Simultaneous Determination of 14 β-Receptor Agonists Residues in Mutton by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
2023-12-12ZheMENGJianhuaWANGBoLIUYuhangGUOHaoshuangDONGPingyangSHANDaweiWANGYajuanSONG
Zhe MENG Jianhua WANG Bo LIU Yuhang GUO Haoshuang DONG Pingyang SHAN Dawei WANG Yajuan SONG


Abstract[Objectives] A high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was established for the determination of 14 β-receptor agonist residues in mutton. [Methods] Samples were hydrolyzed by β-glucuronidase and extracted with 5% acetic acid-acetonitrile (1:99, V/V) solution. An Eclipse plus C18 column was used for separation, and the MRM mode was used for qualitative analysis, and the external standard method was used for quantitative analysis of matrix standard solutions. [Results] Under the optimal conditions, the retention time of the 14 kinds of β-receptor agonists ranged from 1.0 to 9.5 min. When the mass concentration was in the range of 0.05-0.50 μg/ml, the linear relationship of β-receptor agonists was good, with correlation coefficients (r)≥0.999 2. The detection limits of the method were in the range of 0.04-0.87 μg/kg, and the quantitative limits were in the range of 0.35-1.86 μg/kg. The average recovery values were in the range of 82.8%-108.9%, with RSDs (n=6) in the range of 1.9%-6.7%. [Conclusions] The method is simple, sensitive, reproducible, accurate, and can be used for simultaneous determination of the 14 kinds of β-receptor agonist residues in mutton.
Key wordsMutton; High performance liquid chromatography-tandem mass spectrometry; β-receptor agonist; Residue
DOI:10.19759/j.cnki.2164-4993.2023.05.013
β-Receptor agonists, commonly known as lean meat powder, are synthetic adrenaline drugs with a phenylethanolamine structure, as well as drugs, enzyme agonists and hormone molecules, that can enhance molecular activity and promote certain reactions. In clinical medicine, they are used as antiasthmatic drugs to treat diseases related to bronchial asthma, but because they can activate or enhance the activity of the central nervous system, they will cause great harm to human physiology and psychology, including heart failure, irritability, prostatic hypertrophy, diabetes, heart disease, etc., which will seriously damage physical and mental health[1-3]. In 2002, China listed β-receptor agonists in the List of Prohibited Veterinary Drugs and Other Compounds for Edible Animals. In recent years, risk assessment investigations have found that driven by interests, the illegal use of β-agonists is still serious, especially the illegal use of clenbuterol hydrochloride[4-5].
The basic research on multi-residue detection techniques of β-receptor agonists and relevant limit standards in China is still in its infancy, and national standards for simultaneous detection of multiple residues have not yet been established. At present, the detection methods of β-receptor agonist residues mainly include colloidal gold immunochromatography (GICT), enzyme-linked immunosorbent assay (ELISA), high performance liquid chromatography (HPLC), chromatography-tandem mass spectrometry and high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)[6]. The GICT method has low sensitivity and relatively high detection limit, and the detection results are easily influenced by human activities and environmental conditions, and the experimental repeatability is poor. ELISA has poor specificity, and cant analyze multiple components at the same time, so it is easy to appear false positive. HPLC is complicated, time-consuming and expensive, and has high requirements for instruments and equipment and for the professional level of inspectors. HPLC-MS/MS has higher sensitivity and detection limit. On the basis of satisfying trace residue analysis of complex matrixes, it realizes simultaneous analysis of multiple residual compounds, and is the preferred method for residue analysis of β-receptor agonists[7-10]. In this study, 14 kinds of β-receptor agonist residues in mutton were determined by HPLC-MS/MS, hoping to provide reference for the revision of standards and effective monitoring of β-receptor agonists residues in mutton.
Materials and Methods
Materials and reagents
Mutton, commercially available.
Fourteen standard products of β-receptor agonists: cimaterol, terbutaline, salbutamol, fenoterol, ractopamine, clorprenaline, clenbuterol, tulobuterol, penbuterol, zilpaterol, cimbuterol, brombuterol, clenproperol and bambuterol (purity≥99.0%, Testing and Evaluation Center of Chinese Academy of Inspection and Quarantine); formic acid, acetonitrile, NH4AC (chromatographically pure, Sigma Corporation, USA); BLC-1 organic filter membrane 0.22 μm (Beijing Guohuan High-Tech Automation Technology Academy).
