Efficacy and safety of oral Chinese patent medicine combined with sacubitril/valsartan in the treatment of chronic heart failure: A Metaanalysis
2023-12-13TAOShiyiTANGXianwenZHANGLanxinYULintongZHANGJinYANGDeshuangLILinglingHUANGLiWUJiayun
TAO Shi-yi, TANG Xian-wen, ZHANG Lan-xin, YU Lin-tong, ZHANG Jin, YANG Deshuang, LI Ling-ling, HUANG Li, WU Jia-yun✉
1.Beijing university of Chinese medicine, Beijing 100029, China
2.Shenzhen Hospital of Beijing University of Chinese Medicine, Guangdong 518000, China
3.Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing 100053, China
4.The First Hospital of Hebei Medical University, Hebei 050031, China
5.China-Japan Friendship Hospital, Beijing 100029, China
Keywords:
ABSTRACT Objective: To systematically evaluate the clinical efficacy of oral Chinese patent medicine combined with sacubitril/valsartan in treating chronic heart failure (CHF).Methods: CNKI,CSPD, CCD, CBM, PubMed, Web of Science, Cochrane Library and EMbase were retrieved to screen out randomized controlled trials Chinese patent medicine and Western medicine in treating CHF.Manual retrieval was also applied as a supplement.The Cochrane Reviewers Handbook 5.1.0 was used to evaluate the bias risk of the included studies and RevMan 5.4 software was used for Meta-analysis.Results: A total of 1301 patients enrolled in the 13 RCTs were included.According to the results of Meta-analysis, a combination of oral Chinese patent medicine and sacubitril/valsartan could further improve total effectiveness rate (RR=1.23,95%CI[1.16, 1.30], P < 0.001), increase 6 minutes’ walk distance (MD=53.04, 95%CI[33.43,72.64], P < 0.001), improve left ventricular ejection fraction (MD=6.67, 95%CI[5.15, 8.19], P< 0.001) and stroke volume (MD=7.56, 95%CI[3.94, 11.18], P < 0.001), reduce left ventricular end-diastolic dimension (MD=-3.68, 95%CI[-4.57, -2.78], P < 0.001) and N terminal pro B type natriuretic peptide (MD=-434.08, 95%CI[-518.95, -349.22], P < 0.001) and no statistically significant difference in drug safety was found between the sacubitril/valsartan-only group and the combined treatment group (RR=0.73, 95%CI[0.32, 1.65], P=0.45).Conclusion: It’s indicated that a combination of traditional Chinese patent medicine and sacubitril/valsartan had a good clinical efficacy in the treatment of CHF, which had certain guiding significance for clinical practice.
1.Introduction
Chronic heart failure (CHF) is a complex clinical syndrome characterized by reduced pumping function of the heart, which is the end-stage and the main cause of death of various cardiovascular diseases[1].Previous studies showed that there are about 8.9 million patients with heart failure in China[2], with a fatality rate of 4.1%[2]and a 5-year survival rate comparable to that of malignant tumors[3].Sacubitril/valsartan is the first angiotensin receptor-enkephalin inhibitor (ARNi), which can simultaneously inhibit enkephalin and renin angiotensin aldosterone system (RAAS).It has diuretic,vasodilatory and reverse myocardial remodeling effects, and is widely used in clinical treatment of CHF[2, 4].With the continuous progress of modern medical treatment, the in-hospital fatality rate of heart failure patients has decreased significantly, but the re-hospitalization rate is still on the rise[1].How to reduce the readmission rate and mortality of patients with heart failure, further improve the quality of life, improve the long-term prognosis, is still a difficult and important problem in the research.
Drugs have advantages in improving clinical symptoms and quality of life in patients with heart failure[5].With the development and improvement of TCM clinical guidelines for prevention and treatment of heart failure, treatment strategies have become more standardized and standardized[6].A study has shown that oral Chinese patent medicine combined with Sacubitril/valsartan in the treatment of CHF can effectively improve clinical symptoms and cardiac function in patients[6].However, there is a lack of comprehensive analysis and report on this kind of research.Therefore,this study intends to use meta-analysis method to summarize and analyze randomized controlled trial (RCTS) studies on oral Chinese patent medicine combined with Sacubitril/valsartan in the treatment of CHF, in order to provide scientific basis for clinical practice of integrated traditional Chinese and Western medicine.
