Synthesis, Characterization and Water Absorption Analysis of Highly Hygroscopic Bio-based Co-polyamides 56/66
2023-12-28ABDULRahman杜瑞满CHENGKanLIUTaoMUHAMMADUsamaMOHSENQadeer秦鸿彬WANGXueli王学利PANXingyi潘星夷FENGXuMABomou马博谋HEYong
ABDUL Rahman(杜瑞满), CHENG Kan(程 刊), LIU Tao(刘 涛), MUHAMMAD Usama(乌 彤), MOHSEN Qadeer(秦鸿彬), WANG Xueli(王学利), PAN Xingyi(潘星夷), FENG Xu(冯 旭), MA Bomou(马博谋)*, HE Yong(何 勇), *
1 State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Material Science and Engineering, Donghua University, Shanghai 201620, China
2 Innovation Center for Textile Science and Technology, Donghua University, Shanghai 201620, China
3 The People’s Liberation Army Joint Logistic Support Force Quartermaster Energy Quality Supervision Station, Beijing 100071, China
Abstract:This study aims to develop highly hygroscopic bio-based co-polyamides (CPs) by melt co-polycondensation of polyamide (PA) 56 salt and PA66 salt with varying molar fractions. The functional groups and the chemical structure of the prepared samples were determined by Fourier transform infrared (FTIR) spectroscopy and proton nuclear magnetic resonance (1H-NMR) spectroscopy. The relative viscosity was determined with an Ubbelohde viscometer. The melting behavior and the thermal stability of CPs were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Furthermore, the water absorption behavior of CP hot-pressed film was studied. The results reveal that the melting point, the crystallization temperature and the crystallinity of CPs firstly decrease and then increase with the molar fraction of PA66 in CPs. The copolymerization of PA56 with PA66 leads to an obvious increase in water absorption. The CPs with PA66 molar fraction of 50% possess a high saturated water absorption rate of 17.6%, compared to 11.6% for pure PA56 and 7.8% for pure PA66.
Key words:bio-based; PA56; hygroscopic; co-polyamide (CP); melt polycondensation; water absorption
0 Introduction
Aliphatic polyamides (PAs) are commonly used in engineering plastics due to their exceptional mechanical strength, flexibility, toughness, durability,etc.[1-3]. However, most of these polymers are derived from fossil fuels, and thus are finite and non-renewable, and have detrimental effects on the environment[4]. To address this issue, it is necessary to shift towards sustainable and renewable alternatives.
Bio-based PA56, an aliphatic PA partly derived from renewable sources, has been recently introduced into the market and has gained rapid popularity for its potential use in fibers and engineering plastics. Compared with traditional petroleum-based PAs, it has all the advantages, such as excellent mechanical properties, high chemical resistance, thermal stability, ultraviolet light stability, good dyeability and moisture absorption[5-6]. It is suitable for yarn and filament manufacturing and suitable for high-temperature and other harsh environment applications. In addition, the most important thing is that it is bio-based, so it has a unique ability to reduce carbon footprint[5-6].
PA66 is another aliphatic PA. As well known, it is a high-performance polymer owing to its outstanding thermal properties, mechanical strength, chemical resistance and thermal stability[7-9]. Nowadays, PA66 is widely used in various commercial applications such as manufacturing films, fibers and engineering plastics[10-11].
The nonisothermal crystallization kinetics of Co-polyamides 56/66 (CPs) based on PA56 and PA66 have been studied[11], but there is a lack of research on their hygroscopicity to explore their potential applications. Hygroscopic polymers absorb and retain moisture, which is useful in applications such as packaging, textiles and medical devices. Furthermore, they can improve the toughness and impact resistance of the final product. As a result, there is a growing demand for hygroscopic polymers.
In this paper, the highly hygroscopic CPs with high relative viscosity are successfully synthesized through melt polycondensation. The melting and crystallization temperatures of the prepared CPs were determined by differential scanning calorimetry (DSC). The thermal stability was analyzed by thermogravimetric analysis (TGA), and the relative viscosity was measured by an Ubbelohde viscometer. The functional group and chemical structure analysis was conducted through Fourier transform infrared (FTIR) spectroscopy and proton nuclear magnetic resonance (1H-NMR) spectroscopy. Furthermore, the variation in crystallinity of various CPs was evaluated with wide-angle X-ray diffraction (WAXD). Finally, the water absorption behavior of the CP hot-pressed film was investigated.
1 Experiments
1.1 Materials
The diamines 1, 5-pentanediamine (PDA) and 1, 6-hexanediamine (HDA) were purchased respectively from Cathay Industrial Biotech Inc., China and Sinopharm Chemical Reagent Co., Ltd., China. Adipic acid (AA) was purchased from TCI (Shanghai) Development Co., Ltd., China. Concentrated sulfuric acid (H2SO4), ethanol and deuterated sulfuric acid (D2SO4) were bought from Aladdin Industrial Co., Ltd., China. All the chemicals were of analytical reagent grade and used as received without further purification.
