Minimum inhibitory but maximum nonhemolytic concentration of plasma-treated catheters coated with L.tridentata and O.vulgare extracts
2023-12-18FranciscoJavierALONSOMONTEMAYORMiriamDesirVILAMEDINAAlejandroZUGASTICRUZRosaIdaliaNARROSPEDESMarGuadalupeNEIRAVELZQUEZAidENZGALINDOandEduardoALONSOCAMPOS
Francisco Javier ALONSO-MONTEMAYOR,Miriam Desirée DÁVILA-MEDINA,Alejandro ZUGASTI-CRUZ,Rosa Idalia NARRO-CÉSPEDES,*,María Guadalupe NEIRA-VELÁZQUEZ,Aidé SÁENZ-GALINDOand Eduardo ALONSO-CAMPOS
1 Autonomous University of Coahuila,Saltillo 25280,Mexico
2 Center for Research in Applied Chemistry,Saltillo 25294,Mexico
3 University of Monterrey,San Pedro Garza García 66238,Mexico
Abstract Antibacterial extract-coated catheters are promising alternatives to their conventional counterparts,but their hemocompatibility and thermal stability must be studied.Nosocomial bacteria have developed resistance to conventional antibiotics.Herein,the minimum inhibitory but non-hemolytic concentration (MIC-NH) and the thermal stability of Larrea tridentata (L.tridentata) and Origanum vulgare (O.vulgare) extract-coated catheters were studied.Besides,plasma pretreatment was performed to enhance the extract adhesion.Briefly,the extract-coated catheters prevent Staphylococcus aureus colonization without causing hemolysis by using L.tridentata and O.vulgare extracts at MIC-NH(5000 and 2500 μg ml-1,respectively).Moreover,it has been discovered that the extract coating and plasma treatment improved the thermal stability and the extract adhesion,respectively.Thus,this study provides evidence of alternative antibacterial but non-hemolytic extract-coated catheters.
Keywords: antibacterial activity,biocompatibility,blood-contact device,cold plasma,nosocomial bacteria
1.Introduction
The demand for catheters increases among medical devices due to the rising cases of minimally invasive procedures and urinary and cardiovascular diseases[1].However,some types of catheters can also produce human health issues and increase healthcare costs [2].For instance,urinary catheters contact the perineum and create friction against bladder mucosa,leading to an inflammatory response and providing a route for bacterial entry to inner body sections [3].Similarly,bloodstream infection and inadequate blood flow are typical complications of central venous catheterization.It is important to point out that around 75% of the pathogen microorganisms causing infections are Gram-positive,and Staphylococcus aureus (S.aureus) is the most common [4].Moreover,this bacterium is primarily associated with complications of long-term catheterization,such as bloodstream infections with a high mortality rate [5].
Bacteria colonize catheters by forming a surface biofilm that protects them from conventional antibiotics[6].Frequent catheter replacement and sterile insertion have been developed to minimize catheter-related infections,but they would cause patient discomfort and increase treatment costs [1,3].Several antimicrobial catheters based on metallic nanoparticles and antibiotics impregnation strategies,among others,have been approved by the Food and Drug Administration of the United States.However,these catheters are unsuitable for long-term catheterization,their effectiveness decreases over time,and antibiotic-coated catheters cause microbial resistance.Although other antimicrobial strategies are potential solutions against catheter colonization,such as natural compound-based and long-term antibioticbased coatings,most are still premature for clinical applications [4,7].In this concern,hemocompatibility is one of the criteria that determines whether catheters are clinically safe[4].Thus,catheters must have excellent hemocompatibility since they are blood-contact devices [1].
Among natural antibacterial compounds,those obtained from plants have had renewed attention for their capacity to treat different maladies,such as infectious diseases.They are the mainstay for developing solutions to widespread public health issues [8,9] and have been used to develop catheter coatings with antibacterial activity [10-14].Many plant extracts have been used to endow surface antibacterial characteristics to catheters.Aqueous extracts of Aloe vera,Carica papaya,Psidium guajava,Gongronema latifolium,Ocimum gratissimum,Vernonia amygdalina[15],Evolvulus alsinoides[16],and Ziziphora[17,18],ethanolic extracts of Azadirachta indica [19],Camellia sinensis,Eucalyptus,and Salvia rosmarinus [17,18],and methanolic extracts of Mangifera indica,Ocimum gratissimum,and Psidium guajava [20,21],among others,have been used to prevent catheter colonization from clinically relevant bacteria such as Escherichia coli,Klebsiella pneumoniae,Proteus mirabilis,Pseudomonas spp.,Staphylococcus spp.,and urine bacteria.However,although biocompatibility plays an essential role in the clinical applications of materials,the hemolytic activity of extract-coated catheters has not been reported yet [12].
