Retinal laser photocoagulation and intravitreal injection of anti-VEGF for hemorrhagic retinal arterial microaneurysm
2023-12-14YingHuangWeiWeiZhengYingZiLiZuHuaSunBingLin
Ying Huang, Wei-Wei Zheng, Ying-Zi Li, Zu-Hua Sun, Bing Lin
National Clinical Research Center for Ocular Diseases, Eye Hospital, Wenzhou Medical University, Wenzhou 325027,Zhejiang Province, China
Abstract
● KEYWORDS: retinal laser photocoagulation; anti-vascular endothelial growth factor; retinal arterial macroaneurysm
INTRODUCTION
Retinal arterial macroaneurysm (RAM) is an acquired aneurysmal dilatation of retinal arteries, usually within the first three branches, and predominantly affects elderly female patients[1].Rupture of RAM leading to hemorrhage,exudate and edema causes vision loss rapidly if the macula is involved, particularly macular hemorrhage.Lavinet al[2]classified RAMs into three categories: 1) quiescent RAM,with hemorrhage or exudate extending for less than 1-disc diameter and sparing the macula; 2) exudative RAM, in which exudate is the major component measuring more than 1-disc diameter and is responsible for visual loss in cases combined with hemorrhage; 3) hemorrhagic RAM, with hemorrhage extending more than 1-disc diameter, more extensive than any associated exudate and responsible for visual loss.
RAM aneurysms tend to self-heal[3], followed by hemorrhage,exudate and edema that absorb slowly.Blood causes iron toxicity and mechanical damage to the retina in a short time[4].The hemorrhagic type of RAM, in which hemorrhage is responsible for visual loss, needs to be treated as early as possible rather than left to heal independently; otherwise,macular structure and retina cells will be irreversibly damaged by blood, resulting in permanent visual impairment.
There is no standard treatment for RAM thus far.Common therapies include retinal laser photocoagulation and intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents[5-6].Retinal laser photocoagulation for RAM includes conventional and subthreshold lasers.Conventional lasers include direct laser and indirect laser photocoagulation.Direct laser photocoagulation acts on the aneurysm directly and seals it with thermal energy[1].Indirect lasers act around aneurysms,specifically to the incompetent retinal capillaries surrounding the RAM, reducing the blood flow and pressure in the artery as a result of decreased oxygen demand to nearby retinal tissue[7].Subthreshold laser, a kind of lower power laser in the form of pulse, can effectively overcome these side effects of conventional laser[8], and it acts on the edema area to promote the absorption of edema.Indirect laser and subthreshold laser are unable to reach the required retinal level for hemorrhagic RAM because of the large-scale, multilevel reach of the blood from hemorrhagic RAM.In addition, indirect laser treatment was an effective auxiliary therapy for RAM, whereas indirect laser monotherapy was not successful[7].A subthreshold laser has been found to be efficacious for exudative RAM[9-10], but no research on hemorrhagic RAM has been conducted.Direct laser is effective for sealing the aneurysm, but obstruction of aneurysm bleeding may affect the effectiveness of direct laser treatment, even if red laser which has more penetrate power was used[5].
In recent years, anti-VEGF drugs have developed rapidly,although intratreat injection of anti-VEGF might induce systemic complication, especially multiple repeated treatments[11].Focal vascular damage of RAM resulted in localized ischemia with a VEGF-induced increase in the permeability and dilation of the retinal arteries.VEGF inhibitors can prevent the formation of abnormal blood vessels and counteract the VEGF-induced increase in the vascular permeability.In addition, VEGF has also been demonstrated to be a regulator of the coagulation cascade, with its inhibition leading to reductions in both bleeding time and clotting time in an animal model[12].Exudative and hemorrhagic RAM both lead to visual loss and were called RAM.Intravitreal injection of anti-VEGF drugs has been used for the treatment of symptomatic RAM in some studies[13-15].However, the manifestations of the two types are totally different, and they should be discussed separately.
The hemorrhagic type accounts for the majority of symptomatic RAM, and the retinal toxicity of blood is increasingly stronger[4].Patients with hemorrhagic RAM were enrolled in this study and divided into a laser group, an anti-VEGF group, a laser and anti-VEGF combination group, and an observation group.The purpose of this study was to analyze the efficacy of retinal laser photocoagulation and intravitreal injection of anti-VEGF for hemorrhagic RAM.
