Preparation Process of Plumbagin Nanomicelle In-situ Gel
2024-01-08XuemeiLUWanyuZUOYunLIWeiyuWANGRuyinDONGLuyangLUJizhongZHANG
Xuemei LU, Wanyu ZUO, Yun LI, Weiyu WANG, Ruyin DONG, Luyang LU*, Jizhong ZHANG,2*
1. College of Pharmacy, Southwest Minzu University, Chengdu 610041, China; 2. University of Tibetan Medicine, Lhasa, 850000, China
Abstract [Objectives] To prepare plumbagin nanomicelle (PLB-N) in-situ gel, and optimize the formulation and process. [Methods] PLB-N was prepared by self-assembly method, and the optimal formulation of PLB-N in-situ gel was determined by orthogonal experiment design and single factor method. [Results] The optimal preparation process for PLB-N was a drug to lipid ratio of 1:3, a Tween 80 content of 5%, an ethanol content of 7.5% of the hydration medium, a magnetic stirring speed of 2 200 rpm, a stirring time of 30 min, and an ultrasound time of 10 min. The optimal formulation of PLB-N in-situ gel was 22% of poloxamer 407, 6% of poloxamer 188, and 1:1 of PLB-N to water. The encapsulation efficiency of PLB-N prepared with the optimal formula was (95.8%±0.4%), and the average particle size was (75.19±1.14) nm, and the Zeta potential was (-20.73±1.19) mv. [Conclusions] PLB-N in-situ gel had stable and reliable preparation process, uniform content, and broad application prospects.
Key words Plumbagin, Nanomicelle, In-situ gel, Preparation process
1 Introduction
PlumbagozeylanicaL. is derived from the whole herb or root ofP.zeylanicaL., a plant of the family Plumbaginaceae, and is a commonly used ethnic medicine in China[1]. It is commonly used in folk medicine to treat injuries caused by falls, carbuncle and swelling, hepatosplenomegaly, rheumatic pain, bone hyperplasia, stomach and abdominal distension, and psoriasis[2]. In recent years, reports on the chemical composition and clinical pharmacology ofP.zeylanicaL. have shown that it has antibacterial, anti-inflammatory, anti-tumor, anti-liver injury and liver fibrosis, and acaricidal effects[3]. Plumbagin (PLB) is one of the main anti-tumor active ingredients ofP.zeylanicaL., and has achieved good results in the treatment of breast cancer, skin cancer and other tumors[4-6].
At present, domestic and international reports on PLB preparations, such as liposomes[7-9], cyclodextrin inclusion complexes[10-11], microspheres[12], nanoparticles[13-14],etc., are mostly administered orally or by injection, with significant systemic toxicity and side effects.P.zeylanicaL. is more external use in folk medicine, and transdermal administration has a therapeutic effect. PLB has a small molecular weight (Mr 188.18), low melting point (mp 76-78 ℃), and poor water solubility (79.3±1.7) μg/mL. It is easy to sublimate, with a short biological half-life and skin irritation. Therefore, PLB is prepared into nanomicelles, which are formed by amphiphilic molecules. By encapsulating the drug in a hydrophobic core, the solubility of the drug is increased, and the irritation of the drug is reduced, and external environmental damage is avoided, thereby improving the stability of the drug. However, the nanomicelle solution is a liquid formulation with high fluidity and is inconvenient to use. Therefore, PLB-Nin-situgel was prepared for transdermal administration for the first time in this paper, and the addition of gel matrix made up for the defect of liquid preparation that is easy to lose in skin drug delivery. The research aimed to improve the solubility and stability of PLB and lay a foundation for the subsequent development and use of this formulation.
2 Materials
2.1InstrumentsShimadzu High-performance liquid chromatography, Shimadzu (Suzhou) Instrument Co., Ltd.; SHB-III circulating water multi-purpose vacuum pump, Zhengzhou Changcheng Science and Technology Industry and Trade Co., Ltd.; Malvern laser particle size analyzer, UK Marvin Instruments Co., Ltd.; UV spectrophotometer, Beijing Puxi General Instrument Co., Ltd.; KQ-250DE type of CNC ultrasonic cleaner, Kunming Ultrasonic Instrument Co., Ltd.; XW-80A vortex mixer, Shanghai Qingpu Huxi Instrument Factory; TGL-16G high-speed tabletop centrifuge, Shanghai Anting Scientific Instrument Factory; DF-101D-collecting type of constant-temperature heating magnetic stirrer, Zhengzhou Yuchuang Instrument Equipment Co., Ltd.