Larrea tridentata (L.tridentata) and Origanum vulgare(O.vulgare) extracts are low-cost and easily affordable potential options for developing antibacterial catheter coatings since they have exhibited widespread antibacterial activity against S.aureus strains,among other clinically relevant bacteria [22-25].Moreover,L.tridentata and O.vulgare extract-coated catheters have not been reported yet.Thus,the study of these catheters is an interesting and novel clinical topic since their obtention does not require extract purification and simple soaking of catheter pieces with dissolved extract at room conditions is enough to obtain suitable results.
This work hypothesized values of minimum inhibitory concentration (MIC) and minimum inhibitory but maximum non-hemolytic concentration (MIC-NH) of catheters coated with L.tridentata and O.vulgare extracts against an S.aureus strain.Thus,this research aimed to evaluate the antibacterial and hemolytic activity of these catheters.Also,the chemical and morphological characteristics of the extract-coated catheters were studied to explain the extract adhesion.It is important to point out that some catheters were pretreated with low-pressure air plasma to enhance the extract adhesion.Plasma treatment is a suitable option for surface-modifying polymeric material since it can be implemented at lowtemperature and does not affect their bulk characteristics[26].The synergistic effect of plasma pretreatment on the bioactivity of extract-impregnated catheters is first reported here.In addition,the thermal stability of the extract-coated catheters was first evaluated here since it is an essential parameter for heat-sensible coating processing [12].Indeed,it has been reported that some extract compounds have low thermal stability,which may restrict their applicability [14].
2.Experimental details
2.1.Extract obtention
Inflorescences and leaves of L.tridentata and leaves of O.vulgare (named aerial parts) were obtained from the southwest region of Coahuila (Ramos Arizpe,Coahuila,Mexico:25° 32′ 27″ N,100° 56′ 35″ O).Before the extraction,this material was soaked with tap water at room temperature and then open-air dried for 72 h.Next,60 g batches of specific dry aerial parts were milled for 1 min using a commercial blender(Nutribullet,26TS E243921)to obtain a powder.This powder was sealed in hermetic plastic bags.Then,10 g of pulverized extract source was mixed with 50 ml of acetone(Jalmek with>99% purity) and magnetically stirred at room temperature using a magnetic plate stirrer (LabCompanion,HP-3100) for 30 min at 400 r min-1.Later,the solvent and soluble material were separated from the non-soluble one using a funnel made of coarse pore-filter paper,and the solution was poured into Petri dishes.Subsequently,the solvent was separated from the soluble material through an evaporation process at room temperature for 24-48 h.Finally,each extract was dissolved in ethanol up to the stock concentration (5×104μg ml-1)and lesser concentrations: 25,50,75,100,250,500,750,1000,2500,and 5000 μg ml-1.
2.2.Plasma treatment and extract coating
Before the coating process,some pieces of catheter hose with 6 mm diameter (SensiMedical®intravenous instrument) were treated with radiofrequency (13.56 MHz) air plasma.Based on a report [27] and preliminary experiments,a chamber pressure of 0.19±0.01 bar,a work power of 50 W,and 30 min of plasma exposition were the optimal parameters used to treat the catheter pieces.Figure 1 shows the homemade plasma generating system used to treat the catheter pieces.The reactor consisted of a 125 ml flat bottom flask surrounded by a copper coil that induced the electromagnetic field to generate the cold plasma.At the same time,magnetic stirring was performed to ensure a homogeneous exposition of catheter pieces to the plasma glow zone.Here,a cylindrical magnetic stirrer(2.5 cm of length and 0.5 cm of diameter)was energized by an equipment that rotates a natural magnet below the reactor flask.

Figure1.Home-made plasma generating system used to treat the catheter pieces.
Then,each untreated and plasma-treated catheter piece was coated with extract by soaking it with 0.25 ml of L.tridentata and O.vulgare extracts at the stock concentration in a 1.5 ml Eppendorf tube.The ethanol solvent was evaporated entirely at room temperature after 24-48 h.It is important to mention that ethanol,instead of extract solution,was also used for catheter pieces soaking.Once the synergistic effect of plasma treatment was corroborated by the antibacterial and hemolytic activity of extract-coated catheters,batches of 24 plasma-treated catheter pieces were soaked with 1 ml extracts below the stock concentration to estimate the MIC and MICNH values.
2.3.Antibacterial activity evaluation
Disc diffusion method in nutrient agar (BD Bioxon) was performed in sterile plastic Petri dishes with 8 cm of diameter to evaluate the antibacterial activity of the catheter pieces against an S.aureus strain (ATCC 23235).First,1 ml of bacterial dilution was taken from a previously inoculated nutritious broth and added to 9 ml of sterile distilled water.Then,1 ml of the first dilution was dumped in 9 ml of distilled water for the second dilution,and the same process was replicated for a third dilution.Next,four catheter pieces per treatment were arranged equidistantly in each inoculated Petri dish in duplicate,and 200 μl of the third bacterial dilution was inoculated in the nutritious broth and incubated for 48 h at 36°C.Finally,up to 6 measurements were selected to evaluate the capacity of catheter pieces coated with stock-concentrated and diluted extracts to produce inhibition halos (h)against the S.aureus strain.