SUBJECTS AND METHODS
Ethical ApprovalThe study was conducted in adherence with the guidelines established by the Declaration of Helsinki,and it was approved by the Institutional Review Board of Eye Hospital of Wenzhou Medical University, Zhejiang Province,China (No.2020-029-K-27).
ParticipantsThis was a retrospective clinical study.Patients diagnosed with RAM based on fundus examination and fundus fluorescein angiography and/or indocyanine green angiography in the retina department of the Eye Hospital of Wenzhou Medical University between August 2016 and December 2020 were reviewed.The inclusion criteria were 1) hemorrhagic RAM type; 2) a follow-up duration of more than 40d.
Patients who met the following criteria were excluded.1) RAM eyes with any other hemorrhagic retina diseases unrelated to RAM, such as diabetic retinopathy and neovascular age-related macular degeneration; 2) The refractive medium was too turbid to perform fundus examination; 3) Exudative RAM and quiescent RAM; 4) Hemorrhage from RAM only at the subinner limiting membrane (ILM) level with or without yttrium aluminum garnet laser therapy.
Patients were divided into 4 groups corresponding to different treatments: a retinal laser photocoagulation monotherapy group(laser group), an anti-VEGF intravitreal injection monotherapy group (anti-VEGF group), a laser photocoagulation and anti-VEGF intravitreal injection combination therapy group(combination group), and an observation group.Patients who did not benefit enough from monotherapy (laser or anti-VEGF) and accepted the other treatment were classified into the combination group.It was found that some enrolled patients were no longer treated for various reasons, such as fear of treatment and lack of time.The untreated patients were classified into the observation group as the control.
ExaminationsAt baseline, the following data were collected from each participant: visual acuity (VA) expressed as the logarithm of the minimum angle of resolution (logMAR),fundus photography (FP) of the posterior pole (Canon,CR-1 Mark II), macular spectral-domain optical coherence tomography (SD-OCT, Heidelberg, Germany), fundus fluorescein angiography (Heidelberg, Germany) and indocyanine green angiography (Heidelberg, Germany).VA was scored as 2.0 for counting fingers or 3.0 for hand motion.The SD-OCT scan included cross-line mode (6 mm) and volume mode (25-line consecutive scans, 20°×20°) of the macula.Central macular thickness (CMT) was the average thickness of a 1 mm diameter circle centered on the fovea and measured by Heidelberg’s own software using volume mode of 25-line consecutive scans of SD-OCT.Hemorrhage from RAM was multilevel of retina, and the total retinal hemorrhage area (RHA) was detected on fundus fluorescein angiography image using measuring tools of Heidelberg’s own software.
The study period ended at the last follow-up.VA and CMTwere collected at the last follow-up, and these values were labeled final VA and final CMT, respectively.

Table 1 Demographic and clinical features among 4 groups at baseline
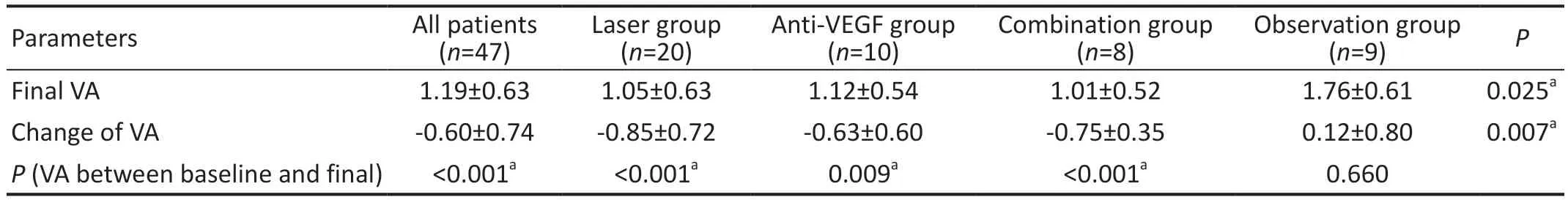
Table 2 Final VA and the change of VA among 4 groups logMAR
InterventionsRetinal laser photocoagulation: A multiwavelength solid-state laser (Lumenis) was used to photocoagulate the aneurysm of RAM directly.Green light was selected for completely exposed aneurysms, while yellow or red light was selected for incompletely exposed aneurysms.The selection principle of the laser parameters was a large spot, low energy and long duration time.The diameter of the sport was 300 μm,the energy was 200 mW, the duration time was 0.5-0.7s, and laser points were emitted repetitively until the aneurysm turned white.
