eight weeks after lesion), all the groups were again tested to demonstrate the therapeutic effect.The evaluation sequence was the vibrissae-elicited forelimb placing and the cylinder tests, the beam walking test, and the corridor test (Soto-Rojas et al., 2020a).Every test was carried out between 10:00 and 14:00 (Additional Table 1).Bench surfaces and appliances were cleaned with 30% ethyl alcohol after each test to avoid influence by odors.
The vibrissae-elicited forelimb placing test evaluates sensorimotor in parkinsonian rats.It consists of brushing the whiskers on the table border to evoke the response of forelimb placement on the table (Soto-Rojas et al.,2020a, b, c).The two forelimbs were independently tested for ten trials by holding the animal by the torso and allowing the forelimbs to hang freely(Soto-Rojas et al., 2020a, b, c).In addition, the behavior was videotaped to count the forelimb placement, which was expressed as the % successful forelimb placing responses per side (Soto-Rojas et al., 2020a, b, c).
The cylinder test also evaluates locomotor asymmetry when the rat is within a transparent acrylic cylinder (Soto-Rojas et al., 2020a, b, c).Then, the rat explores the cylinder walls trying to escape by placing the two forelimbs on the wall.First, we videotaped the behavior to count the first 20 touches made on the walls with the ipsilateral (unimpaired) paw and the contralateral(impaired) paw, and both (simultaneously) paws.Next, the asymmetry was calculated as the percentage of contacts with the ipsilateral forelimb + 1/2 of simultaneous contacts, divided by the total number of contacts (ipsilateral +contralateral + simultaneous; Soto-Rojas et al., 2020a, b, c).
The beam walking test measures the walking speed and balance when the rat travels on a 2 m long and 1 cm wide beam.We use this test to evaluate bradykinesia and postural instability, two signs of motor alterations in PD(Soto-Rojas et al., 2020a, b, c).First, rats were trained on a beam (2 cm wide and 2 m long at a 30° angle) for 2 days before testing.Then, on the test day,the rats were videotaped to count the number of paw faults or slips and thetime spent traversing the experimental beam (2 m long and 1 cm wide at a 30° angle; Soto-Rojas et al., 2020b, c).
The corridor test assesses the olfactory asymmetry while the rat walks along a closed acrylic corridor where capsules (2 cm diameter and 1 cm deep)containing chocolate pellets were evenly distributed every 11 cm on both sides of the floor as an odorous stimulus (Soto-Rojas et al., 2020a, b, c).On the previous day of testing, rats were habituated to the corridor to minimize exploratory behavior and were then food-deprived.On the testing day, a rat was placed in the corridor, and the number of touches on chocolate pellets on each side was counted.The percentage of asymmetric olfactory responses was expressed as the number of contralateral contacts divided by the number of contralateral + ipsilateral touches and the quotient multiplied by 100 (Soto-Rojas et al., 2020a, b, c).
Immunostaining
The following day of the last behavioral test (30 days after transfection or 60 days after lesion), the rats were deeply anesthetized with pentobarbital(50 mg/kg; Laboratorios Aranda, Santiago de Querétaro, México) injected intraperitoneally and euthanized by transcardial perfusion with 4%-PFA in phosphate-buffered saline (PBS) solution.The brains were removed and fixed in 4%-PFA for 24 hours, cryoprotected with 30% sucrose at 4°C and cut into serial coronal 30-µm sections at the level of the SN and striatum using a sliding freezing (–18°C) microtome (Leica Jung Histoslide 2000R).The slices were collected consecutively in a 6-well plate containing PBS (Fernandez-Parrilla et al., 2022).Next, the slices were permeabilized with Triton X-100 in 0.1%-PBS (Triton-PBS) thrice for 10 minutes each and then incubated with 3%BSA (Jackson ImmnunoResearch Laboratories, West Grove, PA, USA) in Triton-PBS for 1 hour at RT to block non-specific binding sites.Finally, the slices were used for staining with immunohistochemistry and immunofluorescence techniques.After incubation in each procedure step described above, the samples were washed for 5 minutes thrice each with PBS.
The immunohistochemistry analysis was performed as described previously(Luna-Herrera et al., 2020; Soto-Rojas et al., 2020c).First, endogenous peroxidases in the slices were eliminated by incubation with 3% hydrogen peroxide in Triton-PBS at RT for 30 minutes.In the specific case of α-synuclein aggregate detection, the slices were incubated with 80% formic acid for 20 minutes at RT before exposure to the primary antibody against α-synuclein(Soto-Rojas et al., 2020c; Additional Table 2).Then, all the slices were incubated at 4°C overnight with the specific primary antibody, diluted with 0.1% Triton X-100 in PBS.The primary antibodies were mouse monoclonal anti-TH (1:1000; Sigma-Aldrich; St.Louis, MO, USA, Cat# T2928, RRID:AB_477569), mouse monoclonal anti-LB-509 α-synuclein (1:500; Abcam,Cambridge, MA, USA, Cat# ab27766, RRID: AB_727020), goat polyclonal anti-Iba1 as a microglia marker (1:500; Abcam, Cat# AB5076, RRID: AB_2224402),and mouse monoclonal anti-GFAP as an astrocyte marker (Clone GA5; 1:500;Cell Signaling Technology, Danvers, MA, USA, Cat# 3670, RRID: AB_561049.Next, the slices were incubated for 2 hours at RT with the secondary antibodies, which were biotinylated horse anti-mouse IgG (1:300; Vector Laboratories, Burlingame, CA, USA, Cat# BA-2000, RRID: AB_2313581, or biotinylated horse anti-goat IgG (1:300; Vector Laboratories, Cat# BA-9500,RRID: AB_2336123).Finally, the color is developed after a 2-hour incubation at RT with the avidin-biotin-peroxidase complex using the manufacturer’s recommendation (ABC Kit; Vector Laboratories), followed by 2 or 3 minutes incubation with 3,3-diaminobenzidine (DAB, Sigma-Aldrich; Additional Table 2).Dopaminergic neuron senescence was assessed in some THimmunohistochemistry samples using Sen-β-Gal staining (Soto-Rojas et al.,2020c).First, a 1:40 dilution of the X-Gal stock solution (5 mM potassium ferrocyanide crystalline, 5 mM potassium ferricyanide trihydrate, and 2 mM magnesium chloride dissolved in PBS) is prepared in X-Gal dilution buffer (4%SA-β-Gal dissolved in dimethylformamide; Sigma-Aldrich).After a 5-minute washing thrice with PBS, the slices were incubated with the X-Gal working solution at 37°C overnight.After the last wash, the samples were mounted on glass slides using Entellan resin (Merck, KGaA; Darmstadt, Germany).All immunohistochemically stained sections were visualized under brightfield microscopy using a light Leica DMIRE2 microscope with 5×, 20×, and 40×objectives (Leica Microsystems), and images were digitized with a Leica DC300F camera (Leica, Nussloch, Germany).