An Excel-programmed two-way analysis of variance supported with the least significant difference (ANOVALSD) was performed to determine the possible significant effects of the stock-concentrated coating and the plasma treatment time(0 and 30 min)on h.It is important to point out that ANOVA was used here to estimate significant differences between h or hemolysis percentage (HP) mean values since some standard deviations intersect each other.The ANOVA ways refer to the amount of the independent variables on which the study focuses (e.g.dissolved extract concentration and plasma treatment time are two independent variables,thus they correspond to a two-way analysis).The enhancement percentage of antibacterial activity after plasma treatment(μ·100) was calculated through equation (1),which was proposed in a theoretical study[26].The activity amount(e.g.expressed by h) of the plasma-treated sample (C1) and the untreated one (C2) must be measured under the same conditions
Similarly,an Excel-programmed one-way ANOVA-LSD was performed to search for significant differences in the antibacterial activity of catheters coated with diluted extracts.
2.4.Hemolytic activity evaluation
The hemolytic activity of catheter pieces coated with the L.tridentata and O.vulgare extract was evaluated according to the ASTM 756-00 method.Blood samples were obtained from human voluntary donors.Donors agreed to the use of their blood in a hemolysis study by signing an informed consent form.The local bioethics committee of the Chemical Science Faculty,of the Autonomous University of Coahuila,approved the study.Blood samples were obtained through venipuncture (4.0 ml) in a tube with heparin as anticoagulant(BD Vacutainer,Franklin Lakes,NJ,USA) and gently homogenized.In this test,150 μl of fresh red blood obtained from a healthy donor was diluted with 1350 μl of AlSever solution (10.45 g dextrose;4 g sodium citrate;2 g sodium chloride;500 ml distilled water).
The catheter samples were placed in contact with the red blood cell solution and incubated for 1 h at 37°C.After that,the red blood cell solution was centrifuged at 2400 r min-1for 4 min.Then,1 ml supernatant was placed on a clean 24-well plate,and the hemoglobin was measured in duplicate at 415 nm using the absorbance reader (OD415nm).This method used red blood cells in distilled water and AlSever solution as positive and negative control (C+and C-),respectively.Finally,six measurements were selected to estimate the capacity of catheter pieces coated with stock-concentrated and diluted extracts to produce HP.This clinical parameter was calculated using equation (2),where the contribution of the color of the extract to the respective catheter OD415nmwas subtracted.This equation is adapted from a usually reported HP formula [1] considering that free hemoglobin and dissolved extract that came off the catheter piece absorb 415 nm wavelength.Thus,to eliminate the contribution of the extract color from the absorbance of the treatment sample (Catheter OD415nm: AlSever solution with free hemoglobin and dissolved extract that came off the catheter piece),the absorbance of a duplicated color control (Color OD415nm: AlSever solution with dissolved extract that came off the catheter piece) has been considered
It is essential to point out that,according to the ASTM 756-00,if the material in contact with blood provokes hemolysis higher than 5%,between 5%and 2%or below 2%,it is considered hemolytic,slightly hemolytic,or non-hemolytic,respectively [28].
An Excel-programmed two-way ANOVA-LSD was performed to determine the possible significant effects of the stock-concentrated coating and the plasma treatment time(0 and 30 min) on HP.Then,an Excel-programmed one-way ANOVA-LSD was performed to search for significant differences in the hemolytic activity of catheters coated with diluted extracts.
2.5.Chemical analysis
A Perkin Elmer Frontier FTIR-ATR spectrometer was used to identify the functional groups on representative catheter samples.Fourier transform infrared spectroscopy with attenuated total reflectance (FTIR-ATR) signals were recorded using a diamond crystal in a 600-3700 cm-1range,16 scans,and 4 cm-1resolution.The FTIR-ATR spectra were normalized for comparison.Besides,the infrared spectrum of the untreated and uncoated catheter surface was compared with a library of pure polymer infrared spectra to identify the primary polymer from which the catheter is made.