Intravitreal injection of anti-VEGF drugs: injectviaa 30-gauge needle at 3.5 or 4.0 mm posterior to the limbus through the pars plana in pseudophakic and phakic eyes,respectively.Anti-VEGF drugs included ranibizumab(Lucentis®, 0.5 mg/0.05 mL, Novar) and conbercept (Lumitin®,0.5 mg/0.05 mL, Chengdu Kanghong Biotechnologies Co.Ltd.).It was used offlabel for RAM.
Statistical AnalysisAll values are presented as the mean±standard deviation (SD).Statistical analysis was performed with SPSS 24.0 (IBM, Chicago, IL, USA) and a 2-sided alpha level of 0.05.P-P plots and Q-Q plots were used to test normality of data distribution.Analysis of variance(ANOVA) was used to compare the mean values of multiple groups of measurement data, such as age, VA, CMT, RHA and follow-up duration.Tukey’s least significant difference(LSD) was used to compare the mean values of 2 groups of measurement data.Chi-square analysis was used to analyze the significant differences in enumeration data, such as sex and lesion eye.A pairedttest was used to analyze the significant difference in VA and CMT between baseline and final.Spearman test analysis was used to analyze the correlation between RHA and VA.
RESULTS
Baseline DataA total of 47 eyes (22 eyes were right eyes and 25 eyes were left eyes) of 47 patients (12 males and 35 females) were enrolled and divided into the laser group (n=20),anti-VEGF group (n=10), combination group (n=8) and observation group (n=9) according to the different therapies.The mean patient age was 71.32±10.06y (49-92y), and the mean follow-up duration was 232.49±222.44d (40-1121d).At baseline, there were no significant differences in age, sex,lesion eye, VA, CMT, RHA, or follow-up duration among the 4 groups (Table 1).OCT images of one patient in the laser group, one patient in the anti-VEGF group and one patient in the observation group were lost at baseline.
All patients in the laser group accepted laser treatment once.In the anti-VEGF group, the number of times anti-VEGF treatment was administered was 2.10±0.99 (1-4), and 30.00%of patients received it only once.In the combination group,one patient received 2 laser treatments, and the other patients received only one laser treatment; the number of anti-VEGF treatments was 2.00±1.41 (1-4), and 55.56% of patients received only one treatment.
Visual AcuityVA improved in 3 treatment groups after treatment, and the final VA was 1.05±0.63, 1.12±0.54, and 1.01±0.52 (logMAR) in the laser group, anti-VEGF group and combination group, respectively.There was a significant difference in VA between baseline and final VA in each treatment group (Table 2).However, the final VA was 1.76±0.61(logMAR) in the observation group, which was worse than

Table 3 Final CMT and the change of CMT among 4 groups
VA: Visual acuity; CMT: Central macular thickness; VEGF: Vascular endothelial growth factor.aP<0.05.baseline and had no significant difference from baseline(Table 2).