2.2ReagentsPLB, self-made, purity>98%; soy lecithin, Beijing Kulaibo Technology Co., Ltd.; polysorbate-80, Chengdu Kelong Chemical Co., Ltd; polyethylene glycol 400, Fuchen (Tianjin) Chemical Reagent Co., Ltd.; P 407 (poloxamer 407), Chengdu Kelong Chemical Co., Ltd.; P 188 (poloxamer 188), Shandong Yousuo Chemical Technology Co., Ltd.
3 Methods and results
3.1PreparationofPLB-NAn appropriate amount of lecithin and PLB were accurately weighed, and a small amount of anhydrous ethanol was added to dissolve. Under magnetic stirring, an appropriate amount of Tween 80 solution and a certain volume of anhydrous ethanol were added. After mixing for 10 min at 2 200 rpm by magnetic stirring, pure water was slowly added to fix the volume, and it continued magnetic stirring for 30 min. Then, it passed through 0.22 μm of microporous filter membrane, thereby obtaining immediately PLB-N.
3.2EstablishmentofHPLCmethod
3.2.1Chromatographic conditions. DiamonsiL C18(2) chromatographic column (5 μm, 200 mm×4.6 mm); column temperature of 30 ℃; velocity of flow 1 mL/min; injection volume of 10 μL; mobile phase of methanol:water=75:25; detection wavelength of 216 nm.
3.2.2Preparation of reference and sample solutions. An appropriate amount of PLB reference material was accurately weighed and dissolved in methanol. After fixing the volume, a PLB reference solution was obtained. 0.3 mL of PLB-N was taken, and an appropriate amount of methanol was added to ultrasonically break the capsule, fix the volume, and centrifuge. The supernatant was taken to pass through 0.22 μm of microporous filter membrane, thereby obtaining PLB-N sample solution. The same method was used to prepare blank nanomicelle solution.
3.2.3Interference test. PLB reference substance, PLB-N, and blank nanomicelles were detected according to the chromatographic conditions of Section3.2.1, andthe chromatographic peaks were recorded (Fig.1). The results indicated that the retention time of PLB was appropriate, and the blank nanomicelles had no absorption. It indicated that the excipients will not interfere with the determination of PLB.
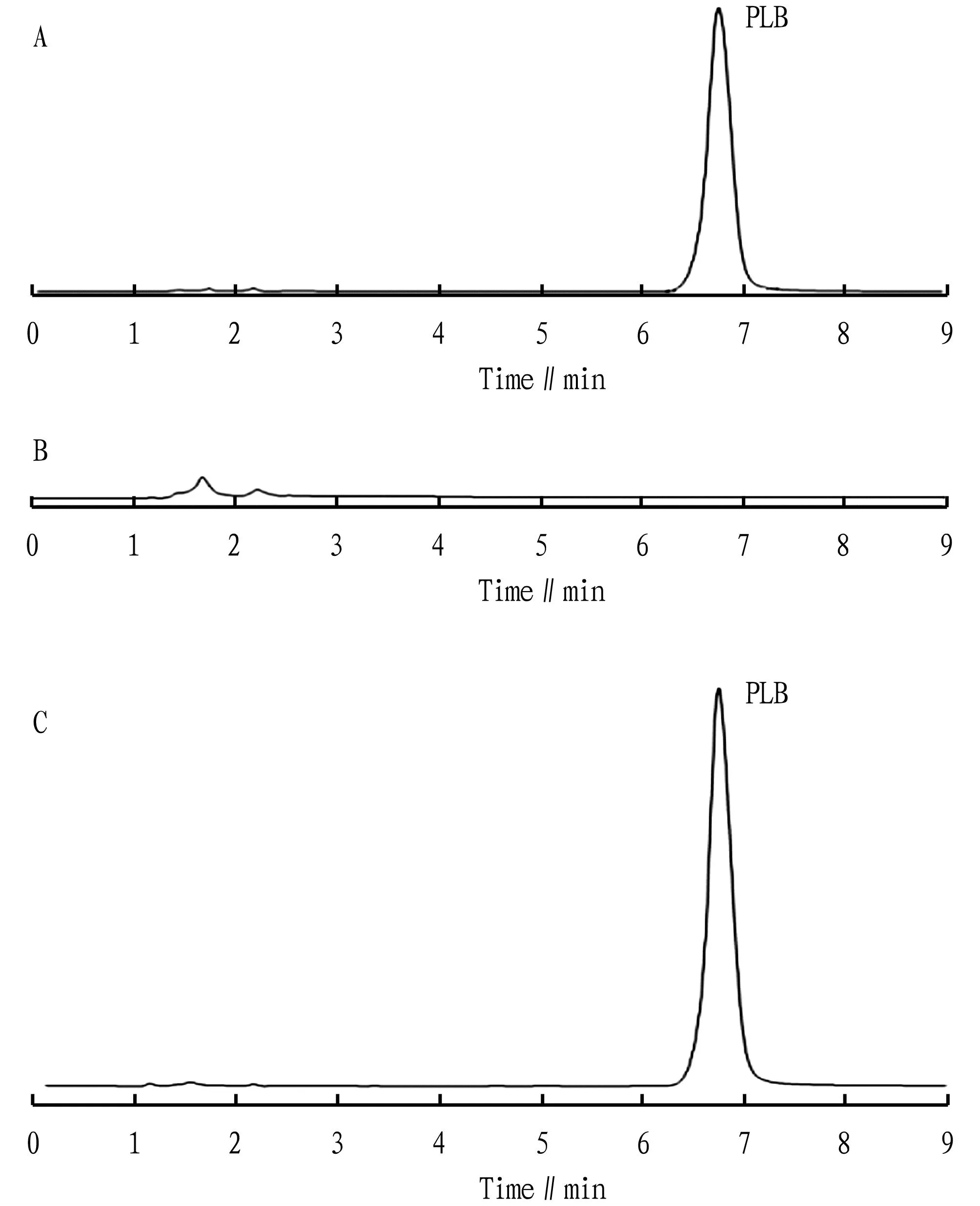