1.2 Preparation of PA56 salt and PA66 salt
The PA56 salt and PA66 salt were prepared separately using the equimolar ratio (1∶1) of PDA to AA and HDA to AA, respectively. Firstly, a measured quantity of AA was dissolved in a flask containing ethanol at 70 ℃. The calculated amount of monomer was added to the flask containing the AA solution. Then the solution was heated under reflux for several hours to promote salt formation. The precipitated salt was collected by filtration and washed with ethanol at least three times to remove any remaining impurities or unreacted monomers. Finally, the prepared PA56 salt and PA66 salt were dried in the vacuum oven for 24 h at 60 ℃.
1.3 Synthesis of PA56, PA66 and a series of CPs
To begin with, for melt polycondensation, the reactants as shown in Table 1 were introduced into the reactor. The reactor was installed in the autoclave and purged with nitrogen three times to remove any oxygen. Once the overhead stirrer was installed, the preheating process commenced and the temperature was raised to 220 ℃ to melt the monomers. The reaction was continued at 220 ℃ for 2 h at a pressure of no more than 1.7 MPa. Following this, the reactor pressure was reduced to the atmospheric level. Furthermore, the temperature was elevated to 270 ℃, and the polymerization reaction was allowed to proceed for 30 min under reduced pressure thus increasing the viscosity. Upon completion of the reaction, the polymer melt was removed from the reactor, cooled, and ground into the powder form. The prepared sample was put into a vacuum oven for 24 h. Figure 1 illustrates the reaction schemes for the above process. The CP samples with the molar ratios of PA56 to PA66 of 70/30, 50/50 and 30/70 named CP-30, CP-50 and CP-70, respectively.
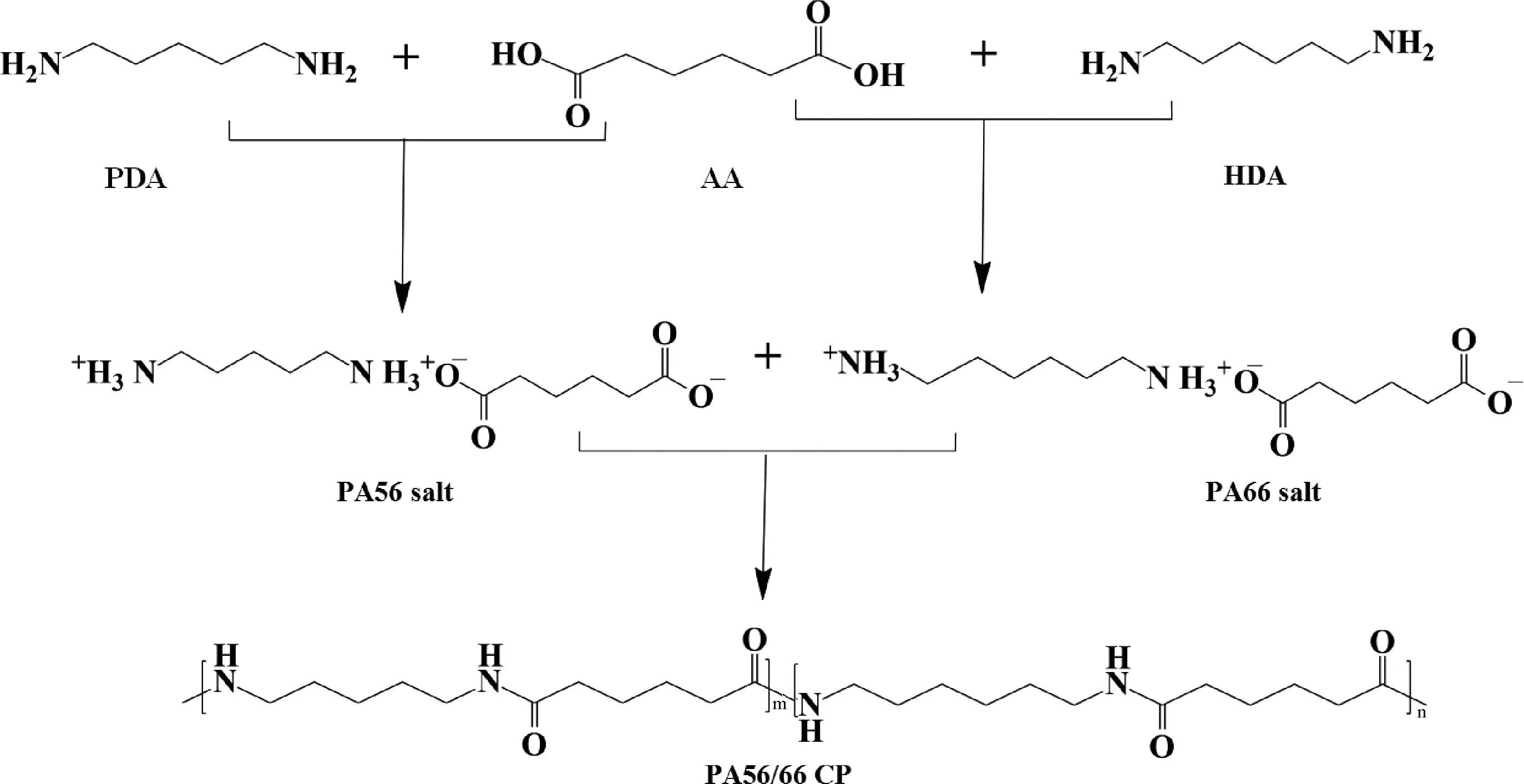
Fig.1 Synthesis of PA56/66 CP

Table 1 Composition of samples
1.4 Characterization
FTIR spectra of PA56, PA66 and CPs were recorded in a Nicolet 8700 spectrometer (ThermoFisher, USA) in a range of 4 000 to 600 cm-1(64 scans) at a resolution of 2 cm-1. A Bruker Avance 400 spectrometer (Bruker, Germany) was used to conduct1H-NMR spectra in D2SO4solvent at room temperature. On a Rigaku D/max 2550 VB/PC X-ray diffractometer (Rigaku, Japan), WAXD measurements were performed utilizing Ni-filtered Cu K radiation at 40 kV and 200 mA.