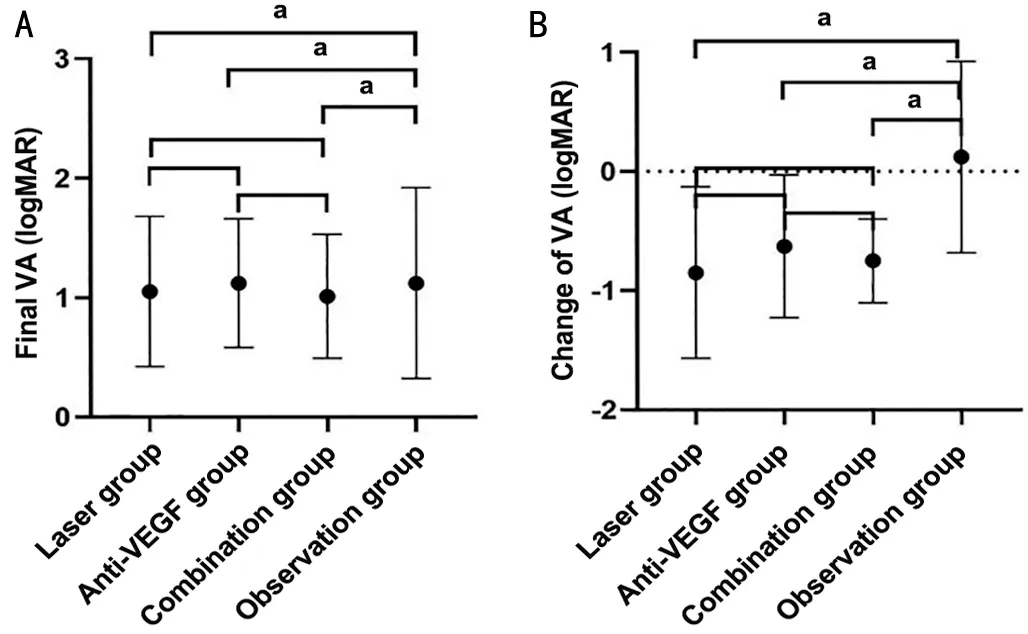
Figure 1 Final VA and change of VA of each group A: Final VA of each group.There were significant differences between laser group and observation group (P=0.005), between anti-VEGF group and observation group (P=0.023), and between combination group and observation group (P=0.012); and no significant differences between laser group and anti-VEGF group (P=0.760), between laser group and combination group (P=0.875), and between anti-VEGF group and combination group (P=0.698).B: Change of VA of each group.There were significant differences between laser group and observation group (P=0.001), between anti-VEGF group and observation group(P=0.018), and between combination group and observation group(P=0.010); and no significant differences between laser group and anti-VEGF group (P=0.404), between laser group and combination group (P=0.733), and between anti-VEGF group and combination group (P=0.702).VA: Visual acuity; VEGF: Vascular endothelial growth factor.aP<0.05.
Both the final VA and the change in VA were significantly different among the 4 groups (Table 2).Of the final VA, there were significant differences between any treatment group and observation group and no significant differences between any 2 treatment groups (Figure 1A).The change in VA was similar to the final VA; there were significant differences between any treatment group and observation group and no significant differences between any 2 treatment groups (Figure 1B).
Central Macular ThicknessThe baseline CMT of all patients was 746.55±272.98 μm and decreased to 245.05±84.93 μm at the final follow-up.The CMT decreased and was significantly different between baseline and the end of the study in each group (Table 3).Neither the final CMT nor the change in CMT was significantly different among the 4 groups (Table 3) or between any 2 groups (Figure 2).
