Hereditary hemorrhagic telangiectasia involving portal venous system: A case report and review of the literature
2023-12-05JunLingWuZhiZhuangZhaoJunChenHanWenZhangZheLuanCongYongLiYiMingZhaoYuJiaJingShuFangWangGangSun
Jun-Ling Wu,Zhi-Zhuang Zhao,Jun Chen,Han-Wen Zhang,Zhe Luan,Cong-Yong Li,Yi-Ming Zhao,Yu-Jia Jing,Shu-Fang Wang,Gang Sun
Abstract BACKGROUND Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant genetic disorder with an incidence of approximately 1 in 5000 in the general population.It is characterized by vasodilation,which affects specific organs,such as the skin,mucous membranes,brain,lungs,gastrointestinal tract,liver,and others.However,HHT rarely involves the portal venous system to cause serious clinical compli-cations.CASE SUMMARY A 68-year-old woman was admitted to the emergency department due to four consecutive days of abdominal pain and bloody stool and was subsequently diagnosed with HHT.Computed tomography angiography confirmed the presence of an arteriovenous fistula (AVFs).Considering this specific manifestation,whole exome sequencing was performed.After a comprehensive evaluation,a selective superior mesenteric artery embolization was prioritized to avoid intestinal ischemia.The postoperative symptoms of the patient were quickly relieved.Unfortunately,two months post-procedure the patient died from intestinal necrosis and abdominal infection related to remaining AVFs.CONCLUSION For patients with diffuse superior mesenteric AVFs,selective mesenteric arterial embolization may lead to positive short-term outcomes.
Key Words: Hereditary hemorrhagic telangiectasia;Portal system;Arteriovenous fistula;Arteriovenous malformation;Selective artery embolization;Case report
INTRODUCTION
Hereditary hemorrhagic telangiectasia (HHT),also known as Osler-Weber-Rendu syndrome,is a common autosomal dominant genetic disease,with an estimated incidence of 1 in 5000[1].HHT is characterized by vascular malformations that affect specific organs such as the skin,mucosa,brain,lungs,gastrointestinal tract,liver,and others.The most common clinical symptoms of HHT are epistaxis,gastrointestinal bleeding,iron deficiency anemia,and characteristic cutaneous-mucosal telangiectasia[2].However,HHT rarely affects the portal venous system to cause severe clinical complications.HHT involving the portal venous system is an extremely rare clinical manifestation,with only six similar cases reported in the literature.Patients exhibiting this clinical presentation often experience severe symptoms and have a poor prognosis.Here,we report a case of HHT with mesenteric arteriovenous fistulas (AVFs).We performed wholeexome sequencing and selective embolization of the mesenteric arteries in the patient,which achieved some positive outcomes.This study aims to analyze the diagnosis and treatment of HHT involving the portal venous system and provide a reference for the effective clinical treatment of these patients.
CASE PRESENTATION
Chief complaints
A 68-year-old female patient presented to the Emergency Department of our hospital with the chief complaint of abdominal pain and bloody stool for four days,worsening for two days.
History of present illness
Prior to admission,there was no obvious cause for abdominal stabbing pain,which continued to worsen and changed to dull pain in the left lower abdomen.She had experienced two episodes of dark red bloody stools (approximately 200 mL each time).After defecation,there was no improvement in abdominal pain,and these symptoms were accompanied by abdominal distension,nausea,and low back pain.The patient had no fever,vomiting,or other symptoms over the course of the disease.
History of past illness
The patient had a history of spontaneous epistaxis for 20 years and intermittent rectal bleeding for 10 years,which had been previously neglected in terms of sufficient attention and medical intervention.
Personal and family history
Some of her family members also had a long-term history of spontaneous epistaxis (Figure 1).

Figure 1 Pedigree chart.Black circles refer to female patients with a history of epistaxis,open circles refer to healthy women;black squares refer to male patients with a history of epistaxis,open squares refer to healthy men.Red circle highlights the patient in this case study.