The Ubbelohde viscometer (Synthware, China) was used to evaluate the relative viscosity of the polymer (0.01 g/mL, in the concentrated sulfuric acid) at (25.00 ± 0.01) ℃. DSC runs were performed on the Polyma DSC214 (NETZSCH, Germany) under ultrapure nitrogen with 5.0-10.0 mg samples encapsulated in aluminum pans. The samples were first heated from 20 ℃ to 300 ℃ (the first heating run) and held for 2 min to erase the thermal history, then cooled from the melt to -20 ℃ at a rate of 20 ℃/min (cooling run) and kept at -20 ℃ for 2 min, and finally heated to a temperature about 20-30 ℃ higher than the melting point of the co-polyamides at a heating rate of 20 ℃/min (the second heating run). In the case of PA56 salt and PA66 salt, the heating run was adjusted to 260 ℃ and the cooling run to 30℃ at 10 ℃/min. The crystallization temperature (Tc) and corresponding crystallization enthalpy (ΔHc) values were taken from the peak and the area of the crystallization exotherm in the cooling run, respectively. The melting temperature (Tm) and melting enthalpy (ΔHm) values were taken from the position of the peak and the area of the melting endotherm in the second heating run, respectively.
TGA was performed using a NETZSCH TG 209F3 thermogravimetric analyzer (NETZSCH, Germany) at a heating rate of 20 ℃/min under nitrogen and a temperature range of 30 to 700 ℃.
Hot-pressed films of PA56, PA66 and CPs with a dimension of about 30.0 mm × 30.0 mm × 0.1 mm were used for the measurement of water absorption. Before the test, the sample was dried at 80℃ in a vacuum oven overnight and the massm0was measured using a balance with a precision of 0.1 mg. It was then immersed in the distilled water at 23 ℃ for a water absorption test. The film massmtwas weighted for a given absorption time oftafter wiping the surface water with a paper towel. The water absorption raterwat timetis determined as
(1)
Three identically sized and weighed specimens were used in the test, and the average value was taken.
