Response of heat inducible heat shock protein 90 gene of Aphis gossypii (Hemiptera: Aphididae) to gossypol and flupyradifurone stresses and mutual effect on transcription factor HSF
2024-01-03LIANGPingZhuoLIRenCHENGShenHangLIDaPengZHANGLeiZHENCongAi
LIANG Ping-Zhuo, LI Ren, CHENG Shen-Hang, LI Da-Peng,ZHANG Lei,*, ZHEN Cong-Ai,*
(1. Department of Entomology, China Agricultural University, Beijing 100193, China;2. The Museum of Chinese Gardens and Landscape Architecture, Beijing 100072, China)
Abstract:【Aim】The aim of this study is to identify heat shock protein (Hsp) gene in Aphis gossypii(AgHsp90) and clarify the response of AgHsp90 to temperature, plant allelochemicals and insecticide stresses. 【Methods】RT-PCR and RACE were used to clone AgHsp90 of A. gossypii, and bioinformatics analysis was conducted. The expression levels of AgHsp90 in different developmental stages (1st-4th instar nymphs and adult) and in adults at 1 h after treatments with -5, 0, 5, 10, 15, 20, 30, 35, 38 and 42 ℃, and at 24 h after treatment with plant allelochemicals (20 mg/L tannic acid, 50 mg/L gossypol, 50 mg/L 2-tridecanone and 50 mg/L quercetin) and pesticide flupyradifurone stress[LC25(2.410 mg/L)] were quantified by qRT-PCR. RNAi of AgHsp90 and heat shock factor (HSF) gene AgHSF of A. gossypii adult was performed for 24 h through feeding method to determine the mortality rate of A. gossypii adults at 24 and 48 h after treatments with 50 mg/L gossypol and LC40 (4.649 mg/L) flupyradifurone through feeding and leaf dipping methods, respectively, explore the effect of AgHsp90 on the susceptibility of A. gossypii to gossypol and flupyradifurone and preliminarily verify the mutual regulation between AgHsp90 and AgHSF. 【Results】One Hsp90 gene of A. gossypii designated AgHsp90 (GenBank accession no.: UOF38310) was obtained with a 2 181 bp ORF in length. The expression levels of AgHsp90 were relatively stable in different developmental stages, with the significant difference happened between the 3rd instar nymph and adult. Temperature stress experiment result revealed that AgHsp90 was obviously high temperature inducible, but low temperature downregulated the expression level of AgHsp90. The expression level of AgHsp90 in A. gossypii adult was not significantly induced by three plant allelochemicals (gossypol, tannic acid, and quercetin) and significantly induced by 2-tridecanone and LC25 flupyradifurone compared with the controls 0.5 mol/L sucrose and Triton X-100, respectively. RNAi of AgHsp90 could remarkably increase the mortality rate of A. gossypii adult resulted from gossypol and flupyradifurone compared with the control fed with dsGFP. AgHsp90 and its transcription factor AgHSF could mutually affect the expression levels using RNAi method. 【Conclusion】 The heat shock protein gene AgHsp90 of A. gossypii is high temperature and flupyradifurone inducible. AgHsp90 is mostly associated with the gossypol and flupyradifurone susceptibility of A. gossypii. The above results indicate that AgHsp90 may play crucial roles in response to high temperature and pesticide flupyradifurone stresses.
Key words:Aphis gossypii; heat shock protein 90; gossypol; flupyradifurone; HSF
1 INTRODUCTION
The melon-cotton aphid,Aphisgossypii(Hemiptera: Aphididae), is highly polyphagous, with wide host range spectrum of nearly 100 host plant species (Capinera, 2000) implying that this pest has to struggle with the toxic substances produced by host crops during evolution. The serious damages on crops are usually caused via direct sap-sucking, honeydew production, and viral vector (Hulléetal., 2020). Due to its generations up to 20 per year, the chemical insecticides are mainly used for controlling this pest. Given the small body size and ectothermic property,A.gossypiiis sensitive to climate change such as elevated atmospheric carbon dioxide and temperature (Shreevanietal., 2017). Therefore,A.gossypiiindividuals have to cope with various environmental stress, including plant allelochemicals, insecticides, and climate factors, especially temperature.

