Effects of Homoharringtonine on the Proliferation and Apoptosis of Melanoma Cells
2024-01-08RongHUBinLIUFanXIAOXianjingZENG
Rong HU, Bin LIU, Fan XIAO, Xianjing ZENG*
1. Department of Dermatology, Affiliated Hospital of Jinggangshan University, Ji’an 343000, China; 2. Department of Oncology, Affiliated Hospital of Jinggangshan University, Ji’an 343000, China; 3. Jinggangshan University, Ji’an 343000, China
Abstract [Objectives] To explore the effects of homoharringtonine (HHT) on the proliferation and apoptosis of melanoma cells. [Methods] Mouse melanoma cell line B16F10 was divided into 5 different dose groups, among which 0 μg/mL HHT was as the negative blank group, and the remaining four groups were added with different concentrations of HHT (20, 40, 80 and 160 μg/mL). After B16F10 cells were treated with HHT for 48 h, the growth inhibition rate of B16F10 cells was observed, and the cell cycle was detected. Cyclin D1, MMP-9 and VEGF mRNA and protein levels were detected by qRT-PCR and Western-blot. [Results] Compared with 0 μg/mL group, after different concentrations of HHT treated B16F10 cells, the inhibition rate and apoptosis rate of B16F10 cells increased, and Cyclin D1, MMP-9 and VEGF mRNA and protein levels in all groups decreased significantly, with statistical significance (P<0.05). [Conclusions] HHT may be involved in the apoptosis of melanoma cells by regulating Cyclin D1 level, affecting cell cycle, and inhibiting VEGF and MMP-9 gene protein levels.
Key words Homoharringtonine, Melanoma, Matrix metalloproteinases, Cyclin
1 Introduction
Originating from neural crista-derived melanocytes, melanoma is characterized by high malignancy, rapid proliferation, strong metastasis and low survival rate, and its incidence is increasing year by year worldwide[1]. The current conventional therapies for melanoma include surgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy,etc.Once the tumor enters the stage of metastasis, the five-year survival rate of patients is only 25%[2]. With the continuous deepening of research, more targeted therapies have been gradually developed in recent years. Although the therapies have been greatly improved, the five-year survival rate of patients is still low, and the problems such as resistance and drug resistance, low response rate and adverse reactions generated during the treatment cannot be ignored. Therefore, finding effective methods is still the focus of current research.
With the development of the modernization of traditional Chinese medicine, traditional Chinese medicine has shown good efficacy in stabilizing tumor volume, blocking cell cycle, inhibiting tumor angiogenesis, regulating the body’s immunity, and alleviating the toxic side effects of chemotherapy[3]. Seeking effective anticancer drugs from traditional Chinese medicine is one of the important ways to improve tumor efficacy. Homoharringtonine (HHT), an alkaloid extracted from the branches and leaves ofCephalotaxus, is a highly effective anti-tumor drug independently developed in China. Recent studies have shown that a variety of tumor cells can be inhibited by HHT[4], and the US FDA (Food and Drug Administration) has approved HHT for the treatment of chronic myelogenous leukemia and myelodysplastic syndrome[5]. At present, there are few studies on the treatment of melanoma with HHT. In this paper, the effects of HHT on the cell cycle and apoptosis of melanoma were discussed to provide a reference for clinical treatment.
2 Materials and methods
2.1 Experimental materialsMain experimental materials included HHT (Hangzhou Minsheng Pharmaceutical Co., Ltd.), melanoma cell line B16F10 (Shanghai Saimo Biological Co., Ltd.), fetal bovine serum (Beijing Solarbio Company), DMEM medium (Hyclone Company), rabbit anti-human cyclin D1, rabbit anti-VEGF monoclonal antibody, rabbit anti-MMP-9 monoclonal antibody, goat anti-mouse β-actin (Abcam Company, USA), VEGF primer, MMP-9 primer, β-actin (Generay Biotech Company), RNA extraction kit, PCR detection kit (Shanghai Biyuntian Biotechnology Co., Ltd.), BCA protein concentration detection kit (Beijing Solarbio Company), and Trizol reagent (Invitrogen Company).