Instrument and equipment
TSQ Altis HPLC-MS/MS, Thermo Fisher Scientific Co., Ltd; VM-0005M multifunctional vortex mixer, LabYeah Biotechnology Co., Ltd; Avanti JXN-30 high-speed freezing centrifuge, Beckman Coulter, Inc.; AN-12 fully-automatic nitrogen blowing concentration instrument, Guangzhou Gdana Instrument Co., Ltd.
Experimental methods
Chromatographic conditions
Chromatographic column: Eclipse plus C18 (50 mm×2.10 mm, 1.8 μm); injection volume 5 μl; column temperature: 40 ℃; mobile phase: A aqueous solution containing 0.1% formic acid, B 0.2% formic acid-acetonitrile solution; gradient elution: 5% B for 0.0-1.0 min, 40% B for 1.0-3.0 min, 95% B for 3.0-7.0 min, and 5% B for 7.0-10.0 min; flow rate: 0.30 ml/min.
MS conditions
Electrospray positive ion source (ESI+) scanning mode; capillary voltage: 2.50 KV; ion source temperature: 200 ℃; desolvation temperature: 520 ℃; desolvation gas flow rate: 1 000 L/h; atomization gas flow rate: 3.00 L/min; dry gas flow rate: 15.00 L/min; scanning time: 0.1 s; multiple reaction monitoring mode (MRM).
Standard solution preparation
For a single β-receptor agonist standard substance with a concentration of 1 000 μg/ml, 100 μl was accurately pipetted and diluted to 10 ml with formic acid to obtain a mixed standard stock solution with a concentration of 10 μg/ml, which was then stored in a refrigerator at 4 ℃. A sample blank solution was pipetted and prepared into 0.05, 0.1, 0.2, 0.4 and 0.5 μg/ml matrix mixed standard working solutions, respectively. The matrix mixed standard working solutions should be prepared freshly for use.
Sample pretreatment
The edible part of the mutton sample was collected and cut into small pieces, which were ground with a food grinder evenly to make the sample to be tested. The sample to be tested was added into packaging containers, which were stored at -20 ℃ for later use.
First, 10.0 g of mutton sample was added into a 50 ml centrifuge tube, which was then added with a mixed standard solution (external standard method), 15 ml of 5% formic acid-acetonitrile (1:99, V/V), and 0.06 ml of β-glucuronidase. The sample mixture was vortex-oscillated for 10 min and ultrasonically treated for 5.0 min. Enzymatic hydrolysis was performed in a water bath in a dark place at 37 ℃ for 10 h, and the inner tube (containing five 4 mm zirconia beads, 200 mg of PSA, 500 mg of anhydrous magnesium sulfate, 100 mg of C18) was tightened to perform centrifugation at 10 000 r/min for 5 min. Another 20 ml of 5% formic acid acetonitrile was added into the residue, and the above operation was repeated once. The extracts were merged, evaporated in a 50 ℃ water bath using a rotary evaporator until almost dry, and then blown dry with a nitrogen analyzer. Finally, 2 ml of 2% formic acid was accurately added to dissolve the residue, and the solution was vortex-mixed for 1 min, and filtered through a 0.22 μm microporous filter membrane for later testing.
Results and Analysis
Optimization of chromatographic conditions
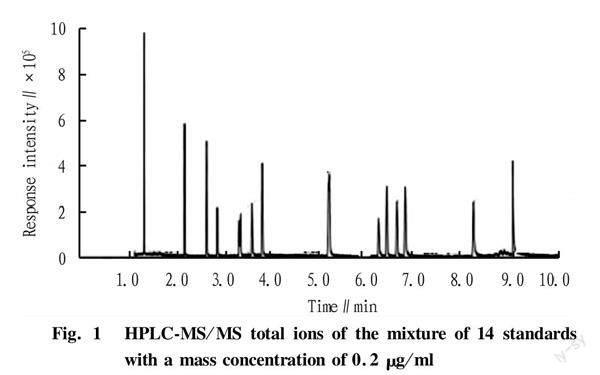
An efficient chromatographic column is the prerequisite for the detection of β-receptor agonist residues. The separation effects of Eclipse plus C18 (50 mm×2.10 mm, 1.8 μm), XTerra C18 (150 mm×2.1 mm, 3.5 μm) and Waters Acquity BEH C18 (50 mm×2.1 mm, 7.0 μm) were investigated, respectively. The results showed that the Eclipse plus C18 column had strong retention of the 14 kinds of β-receptor agonists, exhibiting good peak shapes and complete separation of components, as shown in Fig. 1. Therefore, in this study, Eclipse plus C18 column was selected to analyze the analytes.