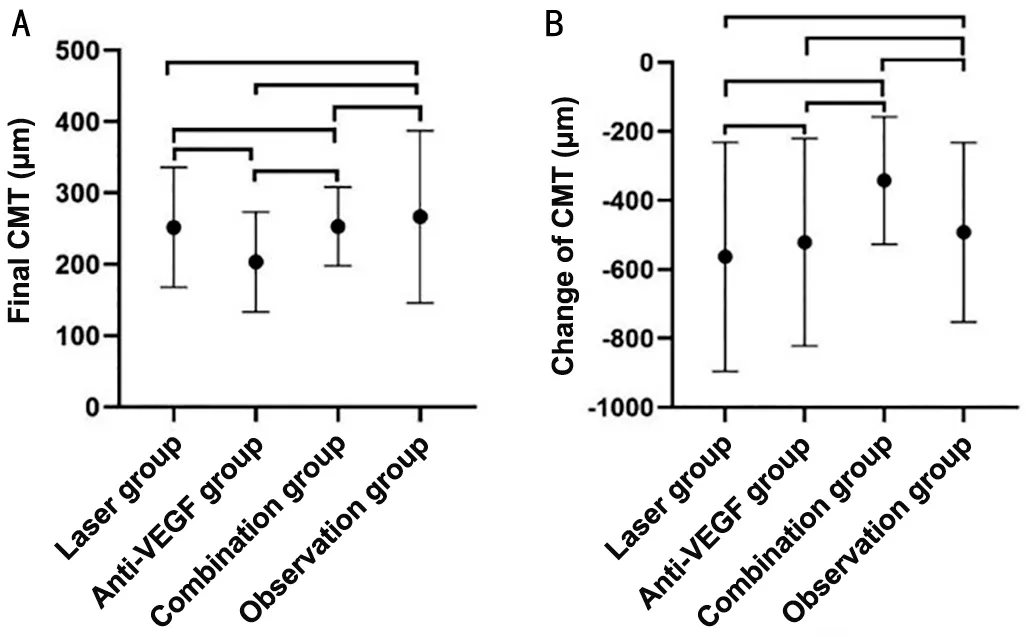
Figure 2 Final CMT and change of CMT A: final CMT of each group.There were no significant differences between laser group and observation group (P=0.679), between anti-VEGF group and observation group (P=0.133), between combination group and observation group (P=0.746), between laser group and anti-VEGF group (P=0.167), between laser group and combination group(P=0.976), and between anti-VEGF group and combination group(P=0.237).B: Change of CMT of each group.There were no significant differences between laser group and observation group (P=0.566),between anti-VEGF group and observation group (P=0.836), between combination group and observation group (P=0.313), between laser group and anti-VEGF group (P=0.727), between laser group and combination group (P=0.081), and between anti-VEGF group and combination group (P=0.215).CMT: Central macular thickness; VEGF:Vascular endothelial growth factor.
Retinal Hemorrhage AreaAt baseline, the mean RHA was 26.46±20.24 mm2across all 47 eyes and 28.99±28.15,25.94±11.58, 19.64±8.97, and 27.45±13.76 mm2in the laser group, anti-VEGF group, combination group, and observation group, respectively.There were no statistical correlations between RHA and baseline VA in the laser group (P=0.113),anti-VEGF group (P=0.578), combination group (P=0.797),or observation group (P=0.743).There were no statistical correlations between RHA and final VA in the laser group(P=0.641), anti-VEGF group (P=0.206), or combination group (P=0.257), but there was a statistical correlation in the observation group (P=0.032).
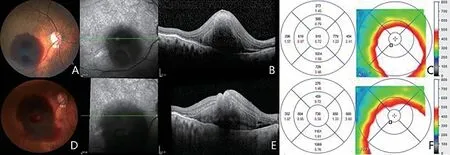
Figure 3 One eye in anti-VEGF group, that RHA increased during follow-up A: FP at baseline; B: Macular OCT at baseline; C: Retina thickness of macular at baseline; D: FP at follow-up, RHA increased than before; E: Macular OCT at follow-up; F: Retina thickness of macular at follow-up.The average thickness of fovea, supra, and temporal macular decreased; the average thickness of nasal and inferior macular increased.It was confirmed that haemorrhage diffused to the nasal and inferior sides.VEGF: Vascular endothelial growth factor; RHA: Retinal hemorrhage area;FP: Fundus photography; OCT: Optical coherence tomography.

Figure 4 One eye in laser group, that RHA increased during follow-up A: FP at baseline; B: Macular OCT at baseline; C: Retina thickness of macular at baseline; D: FP at follow-up, RHA increased than before; E: Macular OCT at follow-up; F: Retina thickness of macular at follow-up.RHA: Retinal hemorrhage area; FP: Fundus photography; OCT: Optical coherence tomography.
The RHA decreased, but the area of exudation increased in one eye in the laser group after treatment.The RHA of 2 eyes in the anti-VEGF group increased due to retinal hemorrhage diffusion during follow-up (Figure 3), and the RHA of 1 eye increased in the laser group during follow-up (Figure 4).One eye in the observation group progressed to vitreous hemorrhage.