Fig.1 HPLC chromatograms of PLB reference substance (A), blank nanomicelles (B), and PLB nanomicelles (C)
3.2.4Examination of linear relationships. 3.5 mg of PLB reference substance was accurately weighed and placed in a 25 mL of volumetric flask. Methanol was added for ultrasonic dissolution, and the volume was fixed. After shaken well, a 140 μg/mL of PLB stock solution was obtained. 2 mL of the stock solution was taken into a 10 mL of volumetric flask, and the volume was fixed with methanol. After shaken well, a 28 μg/mL of PLB test solution was obtained and passed through 0.22 μm of microporous filter membrane. According to the chromatographic conditions of Section3.2.1, the volume of 2, 4, 6, 8, 10, and 12 μL was used for injection detection, and peak area was recorded. Using the injection volume M as the horizontal ordinate and the peak area A as the longitudinal coordinate, linear regression was performed to obtain the linear regression equationA=3×106M+10 157,r=0.999 9 (n=6). The results indicated that PLB had a good linear relationship within the range from 0.056 to 0.336 μg.
3.2.5Stability test. An appropriate amount of PLB-N was taken, and methanol was added for sonicate for 20 min. After the volume was fixed, it was shaken well. According to the chromatographic conditions of Section3.2.1, the peak area was measured at 0, 2, 4, 6, 8, 10, 12, and 24 h, and the sample content andRSDvalue were calculated. The results showed that theRSDvalue was 1.17%, and the prepared solution had good stability.
3.2.6Sample recovery rate test. 9 portions of blank nanomicelles were accurately measured and divided into 3 groups, each containing 0.5 mL. An appropriate amount of PLB reference solution and methanol were accurately added for sonicate for 20 min. After the volume was fixed, it was shaken well, and three groups of solutions with PLB content of 5.6, 14.0, and 28.0 μg were prepared. Using the method of Section3.2.1, the peak area of PLB in three groups of solutions was measured, and the drug content in each sample was calculated, and the recovery rate was obtained. The results showed that average sample recovery rate was 98.2%, with anRSDof 1.06%.