The separation effects of methanol-water and acetonitrile-water as mobile phases were investigated respectively, and the elution effects of methanol-water and acetonitrile-water on the 14 β-receptor agonists were investigated. The results showed that when methanol was used as the organic phase, the peak time of the compounds was delayed and the response values were reduced, while acetonitrile having low viscosity showed stronger elution ability and sharper peak shapes than methanol, so acetonitrile was selected. Following mobile phases were prepared: 0.1% formic acid-0.1% formic acid acetonitrile, 0.1% formic acid-0.2% formic acid acetonitrile, 0.1% formic acid-0.5% formic acid acetonitrile, and 0.2% formic acid-0.1% formic acid acetonitrile, respectively. The results showed that the response and peak shape of each target were optimal when 0.1% formic acid-0.2% formic acid acetonitrile was used as the mobile phase. Under the optimal elution conditions, the retention time of the 14 kinds of β-receptor agonists was between 1.0 and 9.5 min.
Optimization of MS conditions
First, 1 000 μg/L standard solutions of the 14 kinds of β-agonists were injected into the MS system by a syringe pump to optimize the parameters. The first-order mass spectra of the 14 β-receptor agonists were obtained by full scanning in the positive ion mode with the scanning range of m/z 200-500. The ion with the highest response was selected as the quasi-molecular ion, and the declustering potential (DP) was optimized to obtain the strongest parent ion signal. The two ions with strong response were selected as the quantitative ion and the qualitative ion, and the collision energy (CE) was optimized in the multiple-reaction monitoring (MRM) mode. The mass spectrum parameters are shown in Table 1.
Methodology validation
Linear range, detection limit and quantification limit
A series of standard solutions with concentrations of 0.05, 0.1, 0.2, 0.4 and 0.5 μg/ml were prepared, respectively, and standard curves with mass concentrations as the abscissa and the peak area of each analyte as the ordinate were drawn. The linear regression equations and correlation coefficients are shown in Table 2. The results showed that the 14 kinds of β-receptor agonists had a good linear relationship in the concentration range of 0.05-0.50 μg/ml, and the correlation coefficients (r) were all equal to or greater than 0.999 2. The minimum limit of detection (LOD) and the minimum limit of quantitative (LOQ) of the method were calculated with 3 times signal-to-noise ratio (S/N=3) and 10 times signal-to-noise ratio (S/N=10), respectively. The results showed that the LOD values of the 14 kinds of β-receptor agonists were in the range of 0.04-0.87 μg/kg, and the LOQ values were in the range of 0.35-1.86 μg/kg (Table 3).
Recovery and precision of the method
Fourteen kinds of β-receptor agonist standards were added into negative mutton sample matrixes to prepare three samples with added concentration levels of 5, 50 and 100 μg/kg, respectively. Measurement was repeated 6 times for each concentration level to calculate relative standard deviations (RSD) of the measured values. From Table 4, it can be seen that the average recovery values for various added concentration levels were in the range of 82.8%-108.9%, with RSDs in the range of 1.9%-6.7%. The experimental results showed that this method basically met the requirements for rapid determination of β-receptor agonist residues in daily mutton samples.
Zhe MENG et al. Simultaneous Determination of 14 β-Receptor Agonists Residues in Mutton by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
Conclusions and Discussion
In this study, extracting β-receptor agonist residues in mutton with 5% acetic acid-acetonitrile (1:99, V/V) as the extraction solvent by the ultrasonic technique achieved the best effect. The Eclipse plus C18 chromatographic column completely separated the 14 kinds of β-receptor agonists, with good chromatographic peak shapes, and less interference from impurities was observed.