DISCUSSION
The parameters of the conventional laser acting on the aneurysm of RAM directly in this study were consistent with other studies[1].Some studies have reported the efficacy of conventional lasers with or without indirect lasers, but the benefit of vision is unclear.Koinzeret al[7]suggested that a conventional laser had no benefit of vision for RAM; however,Meyeret al[16]reported that vision improved by a conventional laser, which was consistent with our study that the average VA of patients in the laser group improved from 1.90±0.53 to 1.05±0.63 and was significantly different (P<0.001).All the patients in the laser group accepted conventional laser treatment once.Conventional lasers have serious side effects of retinal scarring and choroidal neovascularization in the process of various diseases[8].For RAM, direct laser exposure potentially leads to aneurysm rupture, hemorrhage, and arterial occlusion due to the already thin and distended aneurysm walls[17].
Intravitreal injection for RAM treatment is an off-label use of anti-VEGF agents, including bevacizumab, ranibizumab,aflibercept and conbercept.Some case reports have shown that the use of VEGF inhibitors for hemorrhagic RAM is effective[18-20].Choet al[13]reviewed 23 symptomatic RAMs, of which 16 eyes were hemorrhagic type, and found no difference in anatomic improvement or VA improvement between the bevacizumab-treated and untreated groups.However, a prospective study reported that the aneurysms were sealed and that VA was improved significantly by intravitreal injections of bevacizumab once a month for three consecutive months in both exudative and hemorrhagic RAM; at week 12, the 2 types were not significantly different in VA[14].The results of the anti-VEGF group in our study showed that the VA of patients in the anti-VEGF group improved from 1.75±0.63 to 1.12±0.54, and the difference was statistically significant (P=0.009), which was basically consistent with the research above.The mean number of injections of anti-VEGF was 2.10±0.99 (1-4) in the anti-VEGF group, and nearly one-third of patients were treated only once.A study suggested that the long-term effect of anti-VEGF treatment, approximately 2y, was not as good as the short-term effect for hemorrhagic RAM[21].However, the mean follow-up duration was 232.49±222.44d for all patients and 215.80±183.23d for patients in the anti-VEGF group in our study.The increased RHA of two eyes in the anti-VEGF group resulted from massive retinal hemorrhage diffused during follow-up, and hemorrhage of these two eyes was too massive to cover the aneurysms, so that direct laser was not good enough for these eyes and anti-VEGF treatment was selected.To date, no study has compared the efficacy of laser monotherapy and anti-VEGF monotherapy for hemorrhagic RAM.The results of our study showed that both laser and anti-VEGF treatment were effective for hemorrhagic RAM,and there were no significant differences in the final VA or the change in VA between the laser and anti-VEGF groups.
A study reported that combined treatment of laser and anti-VEGF could improve VA of symptomatic RAM[22].However,in our study, patients in the combination group did not receive both laser and anti-VEGF treatments at the same time or within a short period; instead, the other treatment was added when the effect of the initial monotherapy failed to meet expectations.Thus, factorial design ANOVA was inappropriate to analyze the interaction of laser and anti-VEGF combination therapy.In the combination group, VA improved from 1.76±0.38 to 1.01±0.52,and the difference was statistically significant (P<0.001).Anti-VEGF and laser combination treatment has been reported for recalcitrant exudative RAM.Macular edema of an elderly woman with macular exudative RAM persisted after six injections of bevacizumab, focal laser photocoagulation was performed, and then edema was completely absorbed[23].In our study, 30.00% of patients in the anti-VEGF group and 55.56%in the combination group received anti-VEGF treatment only once, which indicated that combined laser therapy may reduce the number of injections needed for anti-VEGF treatment.
RAM aneurysms tend to self-heal[3].VA was reduced in the observation group in our study, while VA improved in all three treatment groups, including the laser group, anti-VEGF group and combination group.It was indicated that treatment in time,whether laser or anti-VEGF, was necessary for hemorrhagic RAM.
Hemorrhage from RAM can occur at all levels of the retina, including subretinal hemorrhage (SRH), intraretinal hemorrhage (IRH), sub-ILM hemorrhage, preretinal hemorrhage, and vitreal hemorrhage[21,24].It has already been confirmed that yttrium aluminum garnet laser treatment is safe and effective for removing sub-ILM hemorrhage; therefore,RAMs with only sub-ILM hemorrhage and no other levels of hemorrhage were excluded from this study.RAM patients with vitreal hemorrhage at baseline that affected the clarity of fundus examination were excluded from this study.Blood has a negative impact on the retina, causing iron toxicity and mechanical damage[4].Photoreceptor cells are damaged when exposed to the blood from both sides of the subretinal space and the outer plexiform layer by SRH and IRH[4].Both IRH and SRH have been found to be closely associated with poor visual outcomes[4].