ImageJ software v.1.46r (National Institutes of Health, Bethesda, MD, USA;Schneider et al., 2012) was utilized to quantify the number of TH+cells,the total area density of Sen-β-Gal staining, and pathological α-synuclein aggregates.Five anatomic levels (1 caudal, 2 medial, and 2 rostral) were used to count TH+cells and TH+area density in the SN and TH+area density in the striatum.Color deconvolution: methyl green DAB, an ImageJ plugin, was utilized for color decomposition from the double staining of Sen-β-Gal with TH to quantify the area density of Sen-β-Gal staining in three anatomic levels(1 caudal, 1 medial, 1 rostral) of the SN according to the Paxinos and Watson stereotaxic rat brain atlas (Paxinos and Watson, 2004).Color deconvolution:Haematoxylin-3, 3′-diaminobenzidine (DAB) plugin of ImageJ software was utilized to quantify the area density of TH in the SN and the striatum and pathological α-synuclein aggregates in the SN.Finally, the mean value was calculated from the quantification in the three or five levels per nucleus per rat and then used to calculate the mean value of the three rats used per experimental condition (Additional Figure 1).Immunofluorescence assays were described elsewhere (Fernandez-Parrilla et al., 2022).For double immunofluorescence assays, the slices were permeabilized 3 times for 10 minutes each with Triton-PBS, and non-specific binding sites were blocked using 3% BSA in Triton-PBS for 1 hour at RT.After that, the slices were rinsed using Triton-PBS 3 times for 10 minutes each,then incubated with the suitable pair of primary antibodies overnight at 4C(Additional Table 3).The primary antibodies were mouse monoclonal anti-TH(1:1000; Sigma-Aldrich, Cat# T2928, RRID: AB_477569), rabbit polyclonal anti-TH (1:1000; Millipore, Temecula, CA, USA, Cat# AB5986, RRID: AB_92190),mouse monoclonal anti-V5 (1:500; Thermo Fisher Scientific, Cat# R960-25,RRID: AB_2556564), rabbit polyclonal anti-hCDNF (1:1000; ProSci, San Diego,CA, USA, Cat# 4343, RRID: AB_10909695), rabbit polyclonal anti-GFP (1:1500;Abcam, Cat# ab290, RRID: AB_303395), rabbit polyclonal anti-βIII-tubulin(1:300; Sigma-Aldrich, Cat# T2200, RRID: AB_262133), rabbit polyclonal anti-rNurr1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat# sc-5568, RRID: AB_2267355), rabbit monoclonal anti-BDNF (1:400; Abcam, Cat#ab108319, RRID: AB_10862052, mouse monoclonal anti-LB-509 α-synuclein(1:500; Abcam, Cat# ab27766, RRID: AB_727020, mouse monoclonal anti-GFAP (Clone GA5; 1:500; Cell Signaling Technology, Cat# 3670, RRID:AB_561049, rabbit polyclonal anti-C3 (1:100; Abcam, Cat# ab11887, RRID:AB_298669), and rabbit polyclonal anti-S100A10 (1:100; Thermo Fisher Scientific, Cat# PA5-95505, RRID:AB_2807307).After incubation, the samples were rinsed and incubated for 2 hours at RT with the suitable secondary antibodies (Additional Table 3), which were donkey anti-mouse AMCA H+L IgG (1:300, Jackson ImmunoResearch Laboratories, West Grove PA, USA,Cat# 715-155-151 RRID: AB_2340807), Texas red horse anti-mouse H+L IgG(1:800; Vector Laboratories, Cat# TI-2000, RRID: AB_2336178, Texas red goat anti-rabbit H+L IgG (1:800; Vector Laboratories, Cat# TI-1000, RRID:AB_2336199, Alexa Fluor 488 chicken anti-rabbit H+L IgG (1:300; Invitrogen Molecular Probes; Eugene, Oregon, USA, Cat# A-21441, RRID: AB_2535859,Alexa Fluor 488 chicken anti-mouse (1:300; Invitrogen Molecular Probes,Cat# A-21200, RRID: AB_2535786, and Cy5-conjugated goat anti-mouse IgG(1:300; Invitrogen Molecular Probes, Cat# A-10524, RRID: AB_2534033).Some brain slices and N1E-115 cells were counterstained with 1 µM Hoechst 33258 solution in PBS (Sigma-Aldrich) for 5 minutes for nuclear identification.After washing with PBS, the samples were mounted on glass slides using VECTASHIELD (Vector Laboratories).Negative controls were substantia nigra pars compacta (SNpc) without transfection or transfected with pEGFP-N1 and then subjected to indirect immunofluorescence for GFP (Additional Table 3).The slices of α-synuclein-immunofluorescence were counterstained with 0.05% Thioflavin T in 60% ethanol (Sigma-Aldrich) for 8 minutes, followed by five washes with 70% ethanol and MilliQ water to show the insoluble misfolded α-synuclein (Soto-Rojas et al., 2020c).
The immunofluorescence was analyzed with an SP8 confocal microscope(Leica TCS SPE; Heidelberg, Germany) to analyze at excitation-emission wavelengths of 405–510 nm (Hoechst 33258), 488–522 nm (Alexa 488),596–620 nm (Texas red), and 650–670 nm (Cy5).Ten to twenty consecutive 1µm optical sections in the XYZ plane were taken.The images were processed with the LAS AF software (Leica Application Suite; Leica Microsystems).Also,a Leica DMIRE2 microscope (Leica Microsystems) was used to observe the immunofluorescence through the filters A for Hoechst 33258 and AMCA, K3 for thioflavin T and Alexa 488, and TX2 for Texas-Red.Images were digitalized with a Leica DC300F camera (Leica Microsystems).The immunofluorescence area density (IFAD) for the double fluorescence assays was measured by ImageJ software v.1.46r in three anatomic levels of the SNpc per rat (n= 3 rats per experimental condition) according to the Paxinos and Watson stereotaxic rat brain atlas (Paxinos and Watson, 2004).In addition, background intensity was eliminated from the immunohistochemically stained area using the ImageJ Algorithm Subtract Background set up at 50 pixels.The mean value was calculated from the quantification in three levels of the SN per rat and then used to calculate the mean value of the three rats.
Statistical analysis
Data were represented as the mean ± standard deviation (SD).Once their normal distribution was confirmed with the Shapiro-Wilk and equal variance tests, comparisons involving multiple groups were performed using one-way analysis of variance followed by Tukey’spost hoctest.The statistical analysis was accomplished using GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com).P< 0.05 was considered a significant difference.
Results
Transcriptional activity of rNurr1-V5 in vitro
The pTracer-rNurr1-V5 plasmid transfected in N1E-115 cells expressed rNurr1-V5 and GFP, also encoded in this plasmid (Additional Figure 2A).Moreover, the pSuperscript-rNurr1 plasmid, a positive control, expressed only rNurr1 (Additional Figure 2A).As N1E-115 cells naturally lackNurr1(Additional Figure 2A; Espadas-Alvarez et al., 2017), hCDNF expression driven by the Nurr1-dependent NBRE3x promoter in the pNBRE3x-hCDNF plasmid only occurred with the cotransfection of pTracer-rNurr1-V5 or pSuperscriptrNurr1 plasmids (Additional Figure 2B).As expected, hCDNF was not detected with the transfection of only pNBRE3x-hCDNF in N1E-115 cells(Additional Figure 2B).These results demonstrate that the plasmid pTracerrNurr1-V5 expresses a transcriptionally active rNurr1-V5 protein.
Physical properties of NTS-polyplex NPs
For transfectionin vivo, the plasmids pTracer-rNurr1-V5 and its control pEGFP-N1 were compacted into NPs using the NTS-polyplex system.Field emission scanning electron microscopy analysis showed that the size of NTSpolyplex NPs fits a Gaussian curve with a mean of 99.11 nm for NPs harboring the pEGFP-N1 plasmid and 56.92 nm for the pTracer-rNurr1-V5 plasmid(Additional Figure 3A).However, DSL studies showed a bigger hydrodynamic diameter due to the hydration effect, of 211.15 nm for NPs with the pEGFP-N1 plasmid and 272.09 nm for NPs with the pTracer-rNurr1-V5 plasmid (Additional Figure 3B).Nevertheless, those NPs had a positive electrical charge of 5.01 to 10.37 mV (Additional Figure 3C), allowing repulsion among NPs in DMEM.
Unilateral gene transfection via NTS-polyplex NPs yields bilateral expression in nigral dopaminergic neurons
In healthy rats, the unilateral injection of NTS-polyplex NPs containing the plasmid pTracer-rNurr1-V5 led to rNurr1-V5 and GFP expression in TH+cells of both substantiae nigrae (Additional Figure 4).Similarly, EGFP expression in TH+cells was also bilateral by the local unilateral transfection of the control plasmid pEGFP-N1 (Additional Figure 4).The bilateral gene expression after a unilateral transfection suggests that NTS-polyplex NPs traversed to the opposite side.Accordingly, the unilateral administration of double-fluorescence-labeled NTSpolyplex NPs also led to fluorescent marks of propidium-iodide labeled pDNAs and FAM-KPRa within nigral TH+cells of both brain sides (Additional Figure 5),thus accounting for the bilateral transgene expression.

Figure 1 |Local unilateral gene transfection leads to bilateral transgene expression in nigral dopaminergic neurons of parkinsonian rats.(A) Representative micrographs of the injured substantia nigra (Injected side) transfected with NTS-polyplex NPs containing the plasmids pTracer-rNurr1-V5 (prNurr1) or pEGFP-N1 (pEGFP).Non-injected side = the contralateral substantia nigra of the same rat.V5 (green) and TH (red) were identified by indirect immunofluorescence on day 30 after NPs treatment.The scale value is equal for all micrographs.(B) Scatter plots of immunofluorescence area density (IFAD) for V5 and TH measured from micrographs of panel A with the software ImageJ.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).*P < 0.0001, healthy group vs.other groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: Rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracerrNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside; NPs: nanoparticles; ns: not significant;NTS: neurotensin; TH: tyrosine hydroxylase; V5: tag allows detection of rNurr1.