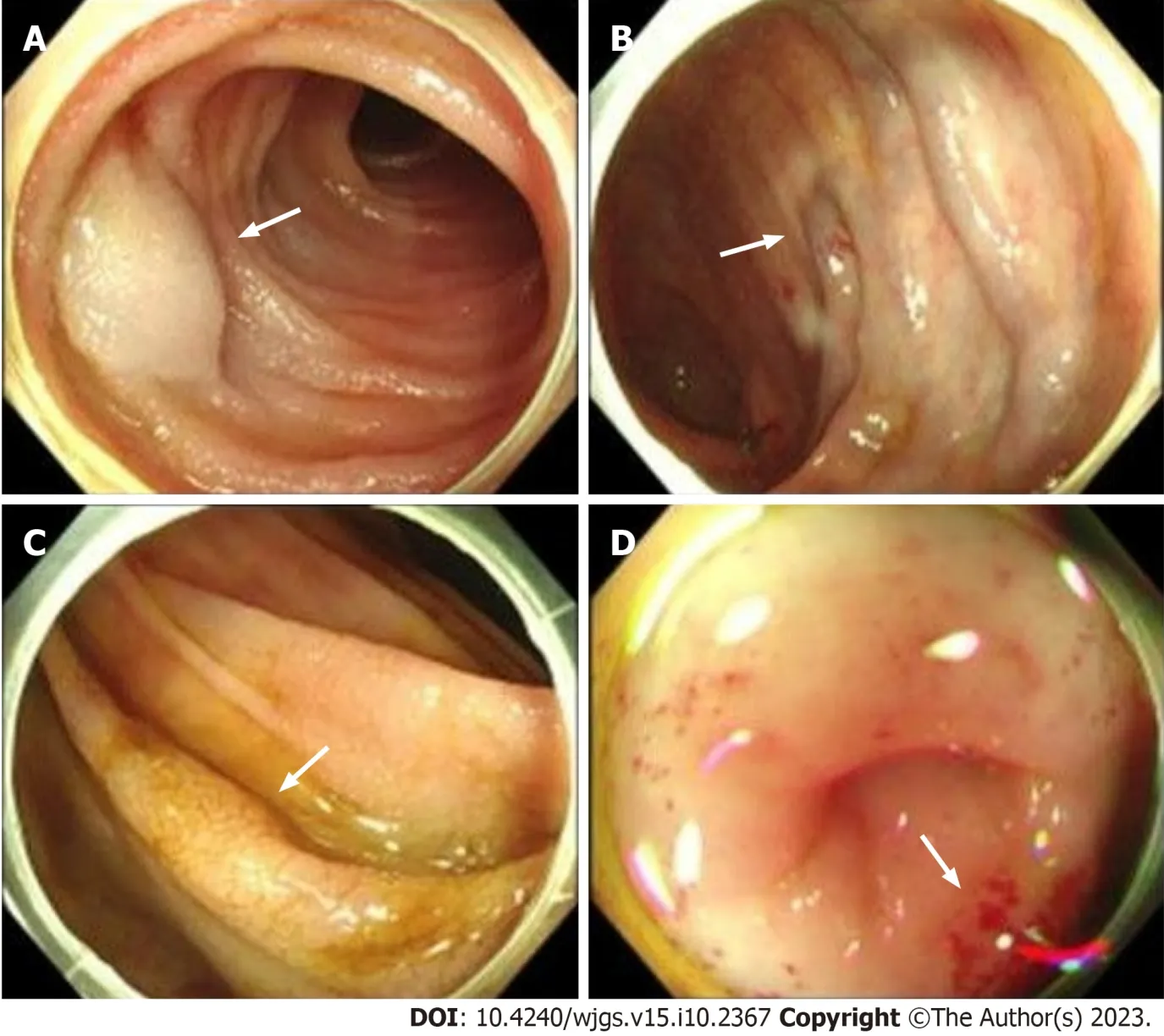
Figure 2 Endoscopy images. A: Aneurysm (arrows) on intestinal mucosa;B: Varicose vein (arrows) on intestinal mucosa;C: Cracked and scale-like mucosa(arrows) on intestinal mucosa;D: Scattered sites of bleeding sites (arrows) on intestinal mucosa.