The previous studies aboutHspsinA.gossypiiwere mainly focused onHsp70 superfamily. However, the information about Hsp90 gene structure, and the role in response to temperature and toxic stresses inA.gossypiistill remained unclear. In this study, the gene characterization, phylogenetic relationship, developmental expression profile, and the transcription induction ofAgHsp90 under thermal, plant allelochemicals and insecticide stresses were investigated. The function ofAgHsp90 ofA.gossypiiin response to plant allelochemicals and insecticide stress and the mutual effect betweenAgHsp90 andAgHSFwas preliminarily verified using RNAi method. Our findings are expected to increase our understanding of Hsp90 role inA.gossypii, which may be important for interpreting that howA.gossypiiadapts to thermal and toxic stress.
2 MATERIALS AND METHODS
2.1 Insects and chemicals
The origination ofA.gossypiiwas provided by ZHANG Dong-Hai (College of Agriculture, Shihezi University) and collected from cotton (Gossypiumhirsutum) field in Xinjiang Province, China. The individuals were reared on fresh cotton seedlings in the laboratory. Constant environmental conditions for maintainingA.gossypiiwere 20-23 ℃ and 60% relative humidity with a photoperiod of 16L∶8D.
Chemicals including 2-tridecanone, tannic acid, quercetin, and gossypol were obtained from Sigma-Aldrich (St. Louis, MO). Insecticide flupyradifurone with the purity of >96% was purchased from Bayer CropScience Co. Ltd. (Monheim am Rhein, Germany).
2.2 Cloning of AgHsp90 and bioinformatics analysis
Total RNA was extracted fromA.gossypiiadults using Trizol Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The quality of RNA was evaluated based on the values of OD260/OD280and OD260/OD230on a NAS-99 Spectrophotometer (ACTGene, USA). Subsequently, 1 μg RNA was used to synthesize the first-strand cDNAs with the PrimeScriptRTReagent kit with gDNA Eraser (TaKaRa Biotechnology, Dalian). The cDNA sequence ofAgHsp90 was obtained via specific primers (Table 1) based on the previous transcriptome information (Lietal., 2017). The full-length cDNA sequence was amplified by using a SMARTerTMRACE cDNA Amplification Kit (Clontech, Palo Alto, CA, USA). Lastly, the full-length open reading frame (ORF) sequence ofAgHsp90 was verified by PCR. The 50 μL reaction agent was composed of 25 μL of 2×Phanta Max Master Mix (Vazyme), 1 μL of each forward and reverse primer (10 μmol/L) (Table 1), 2 μL cDNA template and 21 μL dH2O. The PCR procedure was as follows: 3 min at 94 ℃; 30 cycles Lowercase letters in the primer sequence indicate T7 promoter sequence.