Compared with the healthy control, the TH-IFAD in the UT group was 20% (P< 0.0001) in the ipsilateral SN and 47% (P< 0.0001) in the contralateral side.In contrast, TH-IFAD values in the prNurr1 group were 50% (P< 0.0001) in the ipsilateral SN and 72% (P< 0.0001) in the contralateral side (Figure 1).No significant difference was found between the UT and GFP transfection groups.These results suggest thatrNurr1-V5transfection increased dopaminergic neurons because IFAD values were significantly higher (P< 0.0001) than both control groups (Figure 1).We previously showed that senescence is a death mechanism of nigral dopaminergic neurons activated by the local unilateral BSSG administration,reaching a maximum on day 60 after the lesion in both hemispheres (Soto-Rojas et al., 2020c).Similar results were obtained in this study (Figure 2A and B).The increase in Sen-β-Gal area density was 383% (P< 0.0001) on the injured side and 426% (P< 0.0001) on the contralateral side of the UT group compared with the healthy group (Figure 2C).The pEGFP group was not different from the UT group.In contrast, the Sen-β-Gal area density values in the prNurr1 group were 131% on the injured side and 87% on the contralateral side compared with the healthy group (Figure 2C).The prNurr1 and Healthy groups show no significant difference.These results suggest that prNurr1 reduced the Sen-β-Gal staining in both substantiae nigrae, thus suggesting a reduction in dopaminergic neuron senescence (Figure 2A and B).

Figure 2 |Local unilateral pTracer-rNurr1-V5 transfection bilaterally reduces senescence in dopaminergic neurons of parkinsonian rats.(A) Representative micrographs of the substantia nigra (Injected side) transfected with NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5(prNurr1).Non-injected side = the contralateral substantia nigra of the same rat.TH immunohistochemistry and the Sen-β-Gal counterstaining were made on day 30 after transfection (60 days after lesion).The oval dotted line delimits the SNpc area that was quantified.(B) Representative Sen-β-Gal staining images of TH+ neurons to show the effect of prNurr1.The scale value is equal for all micrographs.(C) Sen-β-Gal area density quantification of micrographs in panel A using ImageJ software.The values are the mean± SD from three anatomical levels (n = 3 independent rats per experimental condition).prNurr1 group vs.UT group (ΩP < 0.001) and pEGFP (£P < 0.01, ¥P < 0.001).*P < 0.01,healthy group vs.UT and pEGFP groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: Rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside;NPs: nanoparticles; ns: not significant; NTS: neurotensin; Sen-β-Gal: senescence-β-Galactosidase; SNpc: substantia nigra pars compacta; TH: tyrosine hydroxylase.
rNurr1-V5 expression mitigates the cytoskeleton loss in nigral dopaminergic neurons of parkinsonian rats
Another evidence of dopaminergic neuron death by the unilateral administration of BSSG is the reduction of the neuronal cytoskeleton in both substantiae nigrae (Soto-Rojas et al., 2020c).Compared with the healthy group, the remaining TH+neurons in the UT group showed TH-IFAD values of 10% (P< 0.0001) in the ipsilateral SN and 24% (P< 0.0001) in the contralateral side.In contrast, TH-IFAD values in the prNurr1 group were 49% (P< 0.0001)in the ipsilateral SN and 64% (P< 0.05) in the contralateral side (Figure 3B).The GFP group showed no significant difference from the UT group in both substantiae nigrae.The βIII-tubulin IFAD values in the UT group were 7% (P< 0.0001) in the ipsilateral SN and 20% (P< 0.0005) in the contralateral side,both compared with the Healthy group.In contrast, βIII-Tubulin IFAD values in the prNurr1 group were 30% (P< 0.0001) in the ipsilateral SN and 55%(P< 0.05) in the contralateral side (Figure 3B).βIII-tubulin IFAD values were statistically significant compared with the UT and GFP values (P< 0.05).These results suggest recovery of the cytoskeleton in dopaminergic neurons by pTracer-rNurr1-V5 gene transfection (Figure 3A and B).
rNurr1-V5 expression recovers the nigrostriatal system in parkinsonian rats

Figure 3 |Local unilateral pTracer-rNurr1-V5 transfection bilaterally mitigates the cytoskeleton loss in dopaminergic neurons of parkinsonian rats.(A) Representative micrographs of the substantia nigra (Injected side) transfected with NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1).Non-injected side = the contralateral substantia nigra of the same rat.Double immunofluorescence against βIII-tubulin (green) and TH (red) was made on day 30 post-transfection.The scale value is equal for all micrographs.(B) Scatter plots of immunofluorescence area density (IFAD) of βIII-tubulin and TH measured from micrographs of panel A using ImageJ software.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).P < 0.05,healthy group vs.other groups in TH (*) and βIII tubulin (£).One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside; NPs: nanoparticles; ns: not significant; NTS: neurotensin; TH: tyrosine hydroxylase.
In the unilateral BSSG model, senescence and reduced cytoskeleton are closely associated with neurodegeneration of both dopaminergic nigrostriatal systems with a predominance of the ipsilateral side to the lesion (Soto-Rojas et al., 2020c).Accordingly, the remaining TH+neurons were 18% (P< 0.0001)and ramifications 20% (P< 0.0001) in the injured SNpc.They were lesser than TH+cells (44%,P< 0.0001) and nigral ramifications (34%,P< 0.0001)in the contralateral SNpc (Figure 4A–C) compared with the health group.In addition, the decrease of nigral TH+neurons was accompanied by low values of TH+area density in the striatum of 23% (injured side,P< 0.0001) and 42%(contralateral side,P< 0.005) in the UT group compared with the healthy group (Figure 5A and B).Compared with the healthy group, TH+area density in the prNurr1 group was 48% (P< 0.0001) in nigral neurons, 60% (P< 0.001)in branching, and 65% (P< 0.01) in striatal terminals on the injured side.On the contralateral side, TH+ area density was 62% (P< 0.0001) in nigral neurons, 70% (P< 0.05) in branching, and 82% (P< 0.05) in striatal terminals compared with the healthy group (Figures 4 and 5).The pEGFP group was not statistically different from UT values.In contrast, prNurr1 group values were significantly higher (P< 0.05) than those of pEGFP and UT (Figures 4 and 5).The recovery occurred throughout both nuclei and could be caused by rNurr1-V5.
rNurr1-V5 expression raises the source of endogenous BDNF in parkinsonian rats
The unilateral BSSG administration also bilaterally decreased BDNFimmunoreactivity in TH+cells (Figure 6A and B), thus confirming that dopaminergic neurons are the primary source of BDNF production in the SNpc (Fernandez-Parrilla et al., 2022).BDNF-IFAD values in the UT group were 37% (P< 0.001) in the lesioned SNpc and 41% (P< 0.005) in the contralateral side compared with the healthy group (Figure 6B).BDNF-IFAD values after pTracer-rNurr1-V5 transfection were 82% in both nuclei compared with the healthy group (Figure 6B).BDNF IFAD values were statistically significant compared with UT and pEGFP groups (P< 0.05).BDNF recovery suggests that it also contributes to mitigating nigrostriatal dopamine neurodegeneration.
rNurr1-V5 expression lessens pathological α-synuclein aggregation in the nigrostriatal system
Unilateral BSSG administration leads to increased levels of pathological α-synuclein aggregates that first appear in the injected SN and later are found in the contralateral side and both striata (Soto-Rojas et al., 2020c).Similar results were found here (Figure 7A and B).Interestingly, pTracerrNurr1-V5 transfection but not pEGFP-N1 significantly reduced pathological α-synuclein aggregates by 81% (P< 0.001) in the injured SN and 84% (P<0.01) in the contralateral SN of parkinsonian rats on day 30 after transfection.Furthermore, a significant decrease also occurred in both striata of the injured side (83%,P< 0.0001) and the contralateral side (69%,P< 0.001; Figure 7A and B).Moreover, pTracer-rNurr1-V5 transfection significantly decreased α-synuclein immunoreactivity and thioflavin T counterstaining in nigral neuron-like cells (Figure 7C and Additional Figure 6).These results suggest that rNurr1-V5 expression decreases pathological α-synuclein aggregation and spreading to the contralateral SN and striatum.