For covalent binding testing of plasma-generated functional groups attached to the untreated and plasma-treated catheter surface,x-ray photoelectron spectroscopy (XPS)analysis was performed on representative samples.An XPS analysis system(Thermo Scientific Modelo K-Alpha+)and a load compensator (flood gun) were used to determine the surface elemental composition of pristine and plasma-treated catheter surfaces at 10 nm.The analysis chamber pressure during the test was 2.6×10-7mbar.A monochromatic x-ray source of Al Kα (1486.68 eV) and an analysis radius of 400 μm were used.High-resolution spectra were obtained with a pass energy of 150 and 50 eV and were conducted from 0 to 1350 eV of binding energy.AVANTAGE software from Thermo Fisher Scientific was used to deconvolution these spectra.The relative concentration (RC) of functional groups associated with a signal i from a region of interest(C1s,N1s,and O1s) was calculated from equation (3) [29].
where A and ATotalare the area ratio of i and the sum of all A(i) from a region of interest (e.g.C1s,N1s,and O1s),respectively.
2.6.Morphological and thermal analysis
The morphological characteristics of representative samples of untreated and plasma-treated catheters were examined in a JEOL conventional scanning electron microscope (SEM)operated with 5-15 kV.Samples were coated with a thin layer of Au-Pd prior to analysis.
A Perkin Elmer TGA 4000 thermogravimetric analyzer was used to evaluate the thermal resistance of representative catheter pieces (1.95±0.41 mg).Thermograms from thermogravimetric analysis (TGA) were obtained at a heating speed of 20°C min-1from 30°C to 600°C under an N2inert atmosphere and at a gas flow of 20 ml min-1.The thermal decomposition behavior was evaluated through the first derivative of the TGA thermogram curve (DTG),calculated by the central finite difference method [30],as equation (4)describes for each derivative point (p)
where i is the current pass,and wt%i+1and wt%i-1are the residual sample weight at time ti+1and ti-1,respectively.
The transitions of such samples (1.12±0.22 mg) under thermal action were studied utilizing a Perkin Elmer DSC 7 differential scanning calorimeter (DSC).The samples were hermetically sealed in standard aluminum-covered pans,held for 1 min at 30°C,and heated from 50°C to 400°C at a heating rate of 10°C min-1under an N2inert atmosphere at a 20 ml min-1flow rate.
3.Results and discussion
3.1.Antibacterial and hemolytic activities of catheters

Table 1.Fisher’s F test statistics with α=0.01 (FReference) and the Fisher’s F (FCalculated) corresponding to the degrees of freedom of the extract coating at the stock concentration(φ1),plasma treatment time(φ2),and their interaction(φ1φ2)from catheter pieces tested to produce h against and S.aureus strain and HP.The significant factor was identified with *.
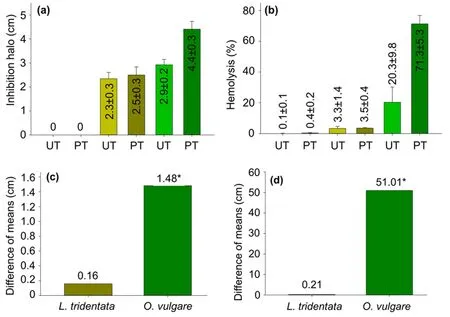
Figure2.(a)Values of h against an S.aureus strain and(b)HP produced by untreated(UT)and plasma-treated(PT)catheters coated with L.tridentata(golden)and O.vulgare(green)extracts at stock concentration.Identification of the significant mean difference(*)of(c)h and(d)HP produced by the extract-coated catheters pretreated with plasma compared with the untreated.The least significant difference calculated was 0.42 cm and 6.33%,with α=0.01 for the comparison of h and HP,respectively.
Figures 2(a) and (b) show that catheter pieces coated with acetonic extracts of L.tridentata and O.vulgare at stock concentration produced h against an S.aureus strain and HP,respectively,where the O.vulgare extract coating had the highest capacity.At the same time,table 1 indicates that stock-concentrated extract coating and plasma treatment time affect the catheter capacity to produce h and HP.Indeed,plasma pretreatment increased the capacity of the catheters coated with extracts at stock concentrations to produce h and HP,as figures 2(c)and(d)indicate.However,this synergistic effect only was significant for O.vulgare extract-coated catheters.It was calculated that the plasma pretreatment improved the capacity of catheters coated with O.vulgare extract at the stock concentration to produce h by μ ·100=51%.At the same time,it was observed that the capacity of the L.tridentata extract-coated catheters to produce h was remarkably lesser than their capacity to produce HP.These HP values were slightly hemolytic(2%<HP ≤5%)for untreated and plasma-treated catheters,which suggest that non-hemolytic HP values can be obtained by reducing enough the L.tridentata extract concentration but remaining its capacity to produce h values.