2.Materials and Methods
2.1 Inclusion criteria
2.1.1 Objects
The Chinese and English literature of oral Chinese patent medicine combined with Sacubitril/valsartan in the treatment of CHF was screened.The subjects in this study met the CHF diagnostic criteria of Chinese Guidelines for Heart Failure Diagnosis and Treatment 2018[7].
2.1.2 Interventions
Both groups were given routine basic treatment of CHF.In addition to conventional basic treatment, the control group was given Sacubitril/valsartan or placebo, and the treatment group was given oral Chinese patent medicine combined with Sacubitril/valsartan.
2.1.3 Outcomes
(1) Total effective rate.Refer to Guiding Principles for Clinical Research of New Chinese Medicines[8] for calculation method of total effective rate: Total effective rate = (number of significant cases + number of effective cases)/total cases *100%; (2) 6-minute walking distance (6MWD); 3) left ventricular ejection fraction(LVEF); (4) stroke output (SV); (5) left ventricular end-diastolic diameter (LVEDD); (6) N-terminal brain natriuretic peptide precursor (NT-proBNP), and adverse drug reactions were recorded.
2.1.4 Sample size and follow-up
There was no limit to sample size and follow-up time for inclusion in this study.
2.2 Exclusion criteria
(1) Research duplication or unclear elaboration or incomplete information or incorrect data; (2) Subjects with severe cardiopulmonary dysfunction, liver and kidney dysfunction,endocrine system diseases, malignant tumors or psycho-nervous system diseases that may affect the test results; (3) pregnant or lactating; (4) Allergic constitution.
2.3 Literature retrieval
Search China National Knowledge Infrastructure (CNKI), China Science Periodical Database, CSPD), Chinese Citation Database(CCD), Chinese Biomedical Miterature Database (Chinese Biomedical Miterature Database, CBM), PubMed, Web of Science,Cochrane Library, EMbase, and manual screening of missing literature.Set search terms in Chinese and English: Chinese patent medicine, Traditional Chinese medicine, Traditional Chinese medicine, Sacubitril/valsartan, nohintol, angiotensin receptor enkephalase inhibitor, heart failure, heart dysfunction, sacubitril/valsartan, heart failure, angiotensin receptor-neprilysin inhibitor,ARNi, randomized controlled trial, RCT, clinical trial, Chinese medicine, TCM, etc.The retrieval period is from the establishment of the database to December 31 2022.
2.4 Data extraction
The literature is summarized and weighed.After the preliminary screening according to the title and abstract, the full text is further reviewed and rescreened.Finally, the literature that meets the standard is obtained.According to the preset data extract table,literature information was extracted, including author and year,gender ratio and age of subjects, sample size, information related to study design, intervention, course of treatment, outcome indicators,baseline comparability, adverse drug reactions, etc.
2.5 Quality evaluation
According to Cochrane 5.1.0, the risk of bias was assessed from randomized allocation methods, allocation hiding, blindness,outcome data, selective reported outcomes, and other biases[9].The process of data extraction and quality evaluation was completed independently by two researchers and then cross-checked.In case of any disagreement, it was negotiated or negotiated with a third.
2.6 Statistical analysis
RevMan 5.4 software was used for analysis.Mean difference(MD) and relative risk (RR) were used as effect sizes for continuity variables and dichotomous variables, respectively, and 95%confidence intervals (CI) were calculated.The corresponding effect model was selected according to the heterogeneity: when the heterogeneity was low, i.e., P>0.10and I2<50% the fixed effect model was selected for analysis.On the contrary, the random effects model was used, and sensitivity analysis and subgroup analysis were used to identify the source of heterogeneity.Funnel plot was drawn to evaluate publication bias.
3.Results
3.1 Literature screening process
As shown in FIG.1 2036 literatures were preliminatively retrieved,835 literatures were obtained after reselection, titles and abstracts were scanned for preliminary screening, 794 literatures that did not meet standards were excluded, and then the full text of the remaining 41 literatures were re-screened, and 13 literatures were finally obtained[10-22].