2 Results and Discussion
2.1 FTIR spectra of CPs

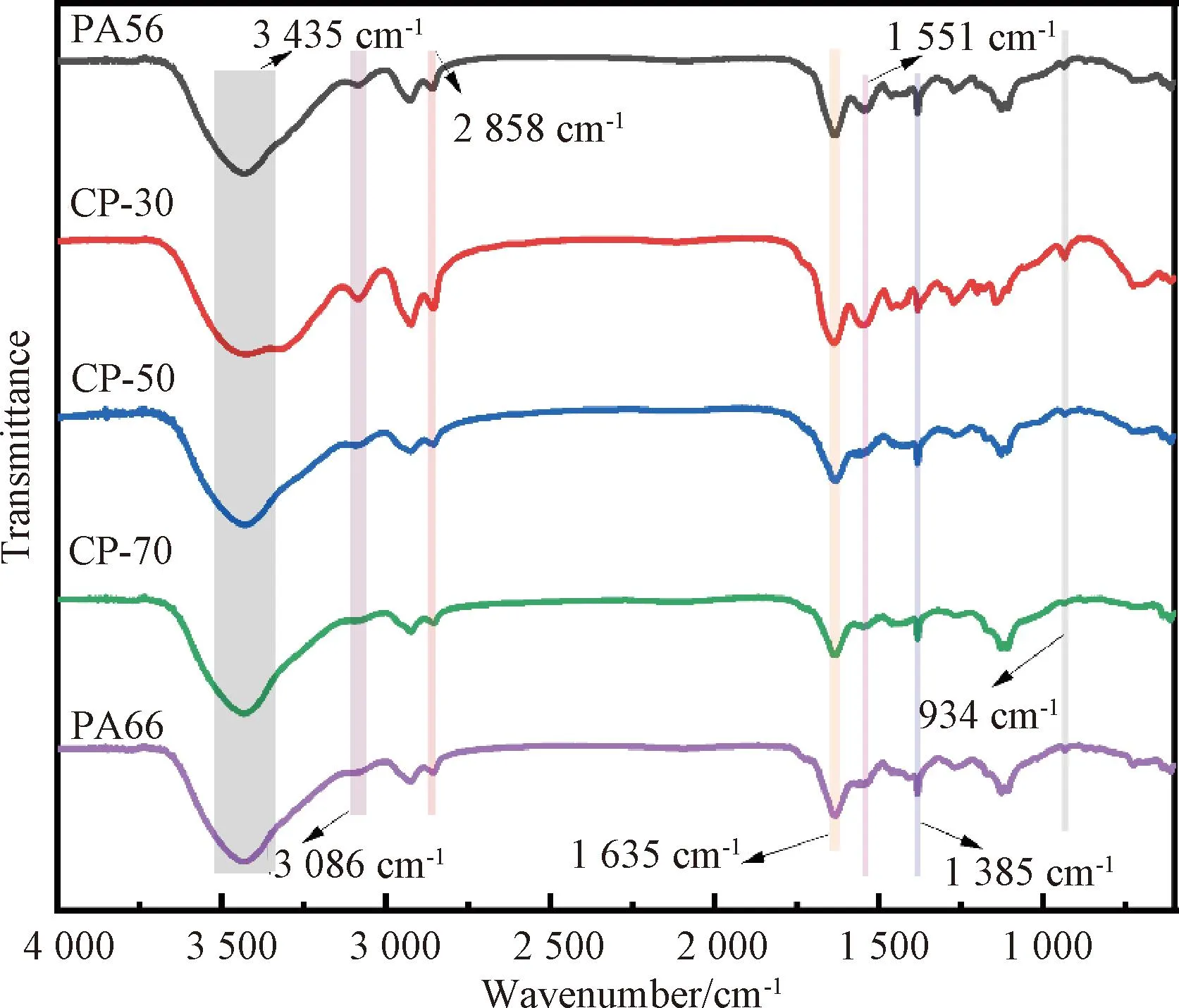
Fig.2 FTIR spectra of PA56, PA66 and CPs
2.2 1H-NMR spectra of CPs
1H-NMR spectroscopy was used to analyze the chemical structures of PA56, PA66 and their subsequent copolymers as exhibited in Fig.3, where the chemical shift multiplied by 106was represented byδ. Overall, five peaks were observed in the spectra obtained for all the samples. The peaks observed at around 3.2 (a) and 2.2 (b) correspond to the adipate adjoining the methylene group. Furthermore, the peaks at around 4.0 (c+c′), 2.3 (d+d′), and 1.9 (e+e′) can be ascribed to the methylene groups present in pentanediamine and hexanediamine units. The overlapping of peaks is caused by the high similarity between PA56 and PA66[15-16]. The molar composition of PA66 incorporated is confirmed by the signal integrals of the characteristic peaks observed ata,b,(d+d′) and e+e′ by using formulas[11]as follows:
(2)

Fig.3 1H-NMR spectra of PA56, PA66 and CPs
(3)
(4)
whereSb,Sd+d′,SaandSe+e′are the signal integral of the peaks atb,(d+d′),aand e+e′, respectively;xandyare the molar fractions of PA66 and PA56 in CPs, respectively;XPA66represents the molar fraction of PA66 in CP. The obtained molar fractions are listed in Table 1.