The capacity of the catheters coated with L.tridentata and O.vulgare extracts at the stock concentration ofproducing h against an S.aureus strain indicates that they have antibacterial activity.It has been reported that phenolic compounds and fatty acids present in the extracts of L.tridentata and O.vulgare damage the cytoplasmic membrane by interrupting the correct functioning of the transport proteins dependent on adenosine triphosphate,preventing the absorption of vital substances for the bacteria as figure 3(a)schematizes [25,26,31,32].It has been explained that treating surfaces with plasma increases the adhesion points through the creation of functional groups [33] and surface wrinkles,improving the extract adhesion to the treated surface[26].Thus,extract coating should be carried out by electrostatic and chemical interactions and mechanical anchoring,as figure 3(b) illustrates.Plasma treatment was used to increase the anchoring points on the catheter surface,improving the extract adhesion and,in turn,the antibacterial activity [34].The extract antibacterial compounds adhered to the catheter surface prevented planktonic bacteria from building a biofilm[17,20].In the same line,it is important to explain that the increment of the hemolytic activity of the extract-coated catheters provoked by the plasma pretreatment is also attributed to the elevated presence of the antibacterial compounds,which tend to kill bacteria and mistakenly the erythrocytes [14].

Table 2.Maximum reported h values caused by catheters coated with the antibacterial extract.

Figure3.(a) Schematic representation of the damage to the cytoplasmic membrane of a bacteria caused by the interaction between said membrane and the antibacterial extract compounds.(b) Schematic representation of the influence of the catheter surface treatment using a non-monomeric plasma on the adhesion degree of an antibacterial extract from chemical and electrostatic interactions and mechanical anchorage.
On the other hand,although the capacity of catheters coated with extracts from diverse plants (e.g.Eucalyptus and Salvia rosmarinus) to produce h against S.aureus and other clinically relevant bacteria has been reported,publications on the antibacterial activity of L.tridentata and O.vulgare catheters against pathogen bacteria were not found,as table 2 summarizes.Moreover,studies on the hemolytic activity of catheters coated with antibacterial extracts were not recovered also.Thus,this study must first contribute to scientific knowledge of the antibacterial and hemolytic activities of plasma-treated and untreated catheters coated with L.tridentata and O.vulgare extracts.Moreover,here is first reported the improvement of antibacterial activity and increment of hemolytic activity of crude extract-coated catheters by using plasma pretreatment.However,it is necessary to point out that non-hemolytic concentrations must be achieved since the catheters coated with extracts at stock concentrations were slightly hemolytic at least.
Once the biological activity of the catheters coated with extracts at stock concentration was corroborated,their MIC and MIC-NH values were determined (see table 3).In this regard,figure 4(a) indicates that the plasma-treated catheters coated with L.tridentata and O.vulgare extracts at 2500 and 1000 μg ml-1,respectively,provoked h against an S.aureus strain.At the same time,figure 4(c)specifies that the capacity of the catheters coated with L.tridentata or O.vulgare extracts at 2500 and 5000 μg ml-1are not significantly different from each other.On the contrary,the capacities of the catheters coated with O.vulgare extract at 1000 and 5000 μg ml-1are statistically different.Thus,the plasma-treated catheters coated with L.tridentata and O.vulgare extracts exhibited MIC values of 2500-5000 μg ml-1and 1000-2500 μg ml-1against an S.aureus strain.Then,about the hemolytic activity of the catheter pieces,figure 4(b)shows that the catheters pretreated with plasma and coated with L.tridentata and O.vulgare extracts produce non-hemolytic HP values up to 5000 and 2500 μg ml-1,respectively.In this sense,figure 4(d)indicates that the capacity of producing HP of the O.vulgare extract coating at 1000 and 2500 μg ml-1was not different from each other.Thus,the plasma-treated catheters coated with L.tridentata and O.vulgare extracts presented MIC-NH values of 5000 and 2500 μg ml-1against an S.aureus strain.

Table 3.Fisher’s F test statistics with α=0.01 (FReference) and the Fisher’s F (FCalculated) corresponding to the degrees of freedom of the extract coating at diluted concentrations from the catheter pieces tested to produce (a) h against and S.aureus strain and (b) HP.The significant factor was identified with *.

Figure4.(a)Values of h against an S.aureus strain and(b)HP produced by plasma-treated catheters coated with L.tridentata(golden)and O.vulgare (green)extracts at diluted concentrations.Significant difference of means(*)of(c)h and(d)HP produced by the extract-coated catheters pretreated with plasma.The least significant difference calculated was 0.2 cm with α=0.01 for the comparison of h.For the comparison of HP,it was 0.6 and 0.8%,with α=0.01,respectively.