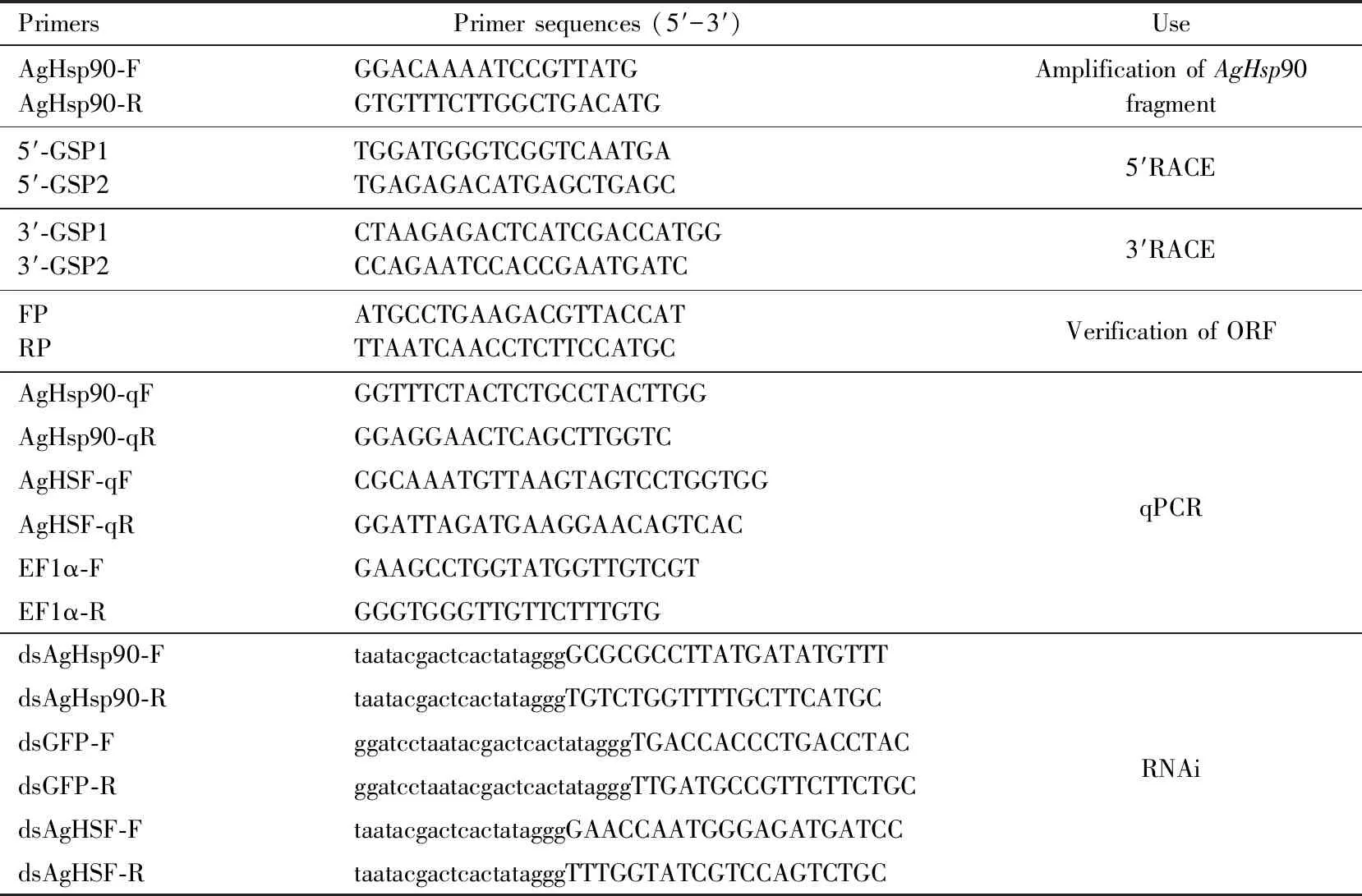
Table 1 Primer information
of 94 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 2 min; 72 ℃ for 10 min. Purification of amplified PCR fragments was done using the TIANGEN Gel Extraction Kit (Beijing). The purified PCR fragment was subcloned into the pMD18-T Vector (TaKaRa) and then sequenced.
Sequence similarity analysis was performed using the BLAST programs available on the NCBI website (https:∥www.blast.ncbi.nlm.nih.gov/Blast). ORF identification was carried out using ORF finder (http:∥www.ncbi.nlm.nih.gov/orffinder/). The HSP features were analyzed using NetPhos (http:∥www.cbs.dtu.dk/services/NetPhos) and Protscale (https:∥web.expasy.org/protscale). The phylogenetic tree was constructed using the neighbor-joining method in MEGA 6.0 software (Tamuraetal., 2013).