Figure 4 |Local unilateral pTracer-rNurr1-V5 transfection recovers dopaminergic neurons and branching in parkinsonian rats.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1)were injected in the injured substantia nigra (Injected side) on day 30 post-BSSG lesion.Non-injected side = the contralateral substantia nigra of the same rat.(A)Representative micrographs of nigral dopaminergic neurons and branching revealed by TH immunohistochemistry on day 30 post-transfection in different anatomic levels(displayed at the left in every row).The scale value is equal for all micrographs.Scatter plots of TH+ cell counting (B) and immunoreactivity area density (C) measured from micrographs of panel A using ImageJ software.The values are the mean ± SD from five anatomical levels (n = 3 independent rats per experimental condition).*P < 0.05, healthy group vs.other groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1;UT: untransfected parkinsonian rats.BSSG: β-sitosterol β-D-glucoside; NPs: nanoparticles;ns: not significant; NTS: neurotensin; TH: tyrosine hydroxylase.
rNurr1-V5 expression attenuates neuroinflammation in the substantia nigra Bilateral neuroinflammation with the participation of activated microglia and neurotoxic A1 astrocytes is another indicator of unilateral BSSG-induced damage (Soto-Rojas et al., 2020c).Similar results were observed on day 60 post-BSSG administration (Figure 8 and Additional Figure 7).The Iba1 area density was 639% (P< 0.0001) in the injured SN and 373% (P< 0.005) on the contralateral side compared with the Healthy group (Figure 8 and Additional Figure 7).pTracer-rNurr1-V5 transfection yields no statistically different values to the Healthy group (ns) in both substantiae nigrae, thus reflecting attenuation of microglial activation (Figure 8).
The reactive astrogliosis was also bilateral after BSSG administration(Additional Figure 8).Furthermore, a significant increase in IFAD for GFAP+(1127%,P< 0.0001 in the lesioned side and 719%,P< 0.0001 in the contralateral side) and C3+(4729%,P< 0.0001 in the lesioned side and 2389%,P< 0.001 in the contralateral side) was observed in the UT and pEGFP groups compared with the Healthy group, suggesting activation of neurotoxic A1 astrocytes (Figure 9 and Additional Figure 9).Compared with the Healthy group, IFAD values in the prNurr1 group were 506% (GFAP;P< 0.0001) and 703% (C3;P< 0.0001) in the injured SN, and 187% (GFAP;P< 0.0001) and 634% (C3;P< 0.0001) in contralateral SN.Therefore, IFAD neurotoxic A1 astrocyte values caused by pTracer-rNurr1-V5 transfection were significantly lower than those of the UT and pEGFP groups, which were no different between them (Figure 9), thus indicating that rNurr1-V5 decreased activation of neurotoxic A1 astrocytes.Only pTracer-rNurr1-V5 transfection significantly increased the double-positive S100A10-GFAP cells (neurotrophic A2 astrocytes) in the injured SN (2582%vs.healthy; 1521%vs.UT;P< 0.0001)and contralateral SN (289%vs.healthy; 450%vs.UT;P< 0.0005; Figure 10 and Additional Figure 10).These results suggest that rNurr1-V5 expression attenuated the BSSG-triggered neuroinflammatory process.
rNurr1-V5 expression reduces motor deficits in parkinsonian rats
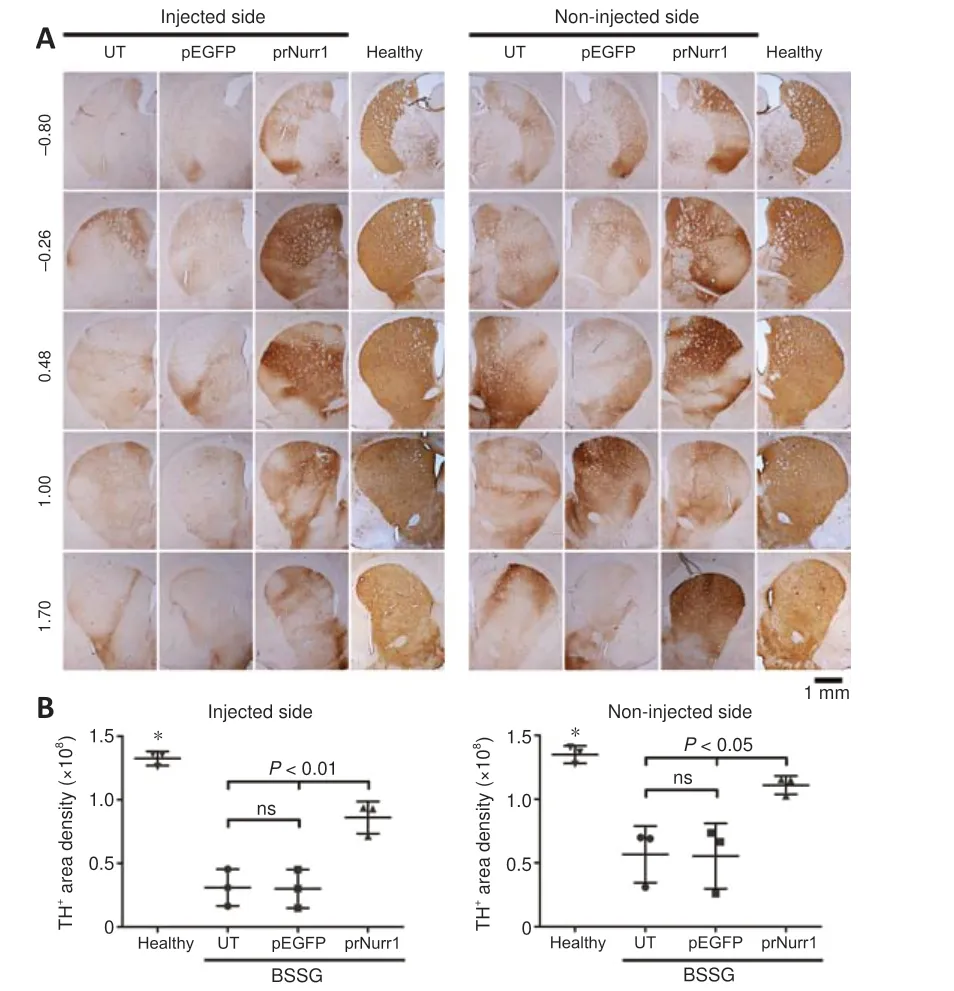
Figure 5| Unilateral pTracer-rNurr1-V5 transfection bilaterally recovers dopaminergic striatal terminals in parkinsonian rats.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1)were injected in the injured substantia nigra (Injected side) on day 30 post-BSSG lesion.Non-injected side = the contralateral substantia nigra of the same rat.(A)Representative micrographs of the striatal dopaminergic ramifications revealed by TH immunohistochemistry on day 30 post-transfection in different anatomic levels (displayed at the left in every row) according to Paxinos and Watson Atlas.The scale value is equal for all micrographs.(B) Scatter plots of TH immunoreactivity area density measured from micrographs of panel A using ImageJ software.The values are the mean ± SD from five anatomical levels (n = 3 independent rats per experimental condition).*P < 0.01, healthy group vs.other groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: Rats with BSSG lesion and transfected with pEGFP-N1;UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside; NPs: nanoparticles;ns: not significant; NTS: neurotensin; TH: tyrosine hydroxylase.
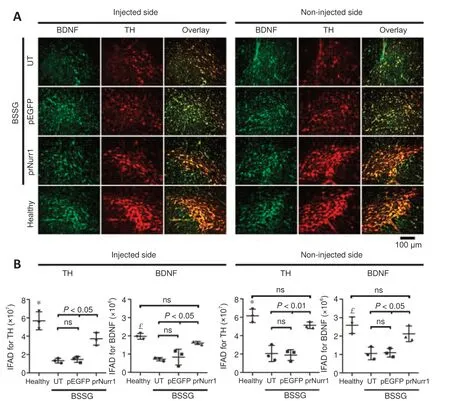
Figure 6 |Unilateral pTracer-rNurr1-V5 transfection bilaterally restores BDNF levels in nigral dopamine neurons of parkinsonian rats.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1) were injected in the injured substantia nigra (injected side) 30 days post-BSSG lesion.Noninjected side = the contralateral substantia nigra of the same rat.(A) Representative micrographs of the SNpc with BDNF (green) and TH (red) double immunofluorescence against BDNF and TH on day 30 post-transfection.The scale value is equal for all micrographs.(B) Immunofluorescence area density (IFAD) for BDNF and TH was determined using ImageJ software.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).P < 0.01, healthy group vs.other groups in TH (*) and BDNF (£).One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: Rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BDNF: Brain-derived neurotrophic factor; BSSG: β-sitosterol β-D-glucoside; NPs: nanoparticles; ns: not significant; NTS:neurotensin; SNpc: substantia nigra pars compacta; TH: tyrosine hydroxylase.