3.2 Research basic features

Fig 1 Literature screening process
Finally, 13 RCTs[10-22] were included, , with a total of 1301 patients, including 652 patients in the treatment group and 649 patients in the control group, male patients: female patients = 1.58:1,a course of treatment for 4 to 24 weeks, all were “randomized”group, and the baseline data of the included study treatment group and control group were comparable, as shown in Table 1.Ten RCTs reported total response rate after treatment[11-13, 15-18 20-22], four reported 6MWD[10, 11, 13, 16], nine reported LVEF[10, 11, 13-16, 19 20, 22], two reported SV[11 20], six reported LVEDD[10, 11, 14, 17, 19,22], nine reported NT-proBNP[11-15, 17, 18 20, 21], and five reported adverse reactions.
3.3 Quality evaluation
The bias risks of 13 RCTs were evaluated, as shown in Figure 2.Five RCTs are random number table method[11, 12, 14, 17, 21], and the rest are not clear about random method.All RCTs make no mention of allocation hiding; Jiang Jun2019[20] mentioned the blind method but did not specify the specific method.The remaining RCTs did not mention the blind method and outcome evaluator blind method.Yang Wenbin 2021[12] Comparative data of cardiac function indexes between the treatment group and the control group were missing,and no data were missing in the remaining RCTs.By comparing the relevant descriptions in the legal part and the outcome part of the literature, all RCTs expected outcome indicators were reported.Default outcome indicators and other bias possibilities are not mentioned in all RCTs.
3.4 Results of Meta-analysis
3.4.1 Total effective rate
The total response rate after treatment was reported in 10 RCTs[11-13, 15-18 20-22], including 512 cases in the treatment group and 507 cases in the control group.Heterogeneity test indicated low heterogeneity between studies (P=0.76, I2=0%).Fixed-effect model analysis showed statistically significant differences between the two groups (RR=1.23, 95%CI[1.16, 1.30], P < 0.001), as shown in Figure 3.These results indicated that oral Chinese patent medicine combined with Sacubitril/valsartan treatment could further improve the total response rate of CHF patients after treatment compared with Sacubitril/valsartan treatment alone.