2.3 Sample collection
Whole bodies of the 1st-4th instar nymphs and adults ofA.gossypiiwere respectively sampled and stored at -80 ℃ for expression analysis.A.gossypiiadults were selected and exposed to 25(CK), -5, 0, 5, 10, 15, 20, 30, 35, 38 and 42 ℃, groups of 50A.gossypiiadults with the fresh cotton leaves were collected into nylon cage in a dry bath, exposed to a selected temperature for 1 h and used for one sample. Each temperature treatment had 3 replicates. After temperature treatment, all the treated adults were immediately frozen in liquid nitrogen and stored at -80 ℃ for RNA isolation.
A.gossypiiadults were collected and fed with the artificial diets containing 20 mg/L tannic acid, 50 mg/L gossypol, 50 mg/L 2-tridecanone, 50 mg/L quercetin and 0.5 mol/L sterile sucrose (CK). The plant allelochemical feeding assays was followed as Maetal. (2019). Each treatment included 3 replicates, and 50 adults were used in each replicate. After feeding on the above artificial diets for 24 h, the survivedA.gossypiiwere collected for RNA extraction. To investigate whetherAgHsp90 was induced by LC25flupyradifurone, sublethal concentration (approximately 25% of mortality rate) of 2.410 mg/L of flupyradifurone was prepared. The LC25value of flupyradifurone againstA.gossypiiadults was calculated based on the previous published bioassay result (Liangetal., 2019), which could suppressA.gossypiidevelopment and fecundity. Triton X-100 treated adult individuals were used as CK. The survived individuals were collected after 24 h of exposure, and flash-frozen in liquid nitrogen and stored at -80 ℃ until use for RNA exaction.
2.4 qRT-PCR
The RNA of the above samples in section 2.3 was extracted using RNA Isolation Solvent (Omega Bio-tek, USA), and cDNAs were synthetized using PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian). qRT-PCR was run on an ABI 7500 System (Applied Biosystems, Foster City, CA, USA) in 20 μL reactions. Each 20 μL reaction was mixed based on the procedure of SYBR®Premix Ex TaqTMⅡ (Tli RNaseH Plus, TaKaRa). Primer pairs used for qRT-PCR were listed in Table 1. The procedure was as follows: 95 ℃ for 30 s; 40 cycles of 95 ℃ for 15 s, and 60 ℃ for 30 s, and then the melting curve analysis was conducted to confirm the specificity of PCR products. To normalize differences, elongation factor 1-alpha gene (EF1α) was used as the reference gene in this experiment (Maetal., 2016). Gene expression level was evaluated using the 2-ΔΔCtmethod (Livak and Schmittgen, 2001). Each experiment was biologically repeated three times and each template was comprised of three technical replications.
2.5 RNAi and bioassay
Control geneGFPfragment, and target gene (AgHsp90 andAgHSF) fragments were amplified using specific primers with T7 RNA polymerase promoter sequence (Table 1). The sequence verified PCR products were used as templates, and the dsRNAs were synthesized by MEGAscript RNAi Kit (Ambion, USA).
A.gossypiiadults were fed with dsRNAs according to the methods described by Maetal.(2017). Briefly, adults were transferred to glass tubes (20 mm in diameter×30 mm long, open at both ends). The liquid artificial diet solution (200 μL) with dsGFP(CK), dsAgHsp90 and dsAgHSFwas added, respectively, between two Parafilm layers stretched over one open end, and Chinese art paper was adhered to the other end. The concentration of dsRNAs used in this experiment was 150 ng/μL. After feeding dsRNAs for 24 h, some survival adults were sampled for RNA extraction to examine the RNAi efficiencies of target genes and other survival adults in the groups of dsAgHsp90 and dsGFPwere fed with 50 mg/L gossypol using sandwich parafilm feeding method and treated with LC40(4.649 mg/L) flupyradifurone by Liangetal.(2019) using leaf dipping methods, respectively. Each treatment contained 80 adults with three replicates. The mortality rates were recorded at 24 h for gossypol group and 48 h for flupyradifurone group, respectively.