2.3 Crystalline structure of CPs
The crystalline structure of the prepared samples is analyzed by WAXD patterns (Fig.4). At room temperature, most PAs having an even number of carbon atoms in their repeat unit possessα-form crystalline. The key identifying feature of theγ-form crystalline of PAs is a diffraction peak at around 2θ= 21.00°, which corresponds to a nearly hexagonal unit cell[5]. Only a single diffraction peak is observed for PA56, which is consistent with theγ-form crystalline of PA56 reported in Refs. [17-18]. The strong diffraction peak for PA56 at 2θ=21.02° corresponds to thed-spacing value of 0.42 nm and (020) crystallographic plane. The strong diffraction for CP-30 at 2θ=20.80° corresponds to thed-spacing value of 0.43 nm depicted in the crystallographic plane value (110). When the PA66 molar fraction increases to over 50%, the diffraction peak widens. The reason should be that the crystal formation of PA56 is disrupted by PA66, leading to the gradual shift of WAXD patterns. As the PA66 molar fraction increases beyond the eutectic point at 50%, two diffraction peaks appear and the WAXD patterns start resembling that of neat PA66. CP-50 and CP-70 show major peaks at 2θ=20.56°, 20.05° respectively and a minor peak at 2θ=22.90°, which correspond to thed-spacing value of 0.43, 0.44 and 0.39 nm, respectively, quite similar to those observed for neat PA66 (2θ=20.03° and 2θ=23.30°), depicting the presence of theα-form crystalline. The two characteristic peaks correspond to triclinic structures connecting through hydrogen bonding[19]. The observed WAXD patterns of CPs (CP-30, CP-50 and CP-70) correspond to the characteristic peaks of the main PA introduced. For example, when the content of PA56 is high, the WAXD pattern shows a characteristic peak unique to