2.2 Cell cultureAt first, 4-6 generations of B16F10 cells were cultured in a high-glucose DMEM cell culture medium containing 10% fetal bovine serum, and it was placed in a constant temperature incubator containing 5% CO2(37 ℃). The medium was changed every day, and subculture was conducted every 3-4 d according to the ratio of 1:3. All the cells used in the experiments were in the logarithmic growth phase.
2.3 Cell groupingThe cells in the logarithmic growth phase were collected, and the number of cells was adjusted to 1×106/mL after being re-suspended with Hanks salt balance solution of 10% fetal bovine serum. The cells were inoculated into a six-well plate at a cell density of 5×104/mL, and then cultured in a constant temperature incubator. The experimental cells were divided into five groups according to HHT concentration, including blank group (0 μg/mL) and low-, medium- and high-concentration HHT groups (20, 40, 80 and 160 μg/mL).
2.4 Cell proliferation assayLogarithmic B16F10 cells were inoculated into 96-well plates with a dosage of 100 μL/well at a density of 1×105cells /mL, and treated with 100 μL of HHT at different concentrations after 24, 48 and 72 h, respectively. Five parallel wells were set up in each group. After CCK-8 reagent was added to the wells, they were continuously cultured for 2-3 h, and the optical density (OD) of each hole was measured at 450 nm. After the influence of blank holes was removed, the growth inhibition rate was calculated according to the following formula: the growth inhibition rate=[(ODvalue of the blank group-ODvalue of drug groups)/ODvalue of the blank group]×100%.
2.5 Detection of cell cycleLogarithmic B16F10 cells were inoculated into a 12-well plate according to cell density, treated with 100 μL og HHT at different concentrations, and washed after culture. Cell concentration was adjusted to 5×105cells/mL. 500 μL Binding Buffer suspension cells were added to the wells. After mixing with 5 μL of Annexin V-FITC, 5 μL of Propidium Iodide was added to the mixture, and they reacted for 15 min at room temperature in a dark place. Flow cytometry was used for observation and detection by Annexin-V and PI double staining method.
2.6 Expression of MMP-9, Cyclin D1 and VEGF genes detected by qRT-PCRAfter 48 h of routine culture, cells were collected, total RNA of cells was extracted by RNA extraction kit, and cDNA was obtained by reverse transcription, and the target gene of qRT-PCR was amplified based on this template. TheCTvalue detected byLC480 was the number of cycles in which the fluorescence intensity of cDNA reached the specified threshold. ΔCt=CTvalue of the target gene-CTvalue of internal reference gene, and mRNA relative expression in each sample=2-ΔCT×100%.

Table 1 Design of PCR primer
2.7 Expression of MMP-9, Cyclin D1 and VEGF protein detected by Western blotAfter 48 h of culture, cells were collected, to which lysate was added, and total protein was extracted by protein extraction kit. The concentration of total protein was determined by BCA method, and then gluing, electrophoresis, membrane transfer, sealing, antibody incubation, and phase development were carried out. β-actin encoded protein level was used as the inner blank, and gray values of target bands were analyzed by gel image processing system. The relative expression level was recorded.
2.8 Statistical analysisAll experiments were independently repeated three times. Statistical software SPSS 20 was used for statistical analysis of all data. One-Way ANOVA was used for comparison among multiple groups, andχ2test was used to compare rates.P<0.05 indicates there exists statistical difference.