Tab 1 Basic features included in the study
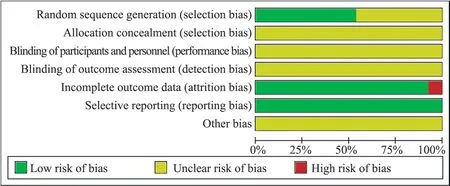
Fig 2 Bias risk assessment of included studies
3.4.2 6MWD
Four RCTs reported 6MWD[10, 11, 13, 16], 170 patients in the treatment group and 173 in the control group.Heterogeneity tests showed high heterogeneity(P=0.03, I2=68%).Random effects model analysis showed statistically significant differences between the two groups(MD=53.04, 95%CI[33.43, 72.64], P<0.001), as shown in Figure 4A.
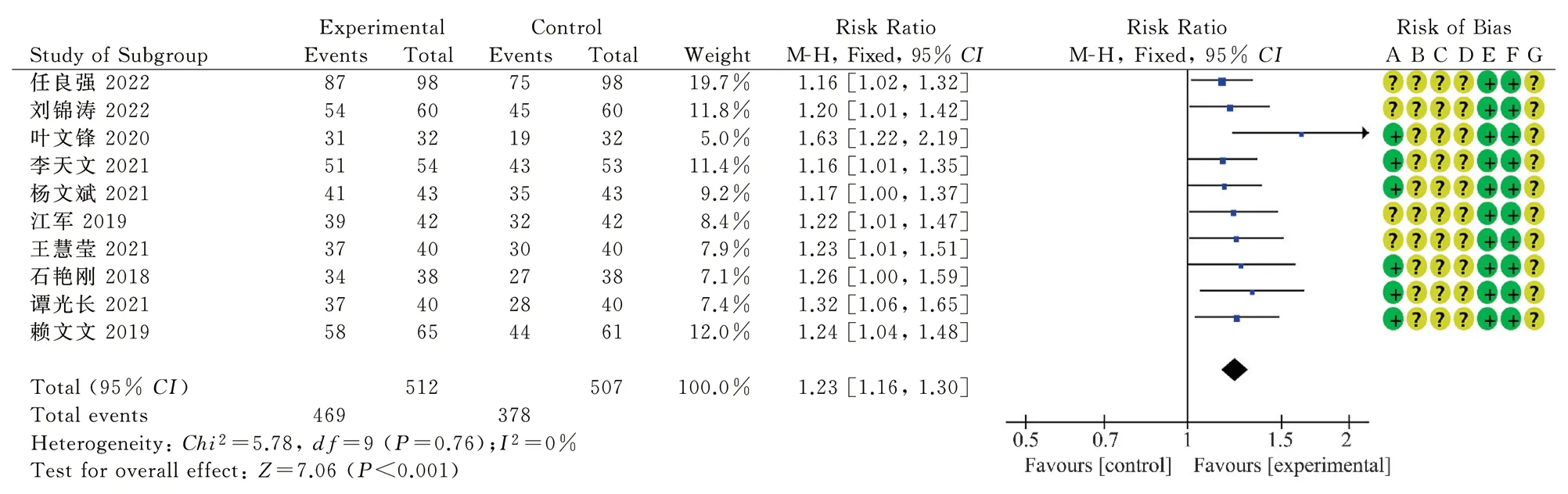
Fig 3 Forest map of total effective rate after treatment in the two groups
Sensitivity analysis showed that the heterogeneity among studies was significantly reduced (P=0.59, I2=0%) after the removal of Liu Juan 2020[10].Fixed-effect model was adopted, suggesting better efficacy in the treatment group(MD=43.48, 95%CI[30.11, 56.85],P<0.001), as shown in FIG.4B, indicating that oral treatment of Chinese prescription medicine combined with Sacubitril/valsartan increased the degree of 6MWD in CHF patients more significantly than Sacubitril/valsartan alone.
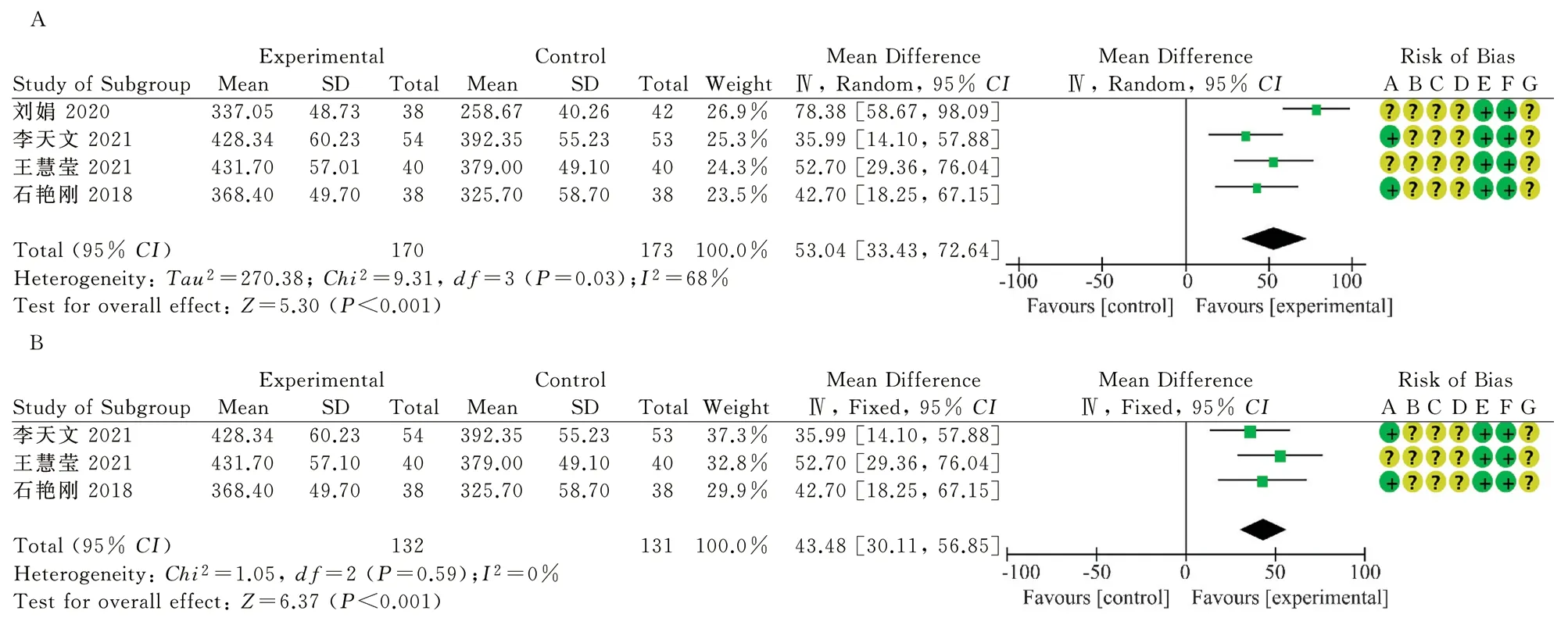
Fig 4 Forest map of 6MWD in the two groups after treatment
3.4.3 Echocardiography
3.4.3.1 LVEF
LVEF were reported in 9 RCTs[10, 11, 13-16, 19 20, 22], 472 patients in the treatment group and 473 in the control group, with high interstudy heterogeneity (P<0.001, I2=77%).Random effects model analysis suggested that the difference between the two groups was statistically significant(MD=6.67, 95%CI[5.15, 8.19], P<0.001), as shown in Figure 5.It is suggested that oral Chinese patent medicine combined with Sacubitril/valsartan treatment is more effective in improving ejection fraction of