Figure 7 | Local unilateral pTracer-rNurr1-V5 transfection decreases the bilateral α-synuclein aggregation in the substantia nigra and striatum.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1) were injected in the injured SN (Injected side) after 30 days post-BSSG lesion.Non-injected side = the contralateral SN of the same rat.(A) Representative micrographs of the SNpc and striatum with α-synuclein aggregates displayed by immunohistochemistry on day 30 post-transfection.The scale value is equal for all micrographs.(B) Scatter plots of α-synuclein aggregation shown through immunoreactivity area density measured from micrographs of panel A using ImageJ software.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).*P < 0.05, healthy group vs. UT and pEGFP groups.One-way analysis of variance followed by Tukey’s post hoc test was used.(C) Immunofluorescence images show a reduction of α-synuclein immunoreactivity (red) and Thioflavin T counterstaining (green) in the SNpc on day 30 after pTracer-rNurr1-V5 transfection.Healthy: rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-Dglucoside; NPs: nanoparticles; ns: not significant; NTS: neurotensin; SN: substantia nigra;SNpc: substantia nigra pars compacta; α-syn: α-synuclein.
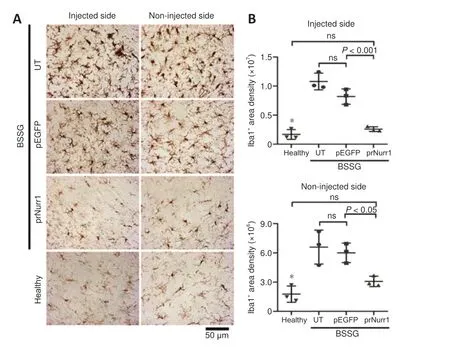
Figure 8 | Unilateral pTracer-rNurr1-V5 transfection halts bilaterally microglial activation in the substantia nigra.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1) were injected in the injured SN (Injected side) on day 30 post-BSSG lesion.Non-injected side= the contralateral SN of the same rat.(A) Representative micrographs of activated microglia revealed by Iba1 immunoreactivity in the SN on day 30 post-transfection.The scale value is equal for all micrographs.(B) Scatter plots of Iba1 immunoreactivity area density measured from micrographs of panel A using ImageJ software.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).*P < 0.01, healthy group vs.UT and pEGFP groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: Rats without lesion;prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside; Iba1: ionized calcium-binding adaptor molecule 1; NPs:nanoparticles; ns: not significant; NTS: neurotensin; SN: substantia nigra.
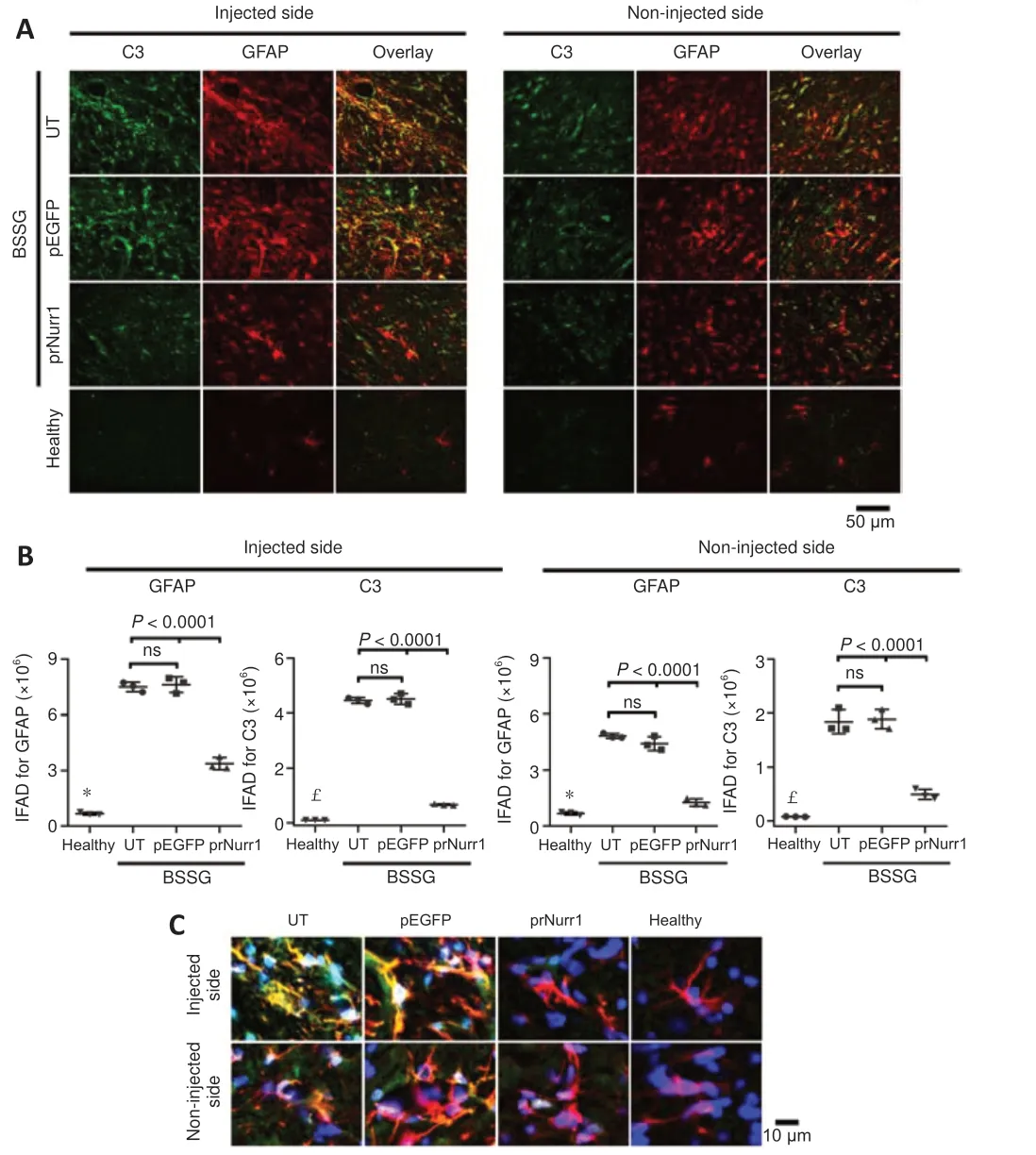
Figure 9 | Unilateral pTracer-rNurr1-V5 transfection bilaterally halts neurotoxic A1 astrocyte induction in the substantia nigra of parkinsonian rats.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1) were injected in the injured SN (Injected side) on day 30 post-BSSG lesion.Non-injected side= the contralateral SN of the same rat.(A) Representative micrographs of the substantia nigra with C3-GFAP double immunofluorescence on day 30 post-transfection.The scale bar is equal for all micrographs.(B) Immunofluorescence area density (IFAD) for C3 (green)and GFAP (red) was quantified using ImageJ software.The values are the mean ± SD from three anatomical levels (n = 3 independent rats per experimental condition).P < 0.0001,healthy group vs.UT and pEGFP groups in GFAP (*) and C3 (£).One-way analysis of variance followed by Tukey’s post hoc test was used.(C) Amplified merged images show the reduction of C3 immunoreactivity in GFAP cells with nuclear Hoechst counterstaining(blue) in the SNpc on day 30 after pTracer-rNurr1-V5 transfection.Healthy: Rats without lesion; prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP:rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG: β-Sitosterol β-D-glucoside; C3: complement component 3; GFAP: glial fibrillary acidic protein; NPs: nanoparticles; ns: not significant; NTS: neurotensin; SN: substantia nigra; SNpc: substantia nigra pars compacta.
A previous work of our group has shown that unilateral BSSG injection leads to sensorimotor deficits associated with bilateral neurodegeneration of the dopaminergic nigrostriatal system (Soto-Rojas et al., 2020b, c).The present work has confirmed similar locomotor deficits (Figure 11).The parkinsonian rats crossed the beam slower (bradykinesia) and slipped (instability) more often during the gait than the healthy controls (P< 0.0001; Figure 11A and B).In addition, the parkinsonian rats exhibited a significantly lessened response(sensorimotor alteration) to the vibrissae stimulation of both the right and left forelimbs (P< 0.0001; Figure 11C and D).They also showed olfactory asymmetry in the corridor test (P< 0.05; Figure 11E) but not in the cylinder test (Figure 11F).Consistently with the dopaminergic nigrostriatal system recovery (Figures 4 and 5), the three motor deficits (akinesia, bradykinesia,and instability) and olfactory asymmetry in parkinsonian rats were reduced by pTracer-rNurr1-V5 transfection reduced on day 30 post-transfection(Figures 4 and 5).Since pEGFP-N1 transfection did not modify motor deficits,the improvement in sensorimotor behavior seems to be directly related to rNurr1-V5 expression.