3 Results and analysis
3.1 Effects of HHT on the proliferation of melanoma B16F10 cellsAs shown in Fig.1, when HHT concentration was 20 μg/mL, the inhibition rate of B16F10 cells increased from (13.05±2.64)% to (28.54±3.74)% after HHT treatment for 24-72 h. As HHT concentration was up to 160 μg/mL, the inhibition rate of B16F10 cells rose from (43.15±5.06)% to (82.75±6.32)%, showing that the difference was statistically significant (P<0.05). It suggests that HHT inhibited the proliferation of B16F10 cells.

Fig.1 Inhibition of B16F10 cells by HHT
3.2 Effects of HHT on B16F10 cell cycleSeen from Fig.2, after B16F10 cells were treated with different concentrations of HHT for 48 h, the number of S-phase cells increased significantly, revealing that B16F10 cell cycle was blocked in the S phase (P<0.001).
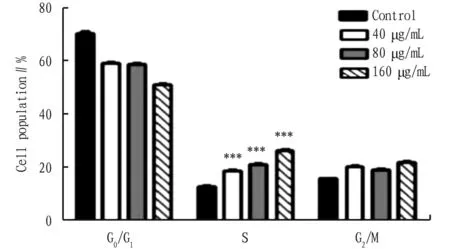
Note: *P<0.001 compared with groups 40, 80 and 160 μg/mL.
3.3 Effects of HHT on the apoptosis of B16F10 cellsAs shown in Fig.3 and Table 2, after B16F10 cells were treated with HHT for 48 h, the apoptosis rate of B16F10 cells gradually rose with the increase of HHT concentration compared with the blank group, and the differences were statistically significant (P<0.05).

Fig.3 Apoptosis of B16F10 cells in different groups

Table 2 Apoptosis rate of B16F10 cells in different groups (%,
3.4 Detection of MMP-9, Cyclin D1, and VEGF mRNA levels in various groups by RT-PCRAs shown in Table 3, with the increase of HHT concentration, MMP-9, Cyclin D1 and VEGF mRNA levels in all groups decreased significantly after B16F10 cells were treated with HHT for 48 h, and the differences were statistically significant compared with the blank group (P<0.05).
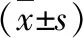
Table 3 MMP-9, Cyclin D1 and VEGF mRNA levels in each group
3.5 Detection of MMP-9, Cyclin D1 and VEGF protein levels by Western blotSeen from Table 4 and Fig.4, with the increase of HHT concentration, MMP-9, Cyclin D1 and VEGF protein levels in all groups dropped significantly after B16F10 cells were treated with HHT for 48 h, with statistical differences compared with blank group (P<0.05).

Note: A: 0 μg/mL; B. 20 μg/mL; C. 40 μg/mL; D. 80 μg/mL; E. 160 μg/mL.
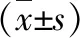
Table 4 MMP-9, Cyclin D1 and VEGF protein levels in each group
4 Discussion
Melanoma is a relatively common malignant tumor in clinical practice. Although great progress has been made in the clinical diagnosis and treatment of melanoma in recent years, the overall survival rate has not yet made a major breakthrough. Blocking cell cycle and inducing cell apoptosis are still one of the important mechanisms of tumor treatment with anticancer drugs.
Traditional Chinese medicine (TCM), as the main method of adjuvant therapy of tumors, has multi-target and multi-channel effects, and can reduce adverse reactions, so it is one of the important ways to improve tumor efficacy. Plant-derived anticancer agents play an extremely important role in the treatment of cancer, and some of them have become the first choice drugs for certain neoplastic diseases, such as HHT, paclitaxel, camptocampine,etc[6]. HHT is an alkaloid extracted fromCephalotaxus, and studies have shown that HHT also has a good killing and inhibiting effect on bladder cancer and liver cancer[7]. The anti-tumor mechanism of HHT is complex. Currently, it is believed that the main mechanism of HHT in tumor treatment is to inhibit the synthesis of tumor DNA and protein, inhibit the binding of tumor cells and fibrinogen, and thus inhibit the proliferation and metastasis of tumor cells[8].