CHF patients than Sacubitril/valsartan treatment alone.There was no significant change in heterogeneity before and after sensitivity analysis, indicating that the results were relatively stable and reliable.Subgroup analysis was conducted according to the type of Chinese patent medicine, course of treatment and the dose of Sacubitril/valsartan, and there was no significant reduction in heterogeneity between studies.
3.4.3.2 SV
Two RCTs reported SV[11 20], 96 in the treatment group and 95 in the control group, with low inter-study heterogeneity (P=0.34,I2=0%).Fixed-effect model analysis suggested that the therapeutic effect was better in the treatment group and the difference between the two groups was statistically significant(MD=7.56, 95%CI[3.94,11.18], P<0.001), as shown in Figure 6.The results indicated that oral Chinese patent medicine combined with Sacubitril/valsartan treatment improved SV more significantly in patients with chronic CHF than Sacubitril/valsartan treatment alone.

Fig 5 LVEF forest map of the two groups after treatment
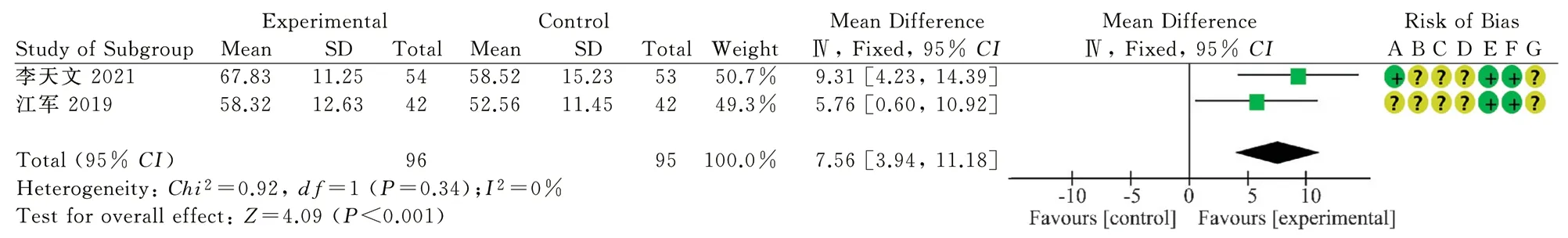
Fig 6 SV forest map of the two groups after treatment
3.4.3.3 LVEDD
LVEDD was reported in six RCTs[10, 11, 14, 17, 19, 22], 332 in the treatment group and 333 in the control group, with high interstudy heterogeneity (P=0.03, I2=61%).Random-effects model analysis showed statistically significant differences between the two groups(MD=-3.68, 95%CI[-4.57, -2.78], P<0.001).Sensitivity analysis showed that after the removal of study Yang Yang 2019[14],there was little heterogeneity among studies (P=0.24, I2=27%)The fixed-effect model was adopted, suggesting that the treatment group had better efficacy(MD=-3.17, 95%CI[-3.64, -2.71], P<0.001)as shown in FIG.7A.The results indicated that oral treatment with Chinese patent medicine combined with Sacubitril/valsartan improved the left ventricular end-diastolic diameter of CHF more significantly than that with Sacubitril/valsartan alone.