PA56. However, the regular arrangement of amide bonds in the PA66 segments is disrupted, which impedes the crystallization of the PA56 sequences. Conversely, with an adequate concentration of PA66 to form a longer fragment, a clear crystallization peak for PA66 starts to emerge.
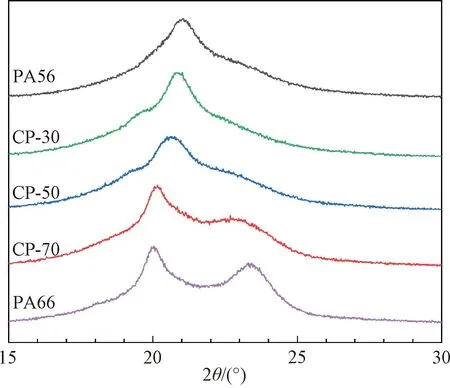
Fig.4 WAXD pattern for PA56, PA66 and CPs
2.4 Thermal properties of CPs
The DSC curves of PA56 salt and PA66 salt are shown in Fig.5 and the detailed results are displayed in Table 2. DSC results obtained from the above-mentioned salts help determine the processing conditions for the polymerization of CPs.
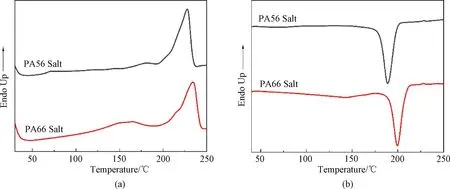
Fig.5 DSC Curves of PA56 salt and PA66 salt: (a) heating curves; (b) cooling curves
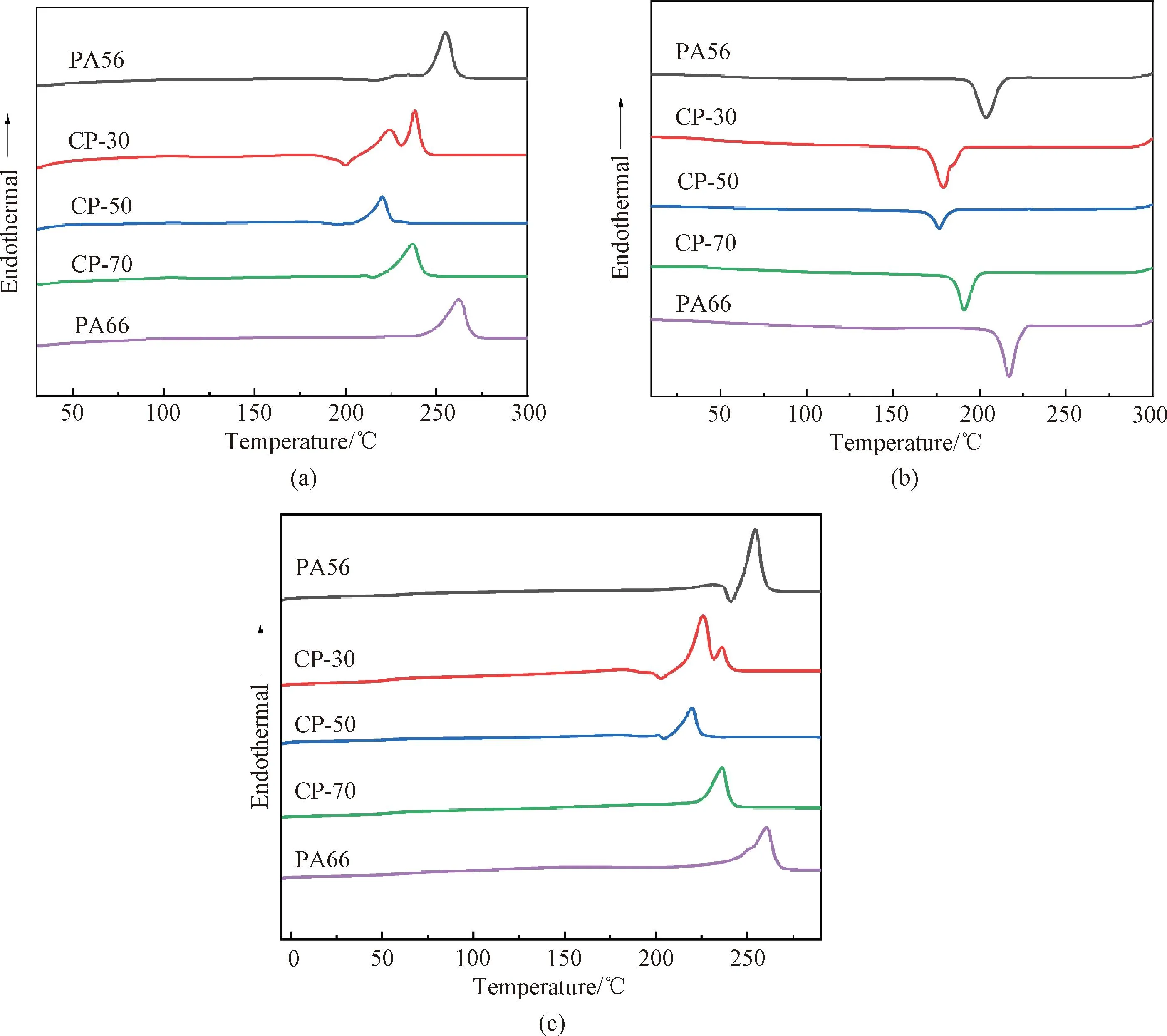
Fig.6 DSC curves of PA56, PA66 and CPs under different conditions: (a) the first heating; (b) cooling; (c) the second heating
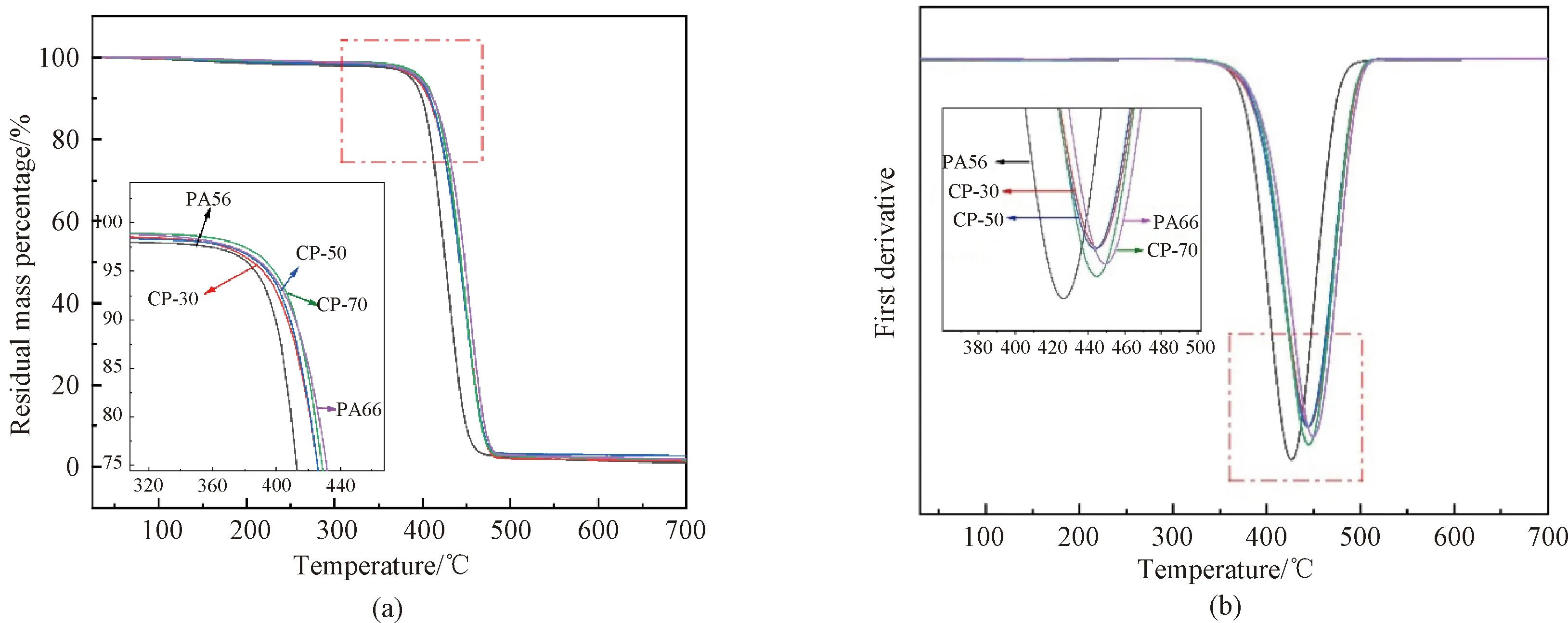
Fig.7 Thermal properties of PA6, PA66 and CPs: (a) TGA curves; (b) DTG curves

Fig.8 Water absorption rates of PA56, PA66 and CPs

Fig.9 Saturated water absorption rate vs. crystallinity for PA56, PA66 and CPs
Figure 6 displays the DSC results for PA56, PA66 and CPs. The melting curve of CP-30 shows dual-melting endotherms, which is typical for PAs, along with a recrystallization phenomenon that is perceived from the curve[20]. Neat PA56 and PA66 have melting points of 255 ℃ and 262 ℃, respectively, and crystallization temperatures of 204 ℃ and 217 ℃ (Table 3), respectively. The increase of PA66 molar fraction in CPs results in a reduction inTmandTc. CP-50 exhibits the lowestTmandTcof 220 ℃ and 177 ℃ (Table 3), respectively. The melting and crystallization enthalpy (ΔHmand ΔHc) of the CPs with intermediate composition are lower than those