He very soon found out that in addition to her natural indolence, she was being as much indulged and spoilt day by day as if the Fairy had been her grandmother, and was obliged to remonstrate17 very seriously upon the subject
3.3DeterminationofencapsulationefficiencyDrug encapsulation efficiency was determined using membrane filtration combined with high-speed centrifugation method[15-16]. 0.2 mL of PLB-N was taken into a 10 mL of volumetric flask, and an appropriate amount of anhydrous ethanol was added for sonicate for 15 min to destroy the micelle structure. Anhydrous ethanol was used to fix the volumn, and the absorbance was measured with a UV spectrophotometer. The content of the encapsulated drug in the micelle was calculated, and it was taken asMtotal. An appropriate amount of PLB-N was taken and passed through 0.22 μm of filter membrane. After centrifuged at 12 000 rpm for 20 min, 0.2 mL of the supernatant was taken in a 10 mL of volumetric flask. An appropriate amount of anhydrous ethanol was added for sonicate for 15 min to destroy the micelle structure. The anhydrous ethanol was used to fix the volume, and the absorbance was measured under UV spectrophotometry. The drug content was calculated and counted asMpackage. According to the formula, the encapsulation efficiency (EE%) and drug loading (DL%) were calculated.
EE%=Mpackage/Mtotal×100%
(1)
DL%=Mpackage/(Mtotal+Mphospholipid)×100%
(2)
3.4Single-factorinspectiontest
3.4.1Effect of magnetic stirring speed on nanomicelles. The effect of magnetic stirring speed on nanomicelles was relatively small. The magnetic stirring speed was set at 800, 1 500, and 2 200 rpm, with a fixed time of 30 min. When the stirring speed reached 1 500 rpm, the particle size was the smallest, but the encapsulation rate was also the smallest at this moment. Taking into account, 2 200 rpm was selected as the magnetic stirring speed.
3.4.2Effect of magnetic stirring time on nanomicelles. The effect of stirring time of 30, 45, and 60 min on particle size and encapsulation efficiency was studied. It was found that the particle size and encapsulation efficiency were not positively correlated with the magnetic stirring time. When the time was 45 min, the encapsulation efficiency was the highest, but the particle size was the largest at this moment. Based on comprehensive analysis, it was determined that the magnetic stirring time was 30 min.
3.4.3Effect of ultrasound time on nanomicelles. The effect of ultrasound time at 0, 5, and 10 min on particle size and encapsulation efficiency was studied. As the ultrasound time increased, the encapsulation efficiency increased, while the particle size gradually decreased, but the difference in encapsulation efficiency was not significant. In order to prepare nanomicelles with high encapsulation efficiency and small particle size, the final ultrasound time for the experiment was chosen as 10 min.