These results indicate that the plasma-treated catheters coated with L.tridentata and O.vulgare extracts at MIC-NH and tested under in vitro conditions prevented the growth of an S.aureus strain without causing erythrocyte lysis.It is chief to highlight that MIC and MIC-NH values have not been reported for extract-coated catheters,although inhibitory concentrations against S.aureus strains have been published for ethanolic and methanolic extracts of L.tridentata[35,36]and acetone and methanolic extracts of O.vulgare [37-40].This pioneer investigation reports MIC and MIC-NH values,critical clinical parameters,for plasma-treated catheters coated with extracts.In addition,it is important to point out that the soaking process reported here allowed to influence the h and HP values with relatively small variation (standard deviation) by controlling the dissolved extract concentration.The standard deviation of h and HP values was lesser than 0.3 cm and 0.5% for catheters soaked with dissolved extracts at 2500 μg ml-1or lesser concentration.
3.2.Chemical characteristics

Figure5.Infrared spectra of the extract of (a) L.tridentata and (b) O.vulgare at stock concentration.(c) Skeletal structure of PET and its similarity degree with the catheter.
Next,a chemical analysis was carried out to corroborate the adhesion of the extract to the catheter surfaces and the formation of functional groups after treatment with plasma.Figures 5(a)and(b)show the infrared spectra of the acetonic extract of L.tridentata and O.vulgare at the stock concentration and the infrared spectra of the catheters coated with said extracts.In addition,the skeletal structure of a commercial polyethylene terephthalate (PET) is shown(figure 5(c)).PET was identified as the main component of the catheter,whose infrared spectrum was similar (82.4%) to a reference spectrum (KODEL CTA A0335).Indeed,signals associated with bonds present in PET were detected.Stretching (v) and bending (δ) vibrations of the C-H in the hydrocarbon chains were correspondingly identified at 2960 to 2870 and 730 cm-1.In addition,the vibrations of C-H in aromatic rings(1480-1390 cm-1),the v and δ of C=O(1719 and 960 cm-1),the v of C-O-C(1268 cm-1),and the v of CO (1110 and 1026 cm-1) in ester groups were also detected[41-44].
After plasma treatment,two new signals were detected at 1216 and 1164 cm-1and were associated with overlapping v of C-F and C-Si,indicating the presence of fluoride and organosilanes [45].It has been reported that incorporating fluorinated polymers in catheters can reduce thrombi due to their low surface energy,good hemocompatibility,and low chemical inertia [46].Thus,detecting these expected vibrations after plasma treatment should result from their exposition when the plasma erodes the surface.
After coating the catheters with extract,a wide signal appeared at 3500-3000 cm-1and a narrow at 1513 and 1400 cm-1.These signals were associated with v and δ (symmetric and asymmetric) of the O-H present alcohols and organic acids in O.vulgare and L.tridentata extracts,as reported (e.g.catechol,encecalinol,phenol,guaiacin,and nordihydroguaiaretic acid) [1,47,48].In addition to the detected PET signals,others at 1690 and 1640 cm-1were respectively associated with the v of C=O of the carbonyl groups and double bonds vibration of alkenes in the organic acids and esters and phthalates of the extracts (e.g.hexadecanoic acid,bis-ethylhexyl-phthalate,and isopropyl cinnamate) [49-51].Compared to the infrared spectrum of the uncoated catheter,the shift of the C=O signal towards lower wavenumbers indicates a bathochromic effect,possibly produced by non-conjugated double bonds in some of the extracts.On the other hand,a signal was detected at 1612 cm-1and was associated with v C=C in an aromatic ring [1].
Furthermore,the v and δ of the bond with an isopropyl,such as that of the reported isopropyl cinnamate,were detected at 1379 and 1252 cm-1,respectively [48].It is noteworthy that carbonyl groups-associated broad signals were detected (1200-1000 cm-1).These broadening must have resulted from overlapping signals displaced by the bathochromic effect.Finally,it is essential to note that the signals increased when the catheter was pretreated with plasma,indicating an improvement in the adhesion of the extract.
The chemical bond energies were evaluated to corroborate the increment of functional groups,which should be anchoring points,on the catheter surface after being treated with plasma.In this sense,the C,Cl,F,N,O,and Si were corroborated through the 1 s orbital photoelectrons detection.In addition,O detected from Auger electrons (KLL) was confirmed only in the plasma-treated catheters,as shown in figure 6(a).Next,high-resolution signals from the C1s,N1s,and O1s regions of interest were deconvolved to identify related chemical bonds.Briefly,the binding energies of C-C,C-H,C-O,C-Cl,O-C=O,C-F2,and O-C-F2in the C1s(see figure 6(b)) of C-N-Oxand C-NH-C in the N1s (see figure 6(c));and of C=O and O-C=O in the O1s (see figure 6(d)) were detected [52-60].