of neat PA56 and PA66. A higher PA66 content disrupted the chain’s orderly arrangement,causing crystal flaws and the formation of tiny crystals. This hurts the melting point and lowers the capacity to crystallize. The eutectic point of the copolymer emerges at the PA66 molar mass fraction of 50% and the copolymer chain structure becomes the most disorganized[11]. This is brought on by the disruption of the asymmetric hydrogen bonds and chain conformation caused by the large chain length difference between PDA and HDA[21-22]. TheTmandTcof CP increase when the PA66 molar fraction in CP surpasses 50%, and the melting and crystallization behavior eventually converge on those of neat PA66 (Table 3).
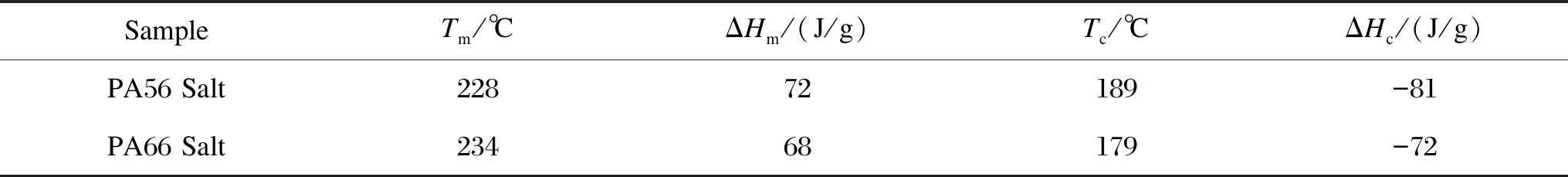
Table 2 DSC results of PA56 salt and PA66 salt
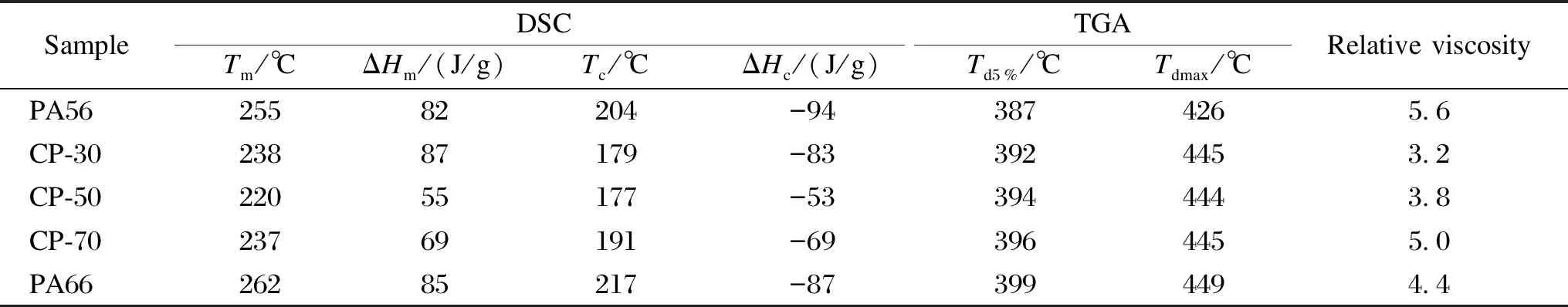
Table 3 DSC results, TGA results, crystallinities and relative viscosities of PA56, PA66 and CPs
TGA and derivative thermogravimetry (DTG) curves for PA56, PA66 and CPs are shown in Fig. 7.Td5%where 5% of the mass is lost, ranges from 387 ℃ to 399 ℃ as listed in Table 3. Up to 150 ℃, a small weight loss is experienced due to the evaporation of free water in CPs. Starting from 150 ℃, there is a gradual mass loss due to the evaporation of crystal water, formed by the hydrogen bonding between water molecules and the amide end of PA[11]. All CPs exhibit exceptional thermal stability.Td5%is over 380 ℃ and near 400 ℃ for all samples.Td5%increases as the molar fraction of PA66 increases from 30% to 70% whereasTd5%is the highest in the case of neat PA66.Tdmaxranges from 426 ℃ to 449 ℃. Neat PA66 exhibits the highest temperature for degradation (449 ℃), which indicates stronger hydrogen bonding and a more perfect crystalline structure[23-24]compared to other samples.