3.5Orthogonalexperimentaldesignforoptimizingformulation

Table 1 Orthogonal experiment factors and levels of PLB-N optimal formulation
3.5.2Determination of optimal formulation. Whether evaluated by encapsulation efficiency or particle size, it was optimal level when ratio of drug to lipid was 1:3 and ethanol content was 10%. When using encapsulation efficiency as an evaluation indicator, the optimal level of Tween content was 3.5%, and influencing factors were A>C>B. When using particle size as an indicator, the optimal level of Tween content was 5%, and influencing factors were A>B>C. According to the range value R, it was found that the main factors affecting encapsulation efficiency and particle size were ratio of drug to lipid and Tween content. After comprehensive analysis, the optimal formulation was determined as A1B3C2. The experimental results were shown in Table 2.
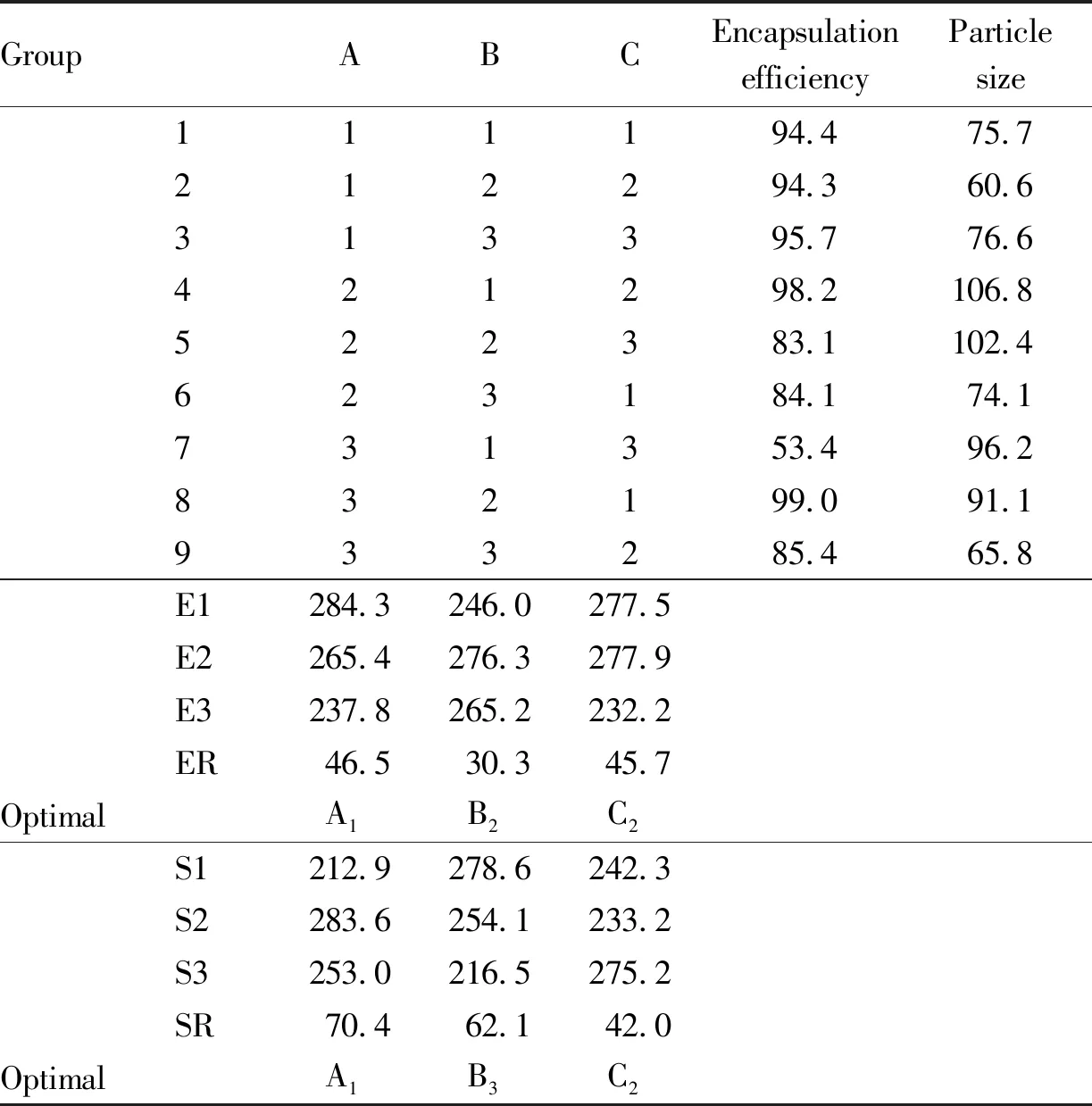
Table 2 Orthogonal experiment design results of PLB-N optimal formulation
3.5.3Validation of optimal formulation. Three batches of PLB nanomicelles were prepared according to the optimal formulation process, with a drug lipid ratio of 1:3 (W/W), a Tween content of 5% (V/V), and an ethanol content of 7.5% (V/V). The encapsulation efficiency was 95.8%, 95.3%, and 96.2%, respectively, while particle sizes were 72.4, 72.9, and 79.2 nm. The difference in encapsulation efficiency and particle size among the three batches of PLB-N was not significant, indicating that PLB-N prepared by the method and formulation had good reproducibility.
3.6InvestigationofphysicalandchemicalpropertiesofPLBnanomicelles
3.6.1Determination of particle size and Zeta potential. The obtained PLB-N was a yellow solution. Using Malvern laser particle size analyzer, the particle size, PDI, and Zeta potential of the prepared nanomicelles were measured by measuring the intensity of scattered light. The average particle size and particle size distribution coefficient of PLB-N measured in the experiment were (75.19±1.14) and (0.276±0.004) nm, respectively, with a Zeta potential of (-20.73±1.19) mV.
3.6.2Stability inspection. Three batches of PLB nanomicelle solutions were prepared and stored in a dark place at 4 ℃. Samples were taken and tested at 0, 7, 15, and 30 d, respectively. The stability of PLB-N stored at 4 ℃ for 30 d was evaluated using encapsulation efficiency, particle size, and PDI as evaluation indicators. The results showed that the encapsulation efficiency and particle size of PLB nanomicelles did not change significantly on the 30thday, with a leakage rate of 2.38%, indicating that the PLB-N prepared by this formulation had good stability under 4 ℃.