Tissue plasminogen activator (t-PA) combined with or without intravitreal gas injection has been used for SRH[25-26].The t-PA is injected into the submacular hemorrhage clot to dissolve the submacular hemorrhage clot, and gas is pressed against the submacular blood to the periphery retina through the appropriate body position to reduce the damage to the macular retinal structure and cells by submacular hemorrhage[27-28].The combination of t-PA and gas treatment for submacular hemorrhage RAM remains controversial[29].Sudden severe vitreous hemorrhage was a serious complication of this treatment[30].In addition, t-PA is effective only for hemorrhage at the subretinal level and not effective for other levels;however, more than 70% RAM with SRH was accompanied by IRH[4].
The level of hemorrhage from RAM could not be distinguished clearly by SD-OCT[4], so we measured the whole RHA in this study.In the observation group, there was a statistical correlation between RHA and final VA (P=0.032); however,there was no statistical correlation between RHA and baseline VA (P=0.919).It was suggested that a large RHA predicted poor natural visual prognosis.In each treatment group, there were no statistical correlations between RHA and final VA or between RHA and baseline VA.It was confirmed that timely treatment was necessary and beneficial for better visual prognosis.We did not analyze the level of blood because of the limited resolution of SD-OCT.Swept-source OCT (SS-OCT)technology has higher resolution and can be used to detect the level of retinal hemorrhage[4].We can choose SS-OCT for follow-up study in the future.
CMT increased due to hemorrhage, exudation and edema at baseline and decreased at the final follow-up in all 4 groups,including the 3 treatment groups and observation group.The CMT of some patients even progressed thinner than normal,which resulted from the death of retinal cells and atrophy of the retinal structure caused by exudation, edema, and especially blood.This was an important reason for the poor visual prognosis of hemorrhagic RAM.
In the observation group, CMT decreased significantly with the absorption of hemorrhage, exudation and edema, but the final VA was worse than baseline.It was speculated that the macular structure was destroyed by hemorrhage, exudation and edema initially, but the function of the cells was normal initially and was destroyed over time.Abnormal function was a main reason for poor vision prognosis.
Both retinal laser photocoagulation and intravitreal anti-VEGF injection were effective for hemorrhagic RAM.Combination therapy reduced the number of injections of anti-VEGF.CMT decreased hemorrhagic RAM both with and without treatment,and RHA was a VA prognosis predictor in the natural history of hemorrhagic RAM.It was a retrospective study, and the sample was small, especially in the anti-VEGF, combination and observation groups, the treatment effet of different methods still need future prospective analysis.The follow-up duration of this study was 232.49±222.44 (40-1121)d in this study, which was not long enough.We still need to increase the follow-up duration in the follow-up study.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Jingjing Zuo, National Clinical Research Center for Ocular Diseases, Eye Hospital,Wenzhou Medical University, Wenzhou, China, for providing statistical support.
Conflicts of Interest: Huang Y,None;Zheng WW,None;Li YZ,None;Sun ZH,None;Lin B,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Dynamic tear meniscus parameters in complete blinking:insights into dry eye assessment
- Effects of diquafosol sodium in povidone iodine-induced dry eye model
- Morroniside ameliorates lipopolysaccharide-induced inflammatory damage in iris pigment epithelial cells through inhibition of TLR4/JAK2/STAT3 pathway
- Role of reactive oxygen species in epithelial-mesenchymal transition and apoptosis of human lens epithelial cells
- Electroacupuncture alleviates ciliary muscle cell apoptosis in lens-induced myopic guinea pigs through inhibiting the mitochondrial signaling pathway
- De novel heterozygous copy number deletion on 7q31.31-7q31.32 involving TSPAN12 gene with familial exudative vitreoretinopathy in a Chinese family