2.6 Data analysis
Data analyses were conducted using the GraphPad InStat 3.0 software. One-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons was used to evaluate the significant differences in the relative expression levels ofAgHsp90 at different developmental stages, at different temperature exposure or under plant allelochemical treatments. The Student’st-test was used to compare the difference in the expression levels ofAgHsp90 between the flupyradifurone treatments and the comparison of RNAi efficiency or sensitivity against gossypol and flupyradifurone.
3 RESULTS
3.1 Molecular characterization of AgHsp90
One Hsp90 gene ofA.gossypiidesignatedAgHsp90 was cloned with the full-length of 2 636 bp (GenBank accession no.: UOF38310). The ORF ofAgHsp90 is 2 181 bp in length, encoding a 727-amino-acid protein with a predicted molecular mass of 83.27 kD and theoretical isoelectric point (pI) of 4.68. The length of 5′-UTR was 162 bp, and the length of 3′-UTR was 293 bp, with a ployA tail and a typical ployA signal sequence AATAAA. The AgHsp90 protein possesses five highly conserved amino acid domains belonging to the Hsp90 family signature, including NKEIFLRELVSNSSDALDKIR (aa 36-56), LGTIAKSGT (aa 103-111), IGQFGVGFYSAYLVAD (aa 127-142), IKLYVRRVFI (aa 356-365) and GVVDSEDLPLNISRE (aa 382-396). At the C-terminus, a cytoplasmic characteristic motif MEEVD was also found in the AgHsp90 sequence.
3.2 Phylogenetic analysis of AgHsp90
Phylogenetic tree is shown in Fig. 1 revealing that Hsp90s were divided into two parts, including the cytosol Hsp90 and the endoplasmic reticulum (ER) Hsp90. AgHsp90 belongs to the cytosol Hsp90 part, and was closest to the tree branch composed ofRhopalosiphumpadiHsp90 (GenBank accession no.: AMQ09609.1) andMyzuspersicaeHsp90 (GenBank accession no.: AWN00452.1), which was in accordance with sequence alignment.