It was found that after plasma treatment,the intensity of the signals associated with the presence of C,F,and O increased markedly,especially for F.However,as table 4 indicates,the atomic concentration of C and O in the plasma-treated catheters is lower than that of the untreated ones,while it was confirmed that their signal intensity was higher than the untreated.Nevertheless,it is necessary to note that a change in atomic concentration is relative,thus,not necessarily implying a change in the amount of the sample matter.Then the noticeable increase of the F concentration should have masked the increment of the C and O concentration after plasma treatment,whose signal intensity did not augment as much as that of the F and not enough to overcome the masking.On the other hand,it is remarkable that the Cl signal,possibly coming from more superficial chlorinated additives than the fluorinated ones,decreased after plasma treatment.It has been explained that O2-based plasma treatment provokes dichlorination [61,62].Additionally,it is essential to clarify that v C-Cl was not detected by infrared analysis since its signals are weak at 700-600 cm-1[61].Finally,the detection of F and Si corroborated their observed homologous infrared signals.

Table 4.XPS analysis-calculated atomic concentration (C,Cl,F,N,O,and Si) on the surface of the untreated and plasma-treated catheter.Functional groups assignment and RC from deconvoluted high-resolution XPS spectra.
As figure 7 schematizes,the plasma treatment should have improved the availability of functional groups such as carboxylic acids,esters,hydroxyls,and fluorides on the catheter surface by the competing functionalization and erosion plasma processes [61].
It has been reported that plasma photoionization produces electrons(e-)and UV photons(γUV)as equations(5)-(7) state,where M is a precursor gas molecule and*denotes an excited state.The ionization energies of the e-and the γUVrise at around 20 and 1.2×10-3eV,respectively.Then,eand γUVfrom plasma must produce chemical changes on the catheter surface,such as functionalization,erosion,and formation of dangling bonds since the dissociation energies of polymer chemical are lesser than 9.3 eV [34].At the same time,it has been reported that reactive species are created from the dissociation of the oxygen and water molecules in air plasma as,for instance,equations (8)-(10) describe [63]
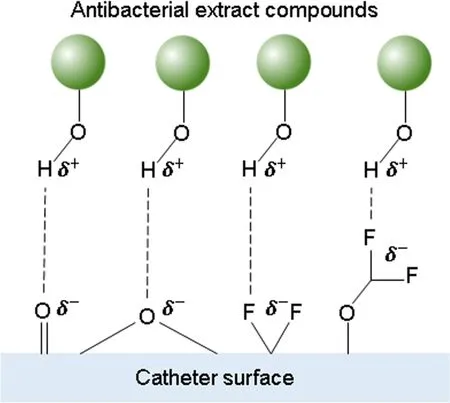
Figure7.Schematic representation of hydrogen bonds formed between the antibacterial extract compounds and the catheter surface.
Considering the above stated and based on reported air plasma-treated catheter[64]and PET surfaces[65,66],chain scission reactions,such as those described by the equations (11)-(13),explain how dangling bonds are created on the PET-based catheter surface after air plasma treatment and react with the plasma reactive species.Then,these reactions create at the chain-ends some oxidized groups like carbonyl,carboxyl,and hydroxyl groups

Figure8.SEM micrographs of untreated and plasma-treated catheters.
Thus,plasma treatment should have enhanced the electrostatic and chemical interactions between the extract and the catheter surface.Although chemical interactions cannot be ruled out,electrostatic interactions should have taken place since these occur spontaneously at room temperature,unlike some chemical interactions.In this concern,it is important to explain that hydrogen bonds are the strongest electrostatic interactions (around 0.2 eV or 5 kcal mol-1),but they are much weaker than typical polymer covalent bonds (around 4.3 eV or 100 kcal mol-1) [67].Hence,FTIR-ATR and XPS analyses confirm the presence of functional groups on the catheter surface as anchoring points for the extract coatings.Moreover,it was demonstrated that the availability of such functional groups is increased after plasma treatment.
3.3.Morphological characteristics
It should be noted that the improvement in the extract adhesion on the plasma-treated catheter surface must be due also to the increase in mechanical anchoring points [68].Indeed,the SEM images of the untreated and plasma-treated catheters(see figure 8)show a change in their surface morphology after plasma treatment.The untreated catheter surface appeared relatively homogeneous and smooth,unlike the plasma-treated,which exhibited numerous irregularities provoked by the eroding plasma effect.Similar results have been reported using non-monomeric plasma (e.g.Ar,He,O2,and air) to modify catheter surfaces[69,70].Thus,SEM analysis results corroborate the formation of catheter surface irregularities as anchoring points for the extract coating after plasma treatment.