Subgroup analysis: (1) There were 4 studies on Qili Qiangxin capsule group according to types of Chinese patent medicine[10, 11,14, 17], with high heterogeneity among studies (P=0.05, I2=63%).By random-effects model, Qili Qiangxin capsule combined with Sacubitril/valsartan was superior to western medicine alone in improving the left ventricular end-diastolic diameter in CHF patients (MD=-3.92, 95%CI[-5.10, -2.74], P<0.001).The other two groups were Qishen Yiqi Pill group and Dengzhan Shengmai Capsule group, each containing 1 study, and heterogeneity analysis was not applicable, as shown in Figure 7B.(2) The group was divided by the dose of Sacubitril/valsartan: It was divided into three groups: 50 mg/d, 100 mg/d and 200 mg/d, among which the 200 mg/d group contained four studies[10, 11, 17, 23], showing low heterogeneity(P=0.35, I2=8%), and the curative effect of the treatment group was better (MD=-3.39, 95%CI[-3.98, -2.81],P<0.001); There was only one study in the 100mg/d and 50mg/d groups, and heterogeneity analysis was not applicable, as shown in Figure 7C.(3) Grouping by course of treatment: They were divided into 3 subgroups bounded by 4 weeks and 12 weeks, and a total of 4 studies were included in 4 to 12 weeks of treatment[10, 11, 17, 19, 22].The heterogeneity was low (P=0.40, I2=0%), and the curative effect of the treatment group was better than that of the control group(MD=-3.08, 95%CI[-3.56, -2.61], P<0.001), as shown in Figure 7D.
3.4.4 NT-proBNP
NT-proBNP was reported in 9 RCTs[11-15, 17, 18 20, 21] in 434 patients in the treatment group and 427 in the control group, with high heterogeneity(P<0.001, I2=99%).Random-effects model analysis showed that the therapeutic effect was better in the treatment group (MD=-434.08, 95%CI[-518.95, -349.22], P<0.001),as shown in FIG.8, indicating that oral Chinese patent medicine combined with Sacubitril/valsartan treatment had a greater reduction in NT-proBNP in CHF patients compared with Sacubitril/valsartan treatment.There was no significant change in heterogeneity before and after sensitivity analysis.Subgroup analysis was conducted according to the type of Chinese patent medicine, course of treatment and the dose of Sacubitril/valsartan, and the heterogeneity was not significantly reduced.
3.4.5 Incidence of adverse reactions
Five RCTs were reported with or without adverse reactions[11-13, 18, 21], including 232 cases in the treatment group and 227 cases in the control group.Adverse reactions occurred in 3 RCTs[12,18, 21] a total of 21 cases, 9 cases (3.88%) in the treatment group,including 2 cases of nausea and vomiting, 2 cases of palpitation,2 cases of hypotension or bradycardia, 2 cases of mild elevated aminotransferase and 1 case of elevated potassium.In the control group, 12 cases (5.29%), including 8 cases of nausea and vomiting, 3 cases of angioedema, and 1 case of palpitation.All adverse reactions were tolerated and no special treatment was given.Fixed-effect model analysis suggested that the safety difference between the two groups was not statistically significant (RR=0.73, 95%CI[0.32, 1.65],P=0.45), as shown in Figure 9.
3.5 Publication bias analysis
There were less than 10 RCTs in each outcome index, and the test efficiency was insufficient.Funnel plot was not drawn.
4.Discussions

Fig 7 LVEDD forest map of the two groups after treatment

Fig 8 NT-proBNP forest map after treatment in two groups

Fig 9 Forest map of incidence of adverse reactions in two groups
Hospitalization and mortality rates in CHF remain high for many years[6].The main pathological mechanism of CHF is dysfunction of the neuroendocrine system, resulting in hypertrophy and fibrosis of myocardial cells, weakening of myocardial remodeling ability, and eventually leading to abnormal myocardial function and structure[1,7].Sacubitril/valsartan is a novel ARNi drug with high bioavailability,which can effectively inhibit the overactivation of neuroendocrine mechanisms[2, 4].However, due to the complex pathogenesis of CHF,the effect of single intervention from the neuroendocrine system is limited.Therefore, it is an important research direction to explore new collaborative treatment methods.There is no disease name of CHF in TCM.According to the clinical manifestations, it can be classified into the categories of “palpitation”, “edema”, “phlegm and drink”, and “asthma syndrome”.The basic pathogenesis is Yang and Yin.In recent years, the efficacy and safety of TCM in treating CHF have been gradually confirmed.Although modern medicine’s understanding of the etiology, pathogenesis and treatment of CHF is not completely the same as that of traditional medicine,a large number of studies have confirmed that Chinese and western medicine can achieve good curative effect in the treatment of CHF[23, 24].