Discussion
This study shows that the unilateralrNurr1-V5gene transfection counteracts bilateral BSSG-induced damage manifested by dopaminergic neurodegeneration, neuroinflammation, α-synuclein aggregates, and consequent sensorimotor deficits in the parkinsonian rat.The recently developed parkinsonian rat model arises from a unilateral BSSG injection(Soto-Rojas et al., 2020b, c).However, the detailed mechanisms of how the insults spread across the brain hemispheres remain unknown.The complexity of the resulting phenotypes and our nescience of their underlying pathology represent limitations for providing detailed insight into the molecular mechanism of the rNurr1-V5 effect.Nonetheless, the present study demonstrates, on the one hand, the consistency of BSSG-induced neuropathology and, on the other hand, the feasibility ofrNurr1-V5gene therapy by NTS-polyplex NPs in a model that more closely resembles the human condition.Furthermore, the complexity of the nigral neuropil and the different cell and subcellular locations of target epitopes impeded an accurate quantification in double immunolabeling micrographs.Future studies,therefore, will include precise techniques to determine the percentage of successfully transfected cells necessary to trigger phenotypic change.

Figure 10 |Unilateral pTracer-rNurr1-V5 transfection bilaterally induces neurotrophic A2 astrocyte in the substantia nigra.NTS-polyplex NPs containing pEGFP-N1 (pEGFP) or pTracer-rNurr1-V5 (prNurr1) were injected in the injured SN (Injected side) on day 30 post-BSSG lesion.Non-injected side = the contralateral SN of the same rat.(A) Representative micrographs of SN with S100A10-GFAP double immunofluorescence on day 30 post-transfection.The scale bar is equal for all micrographs.(B) Immunofluorescence area density (IFAD) for S100A10(green) and GFAP (red) was measured with ImageJ software.The values are the mean± SD from three anatomical levels (n = 3 independent rats per experimental condition).*P < 0.001, healthy group vs.other groups.One-way analysis of variance followed by Tukey’s post hoc test was used.(C) Immunofluorescence images show the increase of S100A10 immunoreactivity in GFAP cells with nuclear Hoechst counterstaining (blue) in the SNpc on day 30 after pTracer-rNurr1-V5 transfection.Healthy: Rats without lesion;prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG:β-Sitosterol β-D-glucoside; GFAP: glial fibrillary acidic protein; NPs: nanoparticles; ns: not significant; NTS: neurotensin; S100A10: S100 calcium-binding protein A10; SN: substantia nigra; SNpc: substantia nigra pars compacta.
The V5 epitope in the C terminus of rNurr1 added to distinguish the transgene protein from the endogenous Nurr1in vivodid not affect its transcriptional activity, as shown by its capability to stimulate hCDNF transgene expression under the control of Nurr1-dependent NBRE3x promoter in N1E-115 cells lacking Nurr1.Furthermore, the rNurr1-V5 construct and pEGFP plasmid were compacted in NPs that comply with the size and surface electrical charge parameters of NTS-polyplex NP technology (Espadas-Alvarez et al., 2017;Aranda-Barradas et al., 2018; Fernandez-Parrilla et al., 2022).The resulting NPs were functional because they internalized in dopamine neurons and enabled transgene expression, i.e., rNurr1-V5 and GFP.Interestingly, the finding of rNurr1-V5 and GFP expression and double fluorescence labeled NTS-polyplex NPs in the SN opposite to the transfection side support the contention that NTS-polyplex NPs indeed spread.Possible propagation pathways could be diffusion through the glymphatic system, which facilitates drug distribution in the brain (Lohela et al., 2022), or microglia, which can internalize exosome-embedded macromolecules and spread them due to their high mobility throughout the brain (Xia et al., 2021).Therefore, the NTSpolyplex NP spread may account for the presence of rNurr1-V5 protein in the contralateral SN, thus explaining its bilateral effect.Future experiments will explore the mechanism of NP propagation.
Our results also found the presence of α-synuclein aggregates associated with neuroinflammation and dopaminergic neurodegeneration on the opposite side of the BSSG administration.These results can be explained by the finding that α-synuclein aggregates start in the BSSG-injected substantia nigra, increase over time, diffuse all over the brain in a prion-like manner,and trigger pathogenesis in the brain areas where they lodge (Soto-Rojas et al., 2020c; Morales-Martinez et al., 2022).The widespread presence of α-synuclein aggregates and their ability to trigger neuroinflammation and neuronal apoptosis where seeded has been documented in other experimental models (Duffy et al., 2018; Lin et al., 2023).Interestingly, the unilateralrNurr1-V5gene transfection lessened α-synuclein aggregates and neuropathology not only on the treated side but also in the contralateral SN.The effects of rNurr1-V5 likely result from suppressing α-synuclein expression and its consequent aggregation (Gamit et al., 2023).This suggestion is further supported by the finding that Nurr1 expression restores the expression of hundreds of dysregulated genes in primary dopaminergic neurons, including α-synuclein (Volakakis et al., 2015).
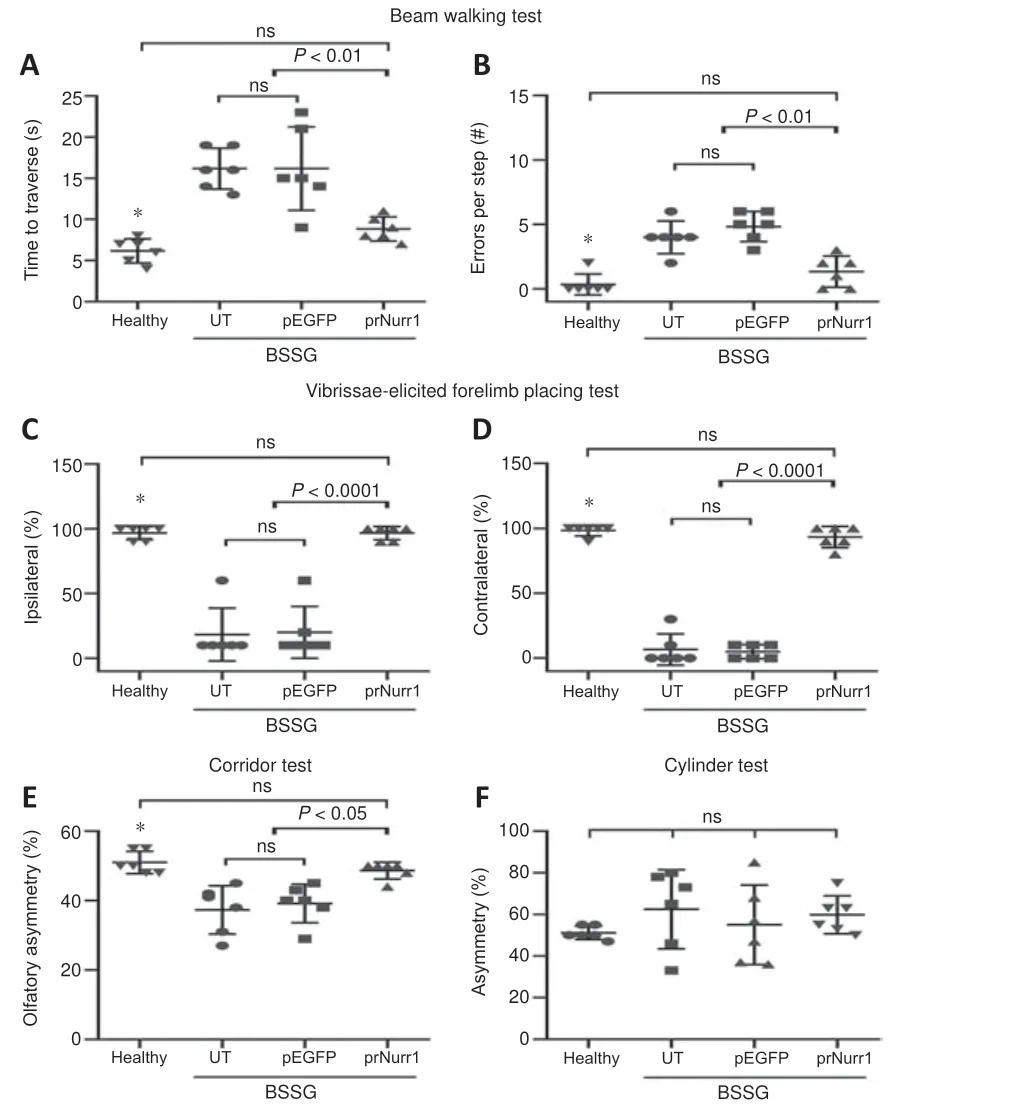
Figure 11 |rNurr1-V5 expression reduces sensorimotor deficits in parkinsonian rats.Rats were transfected with pTracer-rNurr1-V5 or pEGFP-N1 on day 30 post-BSSG lesion and evaluated 30 days after transfection (60 days post-BSSG lesion).(A, B) Time traveled(A) and number of errors per step (B) during displacement on a beam.(C, D) Ipsilateral(C) and contralateral (D) response to the vibrissae stimulation.(E) Asymmetry in discrimination of olfactory stimuli regularly distributed on each side of a corridor floor.(F) Asymmetry in the number of contacts of the forelimb paws on a transparent cylinder wall.Values are expressed as the mean ± SD of 6 independent rats for each experimental condition.*P < 0.05, healthy group vs.UT and pEGFP groups.One-way analysis of variance followed by Tukey’s post hoc test was used.Healthy: rats without lesion;prNurr1: rats with BSSG lesion and transfected with pTracer-rNurr1-V5; pEGFP: rats with BSSG lesion and transfected with pEGFP-N1; UT: untransfected parkinsonian rats.BSSG:β-Sitosterol β-D-glucoside; ns: not significant.