An HPLC-MS/MS method for the determination of 14 β-receptor agonists in mutton was established by optimizing the conditions of chromatography and mass spectrometry. When the mass concentration was in the range of 0.05-0.5 μg/ml, the linear relationship of β-receptor agonists was good, with correlation coefficients (r)≥0.999 2. The detection limits of the method were in the range of 0.04-0.87 μg/kg, and the quantitative limits were in the range of 0.35-1.86 μg/kg. The average recovery values were in the range of 82.8%-108.9%, with RSDs (n=6) in the range of 1.9%-6.7%. The method is simple, sensitive, reproducible, accurate, and can be used for simultaneous determination of the 14 kinds of β-receptor agonist residues in mutton.
Zhe MENG et al. Simultaneous Determination of 14 β-Receptor Agonists Residues in Mutton by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
References
[1] CHENG QP, HAN F, WANG Y, et al. Research progress on β-agonists in food-borne animal tissues[J]. Journal of Food Safety and Quality, 2019, 10(2): 385-393. (in Chinese).
[2] ZHANG RY, LIANG MJ, ZHAO L, et al. Determination of β-agonists in food by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Food Hygiene, 2017, 29(2): 167-171. (in Chinese).
[3] CABAN M, STEPNOWSKI P, KWIATKOWSKI M, et al. Determination of β-blockers and β-agonists using gas chromatography and gas chromatography-mass spectrometry: A comparative study of the derivatization step[J]. J Chromatogr A, 2011, 1218(44): 8110-8122.
[4] SUO DC, WEI SL, XIAO ZM, et al. Simultaneous determination of 18 β-agonists in blood products for feeds by liquid chromatography tandem mass spectrometry[J]. Scientia Agricultura Sinica, 2019, 52(24): 4613-4623. (in Chinese).
[5] YANG M, YIN Y, HU XJ, et al. Determination of 14 β-agonists in infant formulas using QuEChERS-high performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry, 2019, 40(11): 273-277.
[6] GAO Z, ZHANG Y, ZHANG YN, et al. Progress in the detection of residual β2-receptor agonists in animal tissues[J]. Chin J Sports Med, 2014, 33(4): 370-378.
[7] CHEN HL, XIE WP, WANG CL. Determination of β2-agonists in pig liver by liquid chromatography-tandem mass spectmetry conbined with acid hydrolysis for extraction[J]. Physical Testing and Chemical Analysis (Part B: Chemical Analysis), 2018, 54(12): 31-35. (in Chinese).
[8] WANG GN, WU NP, HE X, et al. Magnetic graphene dispersive solid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry for determination of β-agonists in urine[J]. Journal of Chromatography B, 2017(1067): 18-24.
[9] YE P, YUE ZF, XIAO CG, et al. Determination of 28 β2-agonists in pork by liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality, 2013, 4(3): 682-688. (in Chinese).
[10] LI DN, YAN F, WU JP, et al. Simultaneous determination of seven β2-agonists in livestock manure by on-line cleanup liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2014, 42(12): 1797-1803. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
Received: June 28, 2023Accepted: August 29, 2023
Supported by The Fourth Batch of High-end Talent Project in Hebei Province; Tangshan Science and Technology Entrepreneurship and Innovation Leading Talent Project (21130243A).
Zhe MENG (1984-), male, P. R. China, senior engineer, devoted to research about food quality and safety inspection.
Jianhua WANG (1978-), male, P. R. China, senior engineer, devoted to research about food quality and safety inspection.
Bo LIU (1987-), male, P. R. China, engineer, master, devoted to research about storage and processing of agricultural products.
#These authors contributed equally to this work.
*Corresponding author. E-mail: 574169339@qq.com.
杂志排行
农业生物技术(英文版)的其它文章
- Application of Mass Spectrometry (MS)-coupled Techniques in Pesticide Residue Detection
- Optimization of Extraction Process of Polyphenols from Chrysanthemum morifolium and the Development of Chrysanthemum Rice Wine
- Effects of Heat Treatment on Processing Characteristics of Pork
- Discussion on Construction and Mechanism of Practice Bases for Professional Degree Postgraduates Based on the Integration of Production and Education in Local Universities
- Research on Identification Method of Apple Diseases in Southern Xinjiang Based on Deep Learning and Its System Implementation
- Research Progress on Mechanism of Ovulation Disorder in Polycystic Ovary Syndrome