A meta-analysis was performed according to the Cochrane 5.1.0[9] and the PRISMA Statement[25].A total of 13 studies were summarized and analyzed in this study, which systematically evaluated the RCTs of oral Chinese medicine combined with Sacubitril/valsartan for CHF from the perspectives of total effective rate, 6MWD, LVEF, SV, LVEDD, NT-proBNP and adverse reactions,including Qili Qiangxin capsule, Qishen Yiqi Pills and Dengzhan Shengmai Capsule.The results showed that RCTs of 6MWD, LVEF and NT-proBNP had high heterogeneity, and there was no significant change in heterogeneity before and after sensitivity analysis,suggesting that the results were relatively stable and reliable.For LVEDD, the heterogeneity of each group was low after treatment course and Sacubitril/valsartan dose, suggesting that treatment course and dose may be factors affecting study heterogeneity.RCTs of total response rate and SV had low heterogeneity, and fixed-effect model results all indicated better efficacy in the treatment group.In terms of adverse reactions, there was no significant difference in the safety of oral Chinese patent medicine combined with Sacubitril/valsartan compared with Sacubitril/valsartan alone.In conclusion,oral treatment of CHF with traditional Chinese medicine combined with Sacubitril/valsartan can significantly improve the total effective rate, 6MWD, LVEF, SV, and reduce LVEDD and NT-proBNP,without obvious adverse reactions.
Limitations of the study: (1) 61.54% (8/13) RCTs random method was unknown, and all RCTs assignment hiding and blind method were unknown, which to some extent affected the test reliability;(2) This study preliminarily explored the efficacy and safety of oral Chinese patent medicine combined with Sacubitril/valsartan in the treatment of CHF, and further clarified its clinical effects on patients with different types of heart failure in the future.(3) The treatment course of RCTs included was between 4 weeks and 24 weeks, and long-term follow-up results were lacking.The long-term outcome of CHF patients receiving oral Chinese patent medicine combined with Sacubitril/valsartan remains to be determined.(4) Most of the original studies did not specify the type of TCM syndrome of the research object and lacked the efficacy index of TCM syndrome,which was not enough to provide exact guidance for TCM clinical dialectical treatment.
In conclusion, oral treatment of Chinese patent medicine combined with Sacubitril/valsartan has good efficacy and safety for CHF patients.In the future, more studies with rigorous design ideas and standardized reporting forms should be conducted to evaluate clinical intervention effects of drugs from multiple time nodes and long-term follow-up results, reduce research bias, and provide highquality evidence-based evidence for oral Chinese patent medicine combined with Sacubitril/valsartan the treatment of CHF.
Conflict of interest: All authors declare that there is no conflict of interest
Authors’ Contribution
Tao Shiyi: conception and design, data collection, statistical analysis and writing; Zhang Lanxin, Yu Lintong: Data verification and statistical analysis; Zhang Jin, Yang Desheng, and Li Lingling:Writing suggestions and revising manuscripts; TANG Xianwen,Huang Li, and Wu Jiayun: overall planning and quality control.
杂志排行
Journal of Hainan Medical College的其它文章
- TCM Intervention on Wnt/β-Catenin signaling pathway in the treatment of diabetic nephropathy
- Research progress on the correlation between intestinal microflora disorder and liver disease
- Research progress on the effects of phthalates on reproductive health of childbearing population and their offspring
- Construction and validation of prognostic model of Cuproptosis-related LncRNA in osteosarcoma
- Study on pharmacodynamics and mechanism of nano-Kuiyangye in treating radiation esophagitis in rats
- Effect of glycyrrhetinic acid on Th1/Th2 balance in cough variant asthma mice