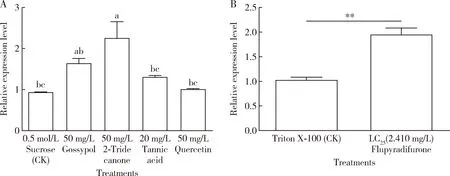
Fig. 4 Relative expression levels of AgHsp90 in Aphis gossypii adults at 24 h after treatment with plant allelochemicals (A) and insecticide flupyradifurone (B) Data in the figure are mean±SE. In Fig. B, asterisks above bars indicate significant difference between two groups (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001)(t-test). The same for Figs. 5-6.
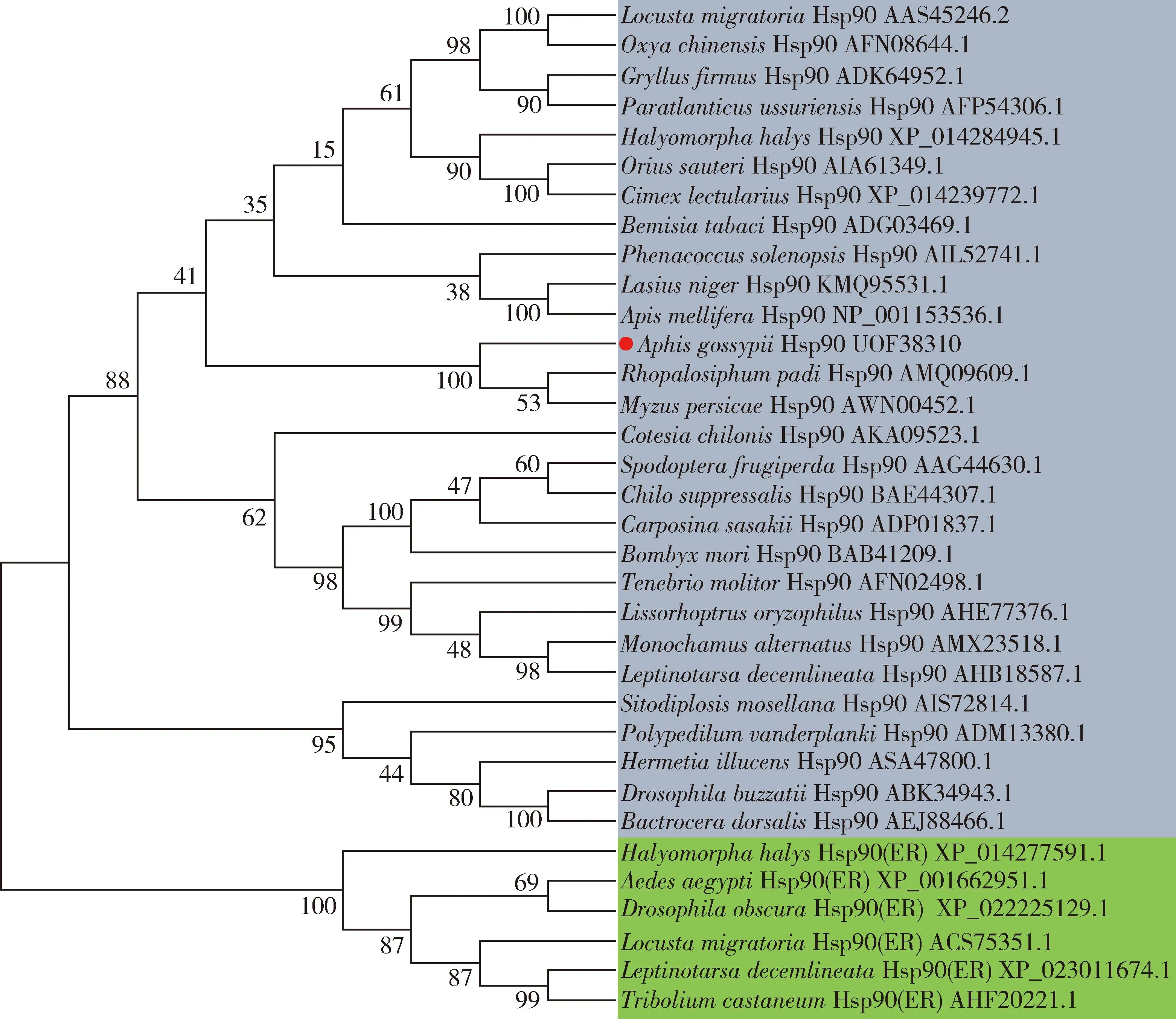
Fig. 1 Phylogenetic tree of Hsp90 family members based on amino acid sequence using neighbor-joining method Aphis gossypii Hsp90 is represented with black circle. Blue indicates the cytosol Hsp90 and green indicates the endoplasmic reticulum (ER) Hsp90.
3.3 Expression levels of AgHsp90 at different developmental stages
The expression levels ofAgHsp90 were relatively stable between different developmental stages ofA.gossypii(Fig. 2). The highest expression level ofAgHsp90 appeared at the 3rd instar nymphal stage, following that at the 4th, 1st, and 2nd instar nymphal stages, with the lowest at the adult stage. Moreover, significant difference only existed in the expression levels ofAgHsp90 between in the 3rd instar nymph and adult (P<0.05).
3.4 Thermal responses of AgHsp90
The expression level ofAgHsp90 was highly upregulated by high temperatures (30, 35, 38 and 42 ℃) and slightly downregulated by low temperatures (-5, 0, 5, 10 and 20 ℃) compared to 25 ℃(CK)(P<0.05)(Fig. 3). It is noteworthy thatAgHsp90 was strongly induced at 42 ℃ with 30-fold increased expression level as compared to that at 25 ℃ (Fig. 3).