Cell cycle is the basic process to ensure the life activities of cells, and regulates the physiological states of cell proliferation, differentiation, senescence and death through the phase change of cell cycle. Abnormal cell cycle is one of the characteristics of malignant tumors. Inhibiting the proliferation of tumor cells by inducing cell cycle arrest has been a hot topic in recent years. In the process of cell division and proliferation, cycle proteins Cyclins and cyclin-dependent kinase CDK play important regulatory roles[9]. Studies have shown that Cyclin D1 is a key protein regulating the G1 phase of the cell cycle, and has been recognized as a proto-oncogene. Overexpression of Cyclin D1 is closely related to the occurrence of tumors, and S-phase arrest plays an important role in the pathogenesis of most tumors[10]. The results of this study show that the apoptosis rate of B16F10 cells treated with different concentrations of HHT significantly increased in the S phase, and the expression level of related cyclinA gene decreased, indicating that the anti-tumor mechanism of HHT may be related to the blocking of B16F10 cells in the S phase to inhibit the expression of Cyclin D1 and thus inhibit the proliferation of A375 cells.
New blood vessels are formed during the continuous growth and metastasis of tumor cells. VEGF is the most effective angiogenic factor among the factors affecting the formation of the new blood vessels, can promote the division and proliferation of vascular endothelial cells, induce the expression of serine protease and interstitial collagenase, and accumulate cytoplasmic calcium to induce angiogenesis, and plays an important role in wound healing, embryonic development, tumor growth and metastasis[11]. Matrix metalloproteinases (MMPs), a class of zinc-dependent endopeptidases, play an important role in the degradation and reconstruction of extracellular matrix. Matrix metalloproteinase-9 (MMP-9) has been shown to play a key role in tumor growth, invasion, metastasis and angiogenesis of tumor tissues, and it is a key step in cancer cell invasion and metastasis. Studies have shown that the increased expression of MMP-9 in cancer tissues increases the ability of tumor cells to degrade extracellular matrix, thus leading to tumor metastasis[12]. The overexpression of MMP-9 is significantly positively correlated with tumor angiogenesis[13]. VEGF and MMP-9 interact in the process of tumor vascular growth, and after VEGF binds to the receptor, the expression of MMP-9 can be up-regulated, and the up-regulated MMP-9 can further release VEGF in tumor stroma. The interaction between VEGF and MMP-9 jointly promotes the formation of blood vessels in tumors[14], inhibits VEGF and MMP-9 expression levels, and has a blocking effect on the growth of tumor blood vessels. The experimental results show that after the intervention of B16F10 cells with different concentrations of HHT, the expression of VEGF and MMP-9 significantly declined (P<0.05), and it was especially obvious in medium- and high-concentration groups, suggesting that HHT could inhibit the secretion of VEGF and MMP-9 by tumor cells, block tumor angiogenesis, and thus inhibit the growth and deterioration of B16F10 cells. The inhibition was dependent on dose and time.
In summary, HHT had good anti-tumor activity on B16F10 cells, and can inhibit cell proliferation, regulate Cyclin D1 gene protein, block cell cycle, inhibit the secretion of VEGF and MMP-9 by tumor cells, block tumor angiogenesis, and induce apoptosis of B16F10 cells, so it has a certain potential role in the treatment of melanoma. Next, it is needed to continue to explore the signaling pathways and molecular mechanisms affecting melanoma cells.
杂志排行
Medicinal Plant的其它文章
- Progress in the Application of Network Pharmacology in Mongolian Medicine Research
- Anti-tumor Effect of Paclitaxel Enhanced by Psoralen at the Cellular Level
- Preparation Process of Plumbagin Nanomicelle In-situ Gel
- Therapeutic Effect of Daphnetin on Mastitis Induced by Staphylococcus aureus in Mice
- Current Status and Prospects of Drugs for Ischemic Stroke Treatment
- Activity Screening Study on the Anti-tumor Effects of Extracts from Mahoniae caulis