3.4.Thermal characteristics
Finally,the thermal characterization of extract-coated catheters was performed to evaluate their thermal stability.Figures 9(a) and (b) correspondingly show that two endothermic heat flux regions were present in the L.tridentata and O.vulgare extract-coated catheters from 240°C-250°C and from 270°C-370°C.At the same time,the extracts presented an endothermic heat flux region associated with moisture removal and volatilization of other components.The first thermal region was attributed to the PET melting temperature (Tm),which has been reported to be from 232°C-250°C[71-73].Moreover,it was observed that the Tmand heat flux necessary for the PET crystallites to melt increased after extract coating and did not depend on the plasma pretreatment.This increment was attributed to the presence of complex compounds in the catheter coatings[74] and indicated an improvement in the thermal stability of the catheters [75],implying that the extracts strongly interact with the catheter surface and penetrate it [76,77].Conversely,the second thermal region (TEndo) was attributed to the volatilization of complex substances such as aromatic extract compounds and potential aromatic catheter plasticizers.Aromatic plasticizers (e.g.lignin,chalcones,and flavones) have been reported to be added to polymer biomaterials to decrease their stiffness while preventing environmental oxidation and degradation from γUVexposure [78].

Figure9.DSC thermograms of (a) L.tridentata and (b) O.vulgare extracts at stock concentration.
Regarding thermal degradation,figures 10(a) and (b)reveal that the maximum degradation rate temperature(Tm˙)of the catheters coated with L.tridentata and O.vulgare extracts at stock concentration,respectively,was at around 309°C,the same as PET [79].Moreover,the degradation of the heatresistant extract compounds was also associated withTm˙[51,74,80,81].On the other hand,Tm˙was not affected by the plasma treatment or the extract coating.In contrast,the maximum degradation rate(m˙)increased after extract coating,and plasma pretreatment improved it.Them˙increment indicates a considerable amount of extract heat-resistant compounds when reachingTm˙as a previous research study explains [82].Finally,it is noteworthy that this pioneering study first describes the thermal behavior of extract-coated catheters and the improvement of the catheters thermal stability after coating them with L.tridentata and O.vulgare extracts.

Figure10.TGA thermograms and DTG of (a) L.tridentata and (b)O.vulgare extracts at stock concentration.
4.Conclusions
The antibacterial and hemocompatible properties of blood contact antibacterial catheters must be corroborated to provide evidence for clinical application.Moreover,their thermal stability must be studied for extract coating processing.The MIC against an S.aureus strain and the MIC-NH,critical clinical parameters of L.tridentata and O.vulgare extractcoated catheters pretreated with plasma,were evaluated to fulfill such requirements.At the same time,chemical,morphological,and thermal analyzes were performed.
This study reports that plasma-treated catheters coated with L.tridentata and O.vulgare extracts have MIC values from 2500-5000 μg ml-1and from 1000-2500 μg ml-1.However,when the hemolytic activity is considered,the MIC-NH values were estimated to be 5000 and 2500 μg ml-1,respectively.At the same time,it is remarked that suitable h and HP values of extract-coated catheters can be achieved with standard deviation smaller than 0.3 cm and 0.5%,respectively,by simple soaking and controlling the concentration of dissolved extract.It is expected that the clinical evidence reported here encourages future studies of the catheter properties needed to offer safe and valid minimally invasive medical devices.
The functional groups and morphological irregularities on the catheter surface,which are anchoring points for the extract coating,increased after plasma treatment,explaining the improvement in the antibacterial activity of the extractcoated catheters.Moreover,extract coating increases theTm˙of the catheter crystallites,implying an improvement in the thermal stability of the catheters.At the same time,this study reports that plasma treatment increases them˙,indicating that the extract adhesion is enhanced.Finally,it is essential to highlight that this original investigation reports the MIC and MIC-NH values for the first time and the thermal behavior of extract-coated catheters pretreated with plasma,providing substantial evidence for clinical applications.
Acknowledgments
The National Council for Science and Technology of Mexico(CONACYT) is thanked for the grant (No.CVU 859503)given to the first author to pursue his doctorate in Materials Science and Technology at the Autonomous University of Coahuila (UAdeC).FONCYT-Fund Destined to Promote the Development of Science and Technology in the State of Coahuila (No.COAH-2020-C14-C058).Nathalia Divenhi Gómez-Rivas is thanked for collaborating in the experimental performance.
ORCID iDs
Francisco Javier ALONSO-MONTEMAYORhttps://orcid.org/0000-0002-0612-9150
Miriam Desirée DÁVILA-MEDINAhttps://orcid.org/0000-0001-8897-0375
María Guadalupe NEIRA-VELÁZQUEZhttps://orcid.org/0000-0002-3850-850X
杂志排行
Plasma Science and Technology的其它文章
- Presheath formation and area design limit satellite-based Langmuir probes
- Study of a collisionless magnetized plasma sheath with nonextensively distributed species
- Effect of spin on the instability of THz plasma waves in field-effect transistors under non-ideal boundary conditions
- Hybrid simulation of q=1 high-order harmonics driven by passing energetic particles in tokamak plasmas
- The advance of magnetic diagnostics system in support of EAST long-pulsed operation
- Numerical study of the self-pulsing of DC discharge: from corona to parallel-plate configurations