3.5 Expression profiles of AgHsp90 exposed to plant allelochemicals and insecticide flupyradifurone
No significant difference in the expression levels ofAgHsp90 inA.gossypiiadults was found in the three plant allelochemical treatment groups (gossypol, tannic acid and quercetin), and 2-tridecanone significantly increased the expression level ofAgHsp90 in comparison with the control group (0.5 mol/L sucrose)(Fig. 4: A). The result revealed that the expression levels ofAgHsp90 were significantly increased in the LC25flupyradifurone-treated group compared with the control group (Triton X-100)(P<0.01)(Fig. 4: B).
3.6 Effects of AgHsp90 RNAi on the mortality rates of A. gossypii adults resulted from gossypol and flupyradifurone stresses
At 24 h afterAgHsp90 RNAi, the expression level ofAgHsp90 inA.gossypiiadults obviously decreased by 27% compared to that of dsGFPgroup (CK)(P<0.05)(Fig. 5: A). Bioassay results exhibited that after feeding the dsAgHsp90, the adult mortality rate significantly increased by 17.2% under 50 mg/L gossypol treatment(P<0.01)(Fig. 5: B), and 22.2% under LC40(4.649 mg/L) flupyradifurone treatment (P<0.01), respectively, compared to that of CK (Fig. 5: B).
3.7 The mutual effect between AgHSF and AgHsp90
SilencingAgHSFcould significantly decrease the expression levels of bothAgHSF(P<0.05) andAgHsp90(P<0.05) compared to dsGFPgroup (CK)(Fig. 6: A). Meanwhile, the expression level ofAgHSFalso significantly dropped byAgHsp90 RNAi (P<0.0001) compared to CK (Fig. 6: B).
4 DISCUSSION AND CONCLUSION
To explicit the function of Hsp90 in protectingA.gossypiiagainst toxics, oneHsp90 gene, designatedAgHsp90 was isolated fromA.gossypiiin this study. The AgHsp90 protein is composed of three main parts: an ATPase domain at the N-terninus, a centrally located binding domain for linking the target protein with N-domain ATP, and a C-part for Hsp90 dimerization (Pearl and Prodromou, 2006). The presence of MEEVD motif at the C-terminus suggested that AgHsp90 is a kind of cytosolic Hsp. Moreover, the phylogenetic result (Fig. 1) also confirmed that AgHsp90 is a cytosol Hsp, which was consistent with the motif analysis.
The temporal expression pattern of insectHsp90 was usually variable in different insect species. In this study, the expression ofAgHsp90 was consistently stable among all developmental stages (Fig. 2), indicating thatAgHsp90 was possibly not involved in the developmental regulation inA.gossypii. Significant difference in the expression level ofAgHsp90 was only found between the 3rd instar nymph and adult (Fig. 2). The possible explanation might be that most insects at the nymphal/larval stage were more sensitive to the environment than those at the adult stage, which might need more Hsps for environment adaptation. TheFohop(a heat shock 90/70 organizing protein gene) expression was also consecutively stable during the developmental stages ofFrankliniellaoccidentalis(Li and Du, 2013). However, the expression level ofSlhsp90 inSpodopteralituraadults was higher than that in larvae (Shuetal., 2011), indicating thatSlhsp90 possibly was involved in the reproduction.
For better adapting to extreme temperature change, the expression levels of insect Hsp genes were commonly enhanced under thermal/cold stresses. In our study, the over-expression ofAgHsp90 was only found under high temperature (Fig. 3), suggesting thatAgHsp90 was mainly coping with thermal stress. The similar phenomenon also appeared inAgHsp70 from the melon aphid,A.gossypii(Saeidietal., 2021),NlHsp90 from the brown planthopper (BPH),Nilaparvatalugens(Chenetal., 2020), andBdHsp18.4 andBdHsp23.8 fromBactroceradorsalis(Douetal., 2017). TwoHsp70s (ZcHsp70-1 andZcHsp70-2), especiallyZcHsp70-1, were highly induced under high temperatures (38 ℃ and 42 ℃) in the melon fly,Zeugodacuscucurbitae(Tanetal., 2023). Meanwhile, insectHsps were elicited in response to high temperature and low temperature, which were important for adapting extreme environmental temperature. For example, hsp90 and hsp70 from the corn earworm,Helicoverpazeawere induced by both heat and cold (Zhang and Denlinger, 2010).BdHsp20.4 andBdHsp20.6 fromB.dorsaliswere up-regulated under heat and cold stresses (Douetal., 2017).Fo-HSP90,Fo-HSP70 andFo-HSP28.9 from the western flower thrips,F.occidentalis(Pergande) were inducible in response to cold and heat stress (Wang HHetal., 2014). Sometimes, some insectHsps could be enhanced only under cold acclimation, such as the Russian wheat aphid,Diuraphisnoxia(Kurdjumov)Hsp70(Saeidietal., 2021).