Counterattacking neuroinflammation is another mechanism by which Nurr1 promotes neuroprotection or neurorepair in parkinsonism models (Decressac et al., 2012; Liu et al., 2017).We, therefore, evaluated the effect ofrNurr1-V5transfection on activated microglia and neurotoxic reactive A1 astrocytes,two indicators of neuroinflammation.In agreement with previous results(Luna-Herrera et al., 2020; Soto-Rojas et al., 2020c), the presence of activated microglia and neurotoxic reactive A1 astrocytes coincided with dopaminergic neurodegeneration in the lesioned SN and the contralateral side sixty days post-unilateral BSSG administration.Microglial transport of pathological α-synuclein via exosomes (Xia et al., 2021) may be responsible for the spread of neuropathology to the contralateral SN (Soto-Rojas et al., 2020c).On the remote sites, α-synuclein secreted by microglia and neurons is known to activate local astrocytes and microglia that, in turn, release proinflammatory cytokines, which trigger the neuroinflammatory process (Pike et al., 2021).In this process, microglial proinflammatory cytokines (Flores-Martinez et al.,2018; Luna-Herrera et al., 2020), microglial NLRP3 inflammasome activation(Valenzuela-Arzeta et al., 2023), and saturated lipids (Guttenplan et al., 2021)released by neurotoxic reactive A1 astrocytes can potentially cause neuron death.
Our study documents that bilateral expression of rNurr1-V5 leads to a significant reduction of activated microglia and neurotoxic A1 astrocytes in both substantiae nigrae, supporting the anti-inflammatory effect of rNurr1-V5(Liu et al., 2017; Oh et al., 2020; Gao et al., 2021).Some mechanisms identified in other neuroinflammation modelsin vitroandin vivo, such as inhibition of NF-κB (Gao et al., 2021), CCL2 (Liu et al., 2017), RasGRP1 (Oh et al., 2020), and α-synuclein (Gamit et al., 2023) have been implicated in the anti-inflammatory effect of rNurr1-V5.The proposed mechanisms need to be evaluated in our model by future studies.Furthermore, rNurr1-V5 induced the neurotrophic A2 astrocytes (S100A10+), which can also contribute to the anti-inflammatory effect by releasing neurotrophic factors such as BDNF(de Pins et al., 2019), GDNF (Gava-Junior et al., 2023), and CDNF (Nadella et al., 2014).Silencing miR-21, a switch for polarization of astrocytes from the A1 phenotype to the A2 phenotype (Su et al., 2019), could be one of the mechanisms of rNurr1-V5-induced neurotrophic A2 astrocytes.
Sixty days after local unilateral BSSG administration, evidence of irreversible dopaminergic neuron degeneration appears bilaterally, including markers of senescence, cytoskeleton disorganization, and loss of dopaminergic cell bodies and ramifications (Soto-Rojas et al., 2020b, c).This latter impairment correlates with the dopaminergic denervation of both striata (Soto-Rojas et al., 2020c).Our study confirmed those structural deficits and further showed that they were reversed by rNurr1-V5 expression, whereas GFP expression had no effect.Together with other studies using different experimental approaches (Decressac et al., 2012; De Miranda et al., 2015; Oh et al.,2015; Hammond et al., 2018; Chae et al., 2022), our results support the Nurr1 neurorestorative effect on dopaminergic neurons.This effect may be mediated by the recovery of RET (Decressac et al., 2011, 2012) and BDNF(as shown here).BDNF, essential for the function and survival of nigral dopaminergic neurons (Hernandez-Chan et al., 2015; Razgado-Hernandez et al., 2015; Fernandez-Parrilla et al., 2022), is directly regulated by Nurr1(Volpicelli et al., 2007).Therefore, BDNF rise could be driven by rNurr1-V5 expression and be responsible for the structural repair of the nigrostriatal system damaged by BSSG neurotoxicity.This suggestion is supported by the capacity of BDNF to suppress neurodegeneration in 6-OHDA acute parkinsonism (Hernandez-Chan et al., 2015) and reverse the structural and functional deficits in 6-OHDA chronic hemiparkinsonism (Razgado-Hernandez et al., 2015).On the other hand, Nurr1, by stimulating RET expression (Conway et al., 2020), can remove the α-synuclein-induced RET inhibition, thus allowing GDNF signaling to cause neuroprotection in dopaminergic neurons (Decressac et al., 2011, 2012).Contrary to the BSSG model, GDNF or neurturin efficiently rescues dopaminergic neurons in the 6-OHDA parkinsonism model that lacks α-synucleinopathy (Gonzalez-Barrios et al., 2006; Reyes-Corona et al., 2017),suggesting that Nurr1 is functional in the 6-OHDA model and highlighting the need to rescue Nurr1 from α-synuclein repression to achieve an efficient neurotrophic effect (Decressac et al., 2011).
Our previous works have shown that unilateral BSSG administration triggers Parkinson’s disease-like locomotor and nonmotor deficits but with differenttimelines.Depression-like behavior evaluated with the forced swimming test and anxiety evaluated with the open field test become consistent after 60 days post-BSSG, which corresponds to a late or severe parkinsonism stage(Soto-Rojas et al., 2020b, c).Thus, depression and anxiety were not evaluated in this study because it was focused on the early parkinsonism stage (30 days post-BSSG; time of transfection), where sensorimotor deficits are wellestablished and still progressive (Soto-Rojas et al., 2020b, c).Our results confirmed that a unilateral intranigral BSSG administration elicits the typical locomotor impairments of early PD in the rat (Soto-Rojas et al., 2020b, c).The poor response of both forelimbs in the vibrissae test and the lack of asymmetry in the cylinder test come from the significant bilateral loss of dopaminergic neurons and striatal denervation.However, the corridor test showed sensorimotor asymmetry on day 60 post-BSSG lesion because the bilateral impact on the olfactory system circuitry appears as late as 120 days in the BSSG model (Soto-Rojas et al., 2020b), even though the oxidative stress and mitochondrial dysfunction in the olfactory bulb start as early as 15 days after BSGG lesion (Morales-Martinez et al., 2022).In addition, the beam test showed bradykinesia and postural imbalance, the two classical signs of PD.Those locomotor deficits were significantly reduced by unilateralrNurr1-V5transfection that structurally improved the bilateral dopaminergic nigrostriatal system.Dopamine resulting from the improved dopaminergic nigrostriatal system can lead to reducing motor deficits.While this study did not measure dopamine, preclinical gene therapy studies with different neurotrophic factor genes and NTS-polyplex NPs have demonstrated that the recovery of the dopaminergic nigrostriatal system correlates with dopamine recovery(Gonzalez-Barrios et al., 2006; Hernandez-Chan et al., 2015; Reyes-Corona et al., 2017; Fernandez-Parrilla et al., 2022).The improved locomotor activity coincides with results in other parkinsonism models after overexpressing Nurr1 alone or combined with Foxa2 (Oh et al., 2015; Liu et al., 2017) or pharmacological stimulation (De Miranda et al., 2015; Hammond et al., 2018).