2.5 Water absorption of CPs
The extent of water absorption is influenced by the concentration of amide groups in the polymer chain and the crystal structure of PA. Higher concentrations of amide groups generally result in greater saturated water absorption, while higher crystallinity leads to lower saturated water absorption[25]. The presence of amide groups in PAs allows for the formation of hydrogen bonds with water molecules, which leads to water absorption[26-27]. For commercial PAs, the saturated water absorption rates are 8.7% for PA6[28], 7.8% for PA66[25], 8.5% for PA610[29], 5.5% for PA612[30], 1.4% for PA11[31]and 1.5% for PA12[32]. The recorded highest saturated water absorption rate is 13.5% for PA55, a newly synthesized bio-based PA[25].
Figure 8 shows the water absorption rate for PA56, PA66 and CPs. It is clear that the water absorption increases rapidly in the first 48 h and levels off after about 96 h for all samples, which indicates that the water absorption is saturated after 4 d under the investigated conditions.
The lowest saturated water absorption rate (7.8%) is for PA66 among all the samples, which is due to its highly ordered structure with the regular arrangement of its amide groups[27]. Due to the larger amide molar fraction in the main chain, the saturated water absorption rate of PA56 (11.6%) is relatively high, nearly half higher than that of PA66. As to CP-30, CP-50 and CP-70, the saturated water absorption rates are 16.4%, 17.6% and 16.3%, respectively, which are much higher even than that of PA56. This result is mainly attributed to the decrease in the crystallinity of CP.
Figure 9 describes the saturated water absorption rate against crystallinity for PA56, PA66 and CPs. The lower the crystallinity, the higher the saturated water absorption rate for CP. The presence of crystalline regions in polymers significantly affects their water absorption capacity. The degree of crystallinity can influence the hydrophilicity, water diffusion and swelling behavior of the material[33]. Crystalline regions affect the diffusion of water molecules and can act as barriers to limit water uptake. Low crystallinity increases the susceptibility to water absorption, making it easier for water molecules to penetrate the amorphous regions of the material[34]. Among CPs, the crystallinity of CP-50 (29%) is the lowest, while the saturated water absorption rate (17.6%) is the highest, and to our knowledge, it is the highest saturated water absorption rate reported so far.
3 Conclusions
In this study, a series of hygroscopic CPs with a relative viscosity higher than 3.0 were successfully synthesized by melt co-polycondensation PA56 salt and PA66 salt. It was found that the melting point, the crystallization temperature and the crystallinity of CP firstly decreased and then increased with the molar fraction of PA66 in CP. The saturated water absorption rates were evaluated to be 16.4%, 17.6% and 16.3% for CP-30, CP-50 and CP-70, respectively, which were much higher than that of both homopolymers, 11.6% for PA56 and 7.8% for PA66. The higher saturated water absorption of CPs should be mainly attributed to the decreased crystallinity arising from copolymerization.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Laser-Induced Graphene Conductive Fabric Decorated with Copper Nanoparticles for Electromagnetic Interference Shielding Application
- Toughness Effect of Graphene Oxide-Nano Silica on Thermal-Mechanical Performance of Epoxy Resin
- Photoactive Naphthalene Diimide Functionalized Titanium-Oxo Clusters with High Photoelectrochemical Responses
- Preparation and Thermo-Responsive Properties of Poly(Oligo(Ethylene Glycol) Methacrylate) Copolymers with Hydroxy-Terminated Side Chain
- Design of Online Vision Detection System for Stator Winding Coil
- Clothing Parsing Based on Multi-Scale Fusion and Improved Self-Attention Mechanism