The over-expression of Hsps in insects was essential in relation to toxics tolerance, especially to insecticide. For instance, AsHSP90AB gene was considered to be responsible for pyrethroid stress inAnophelessinensis(Sietal., 2019). All five Fo-HSP genes (Fo-HSP28.9,Fo-HSP40,Fo-HSP60,Fo-HSP70 andFo-HSP90) were induced when exposed to avermectin at the sublethal concentration (2 mg/mL) inF.occidentalis(Wang HHetal., 2014). The transcripts and protein contents ofAlHSP90 were enhanced by chlorpyrifos, cyhalothrin, imidacloprid, and emamectin benzoate (induction doses=LD50and LD20) inApolyguslucorum(Sunetal., 2014). TheHsp70 and UDP-glucuronosyltransferase (UGT) transcript levels in whiteflyB.tabacifemale adults were over-expressed when exposed to sublethal concentrations of thiamethoxam (Suetal., 2018).MpHsp70 was responsible for lambda-cyhalothrin defense through oxidative stress tolerance inM.persicae(Dongetal., 2022). In our study,AgHsp90 was not induced by some plant allelochemicals, including quercetin, gossypol and tannic acid (Fig. 4: A), which were the compounds that the cotton aphids usually cope with. However, the sensitivity to gossypol was obviously increased whenAgHsp90 knockdown (Fig. 5: B). The inconsistent explanation was that the expression ofAgHsp90 might be variable with the treated chemical concentration or duration time.
HSF was evolutionarily conserved as a primeHsptranscription regulator via binding to HSE located upstream of Hsp genes. Our results showed thatAgHSFsilencing could drastically reduce the expression level ofAgHsp90 (Fig. 6: A). Interestingly,AgHsp90 knockdown also suppressed the expression level ofAgHSF(Fig. 6: B), implying that AgHSF possibly could be binding to HSE located in theAgHsp90 promoter region. In agreement with our finding,AhHsfandAhHsps(AhHsp70 andAhHsp21) fromAgasicleshygrophilacould be affected each other when silencing one of them, indicating the regulation role ofAhHsfon theAhHsp70 andAhsHsp21 transcription (Jinetal., 2020). Similarly,Bthsf1 knockdown inhibited the expression ofBthsf1 and fourBthsps (Bthsp90,Bthsp70-3,Bthsp20 andBthsp19.5) in MEDB.tabaci, suggesting thatBthsf1 was involved in the transcription regulation of these fourBthsps (Baietal., 2022). However, the detailed mutual affecting mechanism betweenAgHSFandAgHsp90 should be further explored.
We provided evidence of one heat shock protein gene namedAgHsp90 responsible for high temperature and toxics adaption in the cotton aphid,A.gossypii. According to the molecular characteristic and phylogenetic analysis, AgHsp90 is cytosol-specific Hsp90. The transcripts ofAgHsp90 were significantly increased under high temperature stress and insecticide flupyradifurone. Besides, the susceptibilities ofA.gossypiiagainst gossypol and flupyradifurone were obviously increased when silencingAgHsp90. In addition, the transcription factorAgHSFandAgHsp90 could be affected each other via RNAi method. These findings are helpful for understanding theAgHsp90 role in adaption to high temperature and toxics stresses.
ACKNOWLEDGEMENTSWe especially thank our supervisor professor GAO Xi-Wu for providing the whole guidance.