Based on the results obtained, it seems thatrNurr1-V5gene transfer into dopaminergic neurons via NTS-polyplex is safe, even with intracerebral administration.This possibility is further supported by the anti-inflammatory effect of hCDNF transfection with NTS-polyplex in the 6-OHDA PD model(Nadella et al., 2014) and studiesin vitroandin vivodemonstrating the safety of NTS-polyplex nanotechnology (Hernandez et al., 2014; Lopez-Salas et al., 2020).However, transgene-induced neurotoxicity is still possible since neurotoxicity was observed with the transfection of pDsRed2DsRed2 plasmid coding for the double DsRed2DsRed2 protein but not with pEGFP-N1 coding for GFP (Lopez-Salas et al., 2020).Therefore, in future studies, it is crucial to evaluate neuroinflammation and neurodegeneration of therNurr1-V5gene overexpression in healthy rats.

Figure 12 |Proposed mechanism of rNurr-V5 therapy in BSSG-induced parkinsonism.Arrowhead lines: simulation.Blunted-end lines: Repression or inhibition.BDNF: Brainderived neurotrophic factor; DAT: dopamine transporter promoter; TH: tyrosine hydroxylase; VMAT2: vesicular monoamine transporter 2; α-Syn: α-synuclein.
Based on the previous knowledge of Nurr1 physiology and pathology explained in previous sections, our findings suggest the following molecular mechanism for the effect of rNurr1-V5 in the unilateral BSSG model (Figure 12).a) In healthy adult conditions, Nurr1 binds to response elements Nurr1 to maintain the dopaminergic function by regulating the expression of TH, other enzymes, and transporters involved in dopaminergic neurotransmission.Similarly, Nurr1 drives the expression of BDNF and RET, which participate in neural survival.b) In the BSSG parkinsonian model, the drop in Nurr1 levels reduces the survival and functional and structural maintenance of dopaminergic neurons.Abolishing Nurr1 repression on α-synuclein expression favors the formation of α-synuclein aggregates, which can diffuse and induce neuroinflammation.Neuroinflammation can also stimulate α-synuclein aggregation, which yields a vicious circle.c) In NTS-polyplex NP gene therapy,rNurr1-V5transgene expression in dopaminergic neurons replaces Nurr1 functions due to its transcriptional activity demonstratedin vitro.The recovered transcriptional activity leads to the expression of TH and BDNF, which can explain the functional and structural improvement of the dopaminergic nigrostriatal system.Furthermore, the possible rNurr1-V5-induced repression of α-synuclein expression could account for the reduction of α-synuclein aggregates, thus reducing neuroinflammation.All the beneficial events contribute to enhancing locomotor behavior.
This study has some limitations that should be noted.The duration of the rNurr1-V5 effect on α-synucleinopathy remains unknown beyond one month after the BSSG lesion.It is also important to note that while rNurr1-V5 expression has been shown to reduce α-synuclein spreading to the striatum,it is still necessary to determine ifrNurr1-V5gene therapy can eliminate α-synuclein aggregates in other cerebral areas (Soto-Rojas et al., 2020c)to prevent their retrograde diffusion to the SN, which could result in the loss of dopaminergic recovery.If rNurr1-V5 production were insufficient,cotransfection of neurotrophic factor genes could complement it, taking advantage of NTS-polyplex NPs’ ability to transfect two genes simultaneously(Arango-Rodriguez et al., 2006).Finally, the study was limited to the intracranial injection of NTS-polyplex NPs in the SN because they do not cross the brain-blood barrier (BBB) when administered via blood circulation.This limitation can be overcome using focused ultrasound, a less invasive procedure, to open BBB transiently (Aly et al., 2023).
In conclusion, the unilateral intranigral BSSG administration provided a suitable PD model to demonstrate thatrNurr1-V5transgene expression in nigral dopaminergic neurons via NTS-polyplex nanotechnology mitigates the four PD hallmarks, i.e., α-synuclein aggregation, neuroinflammation(activated microglia, neurotoxic astrocytes), dopaminergic neurodegeneration(senescence and loss of neuron-cytoskeleton and TH+cells), and sensorimotor deficits.Notably, despite the unilateral injections, the toxin caused bilateral PD phenotypes, and NTS-polyplex NPs caused rNurr1-V5 bilateral expression and rescuing activity.Increased neurotrophic astrocytes and BDNF, also induced by rNurr1-V5 expression, could mediate the rNurr1-V5 effect.The attenuation of α-synuclein aggregates and their consequent pathology by rNurr1-V5 support the hypothesis that α-synuclein aggregates are the leading player in PD and, therefore, must be the primary target of modern therapeutics.Moreover, restraining activated microglia is also crucial because they transport pathological α-synuclein via exosomes (Xia et al., 2021)and (Soto-Rojas et al., 2020b, c) activate neurotoxic reactive A1 astrocytes(Liddelow et al., 2017), which must also be suppressed (Yun et al., 2018) due to their lethal effect on different types of neurons (Liddelow et al., 2017).Our next objective is to investigate the efficacy ofrNurr1-V5gene therapy in severe BSSG-induced parkinsonism, including molecular techniques to measure rNurr1-V5 expression levels and identify its molecular mechanism and nonmotor behavior tests.Once this objective is achieved, we can propose thatrNurr1-V5transgene expression via NTS-polyplex nanotechnology could be a particularly valuable therapy for the recovery of function in PD.
Acknowledgments:We thank Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) for granting scholarship No.426466 to Bismark Gatica García.The authors also thank Anton Paar México, S.A.de C.V for his invaluable technical assistance in the dynamic light scattering characterization of NTS-polyplex nanoparticles.
Author contributions:Conceptualization: DMF, MJB, and BGG; methodology:BGG, LOSR, DRC, CPRO, JUMC, MMB, LE, and JSS; software: BGG, CPRO, IEVA,CLH, and JUMC; validation: DMF and LOSR; formal analysis: DMF, BGG, LOSR,MEGC, and IAMD; investigation: DMF and BGG; resources: DMF, MEGC, and AJEA; data curation: DMF and BGG; writing—original draft preparation:DMF and BGG; writing—review and editing; DMF, BGG, LOSR, MAFP, FELS,DRC, CPRO, MEGC, IAMD, CLH, MMB, IEVA, JSS, AJEA, and LE; visualization:DMF, BGG, LOSR, CPRO, and DRC; supervision: DMF; project administration:DMF; funding acquisition: DMF.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1: Experimental design.
Additional Figure 2: pTracer-rNurr1-V5 plasmid expresses a functional rNurr1-V5 protein in N1E-115 cells.
Additional Figure 3: Size and Z-Potential of NTS-polyplex NPs with the plasmid pEGFP-N1 or pTracer-rNurr1-V5.
Additional Figure 4: Unilateral gene transfection in the substantia nigra leads to bilateral transgene expression in dopaminergic neurons of healthy rats.
Additional Figure 5: Unilateral injection of double fluorescence-labeled NTS-polyplex NPs in the substantia nigra leads to bilateral nanoparticle internalization in nigral dopaminergic neurons of healthy rats.
Additional Figure 6: α-Synuclein immunoreactivity (red) with Thioflavin T(green) counterstaining in the substantia nigra.
Additional Figure 7: Representative micrographs of activated microglia revealed by Iba1 immunostaining in the mesencephalon.
Additional Figure 8: Reactive astrocytosis revealed by GFAP immunohistochemistry in the mesencephalon.
Additional Figure 9: Representative micrographs of neurotoxic astrocytes revealed by double immunofluorescence against C3 (green) and GFAP (red)markers with Hoechst counterstaining (blue).
Additional Figure 10: Representative micrographs of neurotrophic astrocytes revealed by double immunofluorescence against S100A10 (green) and GFAP(red) with Hoechst counterstaining (blue).
Additional Table 1: Overview of the mean values, standard deviation,and sample size per each parameter and treatment group at the date of stratification.
Additional Table 2: Antibodies for immunohistochemistry staining.
Additional Table 3: Antibodies for double immunofluorescence assays.
杂志排行
中国神经再生研究(英文版)的其它文章
- NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases
- Circadian rhythm disruption and retinal dysfunction:a bidirectional link in Alzheimer’s disease?
- Interplay between the glymphatic system and neurotoxic proteins in Parkinson’s disease and related disorders: current knowledge and future directions
- Roles of neuronal lysosomes in the etiology of Parkinson’s disease
- Therapeutic advances in neural regeneration for Huntington’s disease
- The advantages of multi-level omics research on stem cell-based therapies for ischemic stroke