3.7PreparationofPLB-Nin-situgelanddeterminationofgellingtemperature
3.7.1Preparation of PLB-Nin-situgel. PLB-Nin-situgel was prepared by cold solution method. P407 and P188 with different mass percent content were weighed and placed into beaker, and proper amount of pure water and PLB-N were added to make them fully wet. Then, it was placed in a refrigerator at 4 ℃ to fully swell 24 h until clear, agglomerate free and evenly dispersed PLB-Nin-situgel was gotten.
3.7.2Determination of gelation temperature. The gelation temperature was measured using the inverted test tube method. An appropriate amount of gel solution was put into the test tube, and then the test tube was put into the beaker. Ice water was added to pass the liquid level of the test tube, and it was placed for 2-3 min to make the temperature inside and outside the test tube consistent. The mercury ball of a thermometer with an accuracy of 0.1 ℃ was placed into a test tube to completely immerse it in the solution. Then, the beaker was placed in a constant-temperature water bath to raise the temperature to 60 ℃. When thermometer increased every 0.5 ℃, it quickly removed the test tube and turned it upside down to observe the liquid flow inside the tube. The temperature without flow was the gelation temperature.
3.8Investigationofgelationtemperature
3.8.1Effect of P407 concentration on gelation temperature. Fixing the content of P188 at 6%, the gelation temperature at different concentrations of P407 was investigated. The results indicated that as the concentration of P407 increased, the gelation temperature decreased. When P407 was lower than 17%, gel cannot be made at 50 ℃. When P407 was greater than 30%, the fluidity was low at room temperature. When the concentration of P407 was 22%, the gelation temperature at this time was 35.9 ℃, which was close to the body surface temperature. So, the P407 concentration was chosen as 22%.
3.8.2Effect of P188 concentration on gelation temperature. Fixing the content of P407 at 22%, the effect of P188 concentration at 0%, 2%, 4%, 6%, 8%, and 10% on the gelation temperature was investigated. The results showed that with the increase of P188 concentration, the gelation temperature rose. When the concentration was 6%, the gelation temperature was 35.3 ℃. So, P188 concentration was chosen as 6%.
3.8.3Effect of PLB-N ratio on gelation temperature. Fixing the content of P407 at 22%, and the content of P188 at 6%, the effects of PLB nanomicelle addition amounts (V/V) of 1:0.5, 1:0.75, 1:1, and 1:1.25 on the gelation temperature were investigated. The results showed that there was no gelation phenomenon in all ratios when temperature was higher than 50 ℃. Directly adding nanomicelles, the matrix cannot fully swell after 36 h. Fixing total volume of solvent, when water to nanomicelle ratio was 1:1, the gelation temperature was 35.7 ℃.
4 Conclusions
PLB has significant anti-tumor activity, but its toxic side effects were significant. Based on the properties of PLB such as small molecular weight, low melting point, easy sublimation, poor water solubility, and short biological half-life, this study used self-assembly method to prepare PLB-N with high encapsulation efficiency, small particle size, uniform distribution, and good stability. However, PLB-N has high fluidity as a liquid formulation, which makes it inconvenient to use during skin administration. Temperature sensitivein-situgel is a kind of intelligent polymer gel that is sensitive to temperature changes. It can form a non-chemically crosslinked gel through phase transition with the temperature changes of storage conditions and drug application sites[17]. This kind of gel material has good biocompatibility and biodegradability, and good drug slow and controlled release performance effectively improves the bioavailability and reduces the toxic and side effects of drugs. Therefore, the gel matrix was added to prepare PLB-N into a thermosensitivein-situgel for local treatment in this paper. The PLB-Nin-situgel prepared with the optimal formulation has good appearance and uniform content, and its preparation method is simple, stable and feasible, providing a new choice for the development and application of PLB percutaneous drug delivery in the treatment of breast cancer, skin tumors and other diseases.
杂志排行
Medicinal Plant的其它文章
- Progress in the Application of Network Pharmacology in Mongolian Medicine Research
- Anti-tumor Effect of Paclitaxel Enhanced by Psoralen at the Cellular Level
- Therapeutic Effect of Daphnetin on Mastitis Induced by Staphylococcus aureus in Mice
- Current Status and Prospects of Drugs for Ischemic Stroke Treatment
- Activity Screening Study on the Anti-tumor Effects of Extracts from Mahoniae caulis
- Effects of JAG-1 on the Proliferation and Migration of Gastric Adenocarcinoma Cells after TRAIP Knockout