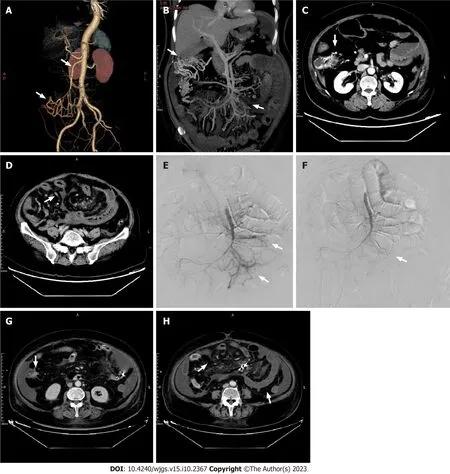
Figure 3 Pre-and post-procedure computed tomography images.A: Computed tomography (CT) angiography examination of abdominal blood vessels.The superior mesenteric vein (upper arrow),and tortuous blood vessels connecting with the superior mesenteric vein (lower arrow);B: Tortuous vessels (left arrow),and the superior mesenteric arteriovenous fistula (right arrow);C: CT examination.Tortuous vessels (arrow);D: Considerable edema of the intestinal segment receiving blood supply from the jejunal and ileal branches of the superior mesenteric artery (arrow);E: Two arteriovenous fistulas (arrows);F-H: After embolization,arteriovenous fistulas were not present (F),the range of tortuous vessels was decreased (G),and edema of the intestinal segment receiving blood supply from the jejunal and ileal branches of the superior mesenteric artery was relieved (H).
Physical examination
During the physical examination,capillary dilatation was identified in the patient’s lips.The patient’s abdomen was distended with tenderness in the lower left quadrant with no signs of rebound tenderness.Bowel sounds were 10 times per minute.No other identifiable abnormalities were found.
Laboratory examinations
The routine blood examination revealed hemoglobin of 115 g/L (normal range: 110-150 g/L),white blood cell count of 8.7× 109[normal range: (4-10) × 109],and 81.6% neutrophils (normal range: 50%-70%).A fecal occult blood test was positive.Coagulation function indexes showed varying degrees of decline.
Imaging examinations
A gastroscopy revealed scattered congestion and edema of the duodenal descending and transverse mucosa,while a colonoscopy showed a blue venous aneurysm or varicose in the ileum at 10 cm from the ileocecal valve.The cecum and ascending colon showed migrated veins and varicose veins (Figures 2A and B).The mucosa was cracked and scale-like(Figures 2C and D).Computed tomography angiography (CTA) of abdominal vessels revealed early mesenteric venous perfusion in the arterial phase that involved the right lower abdomen and left middle abdominal superior mesenteric veins.In the venous phase,there were tortuous vessels around the right upper abdominal intestine,which were connected to the superior mesenteric vein.Tortuous vessels were also visible in the posterior central abdomen (Figures 3A and B).CT showed patchy contrast enhancement in multiple parts of the drainage/supply area of the superior mesenteric artery,mainly involving the jejunal and ileal branches (Figures 3C and D).Whole-exome sequencing was conducted and a comprehensive analysis of the data was performed (Supplementary material).
FINAL DIAGNOSIS
Based on the above information,the patient was ultimately diagnosed with HHT.
TREATMENT
Due to the diffuse congestion and ischemia of the small intestine secondary to the superior mesenteric AVFs (SMAVFs),surgical intervention would involve resection of extensive intestinal segments,leading to adverse effects such as short bowel syndrome,anastomotic stenosis,and others.After being informed of these risks,the patient opted for interventional treatment.Therefore,selective embolization of the SMAVFs was performed.After careful evaluation,it was found that not all sites of the AVFs could be embolized during the selective procedure.If all fistula tracts had been occluded,the patient would have likely developed intestinal ischemic necrosis.Hence,the proportion of embolism had to be appropriate.As no similar cases existed,we selected the most severe shunt site for embolization (Figures 3E and F).
OUTCOME AND FOLLOW-UP
After the procedure,the patient experienced an improvement in abdominal pain,bloating,and diarrhea.An abdominal CT showed reduced intestinal wall edema with no abnormal perfusion at the embolization site (Figures 3G and H).One week later,the patient had recovered well and was discharged.One month after discharge,the patient was readmitted with abdominal pain and bloody stools.Remaining AVFs were identified and evaluated,and were believed to have caused secondary bowel necrosis.However,due to financial difficulties,the patient declined surgery and conservative treatment had to be adopted.One month later,the patient died from bowel necrosis and abdominal infection.
DISCUSSION
After searching various databases,we ultimately identified six cases similar to the one reported herein (Table 1).According to the Curaçao criteria,all six cases can be diagnosed as HHT[3].However,since these reports were from an earlier time period,considerable patient information was missing and no sequencing was performed,which is undoubtedly a significant loss.In this report,we performed whole exome sequencing on the patient and conducted a comprehensive analysis of the data (Supplementary material).Common gene mutations associated with HHT,including endoglin (ENG),activin receptor-like kinase 1 (ACVRL1),and soluble (cytosolic) malate dehydrogenase (SMDH),were not found in this case.This indicates that the patient had a unique gene mutation that had not been detected previously.
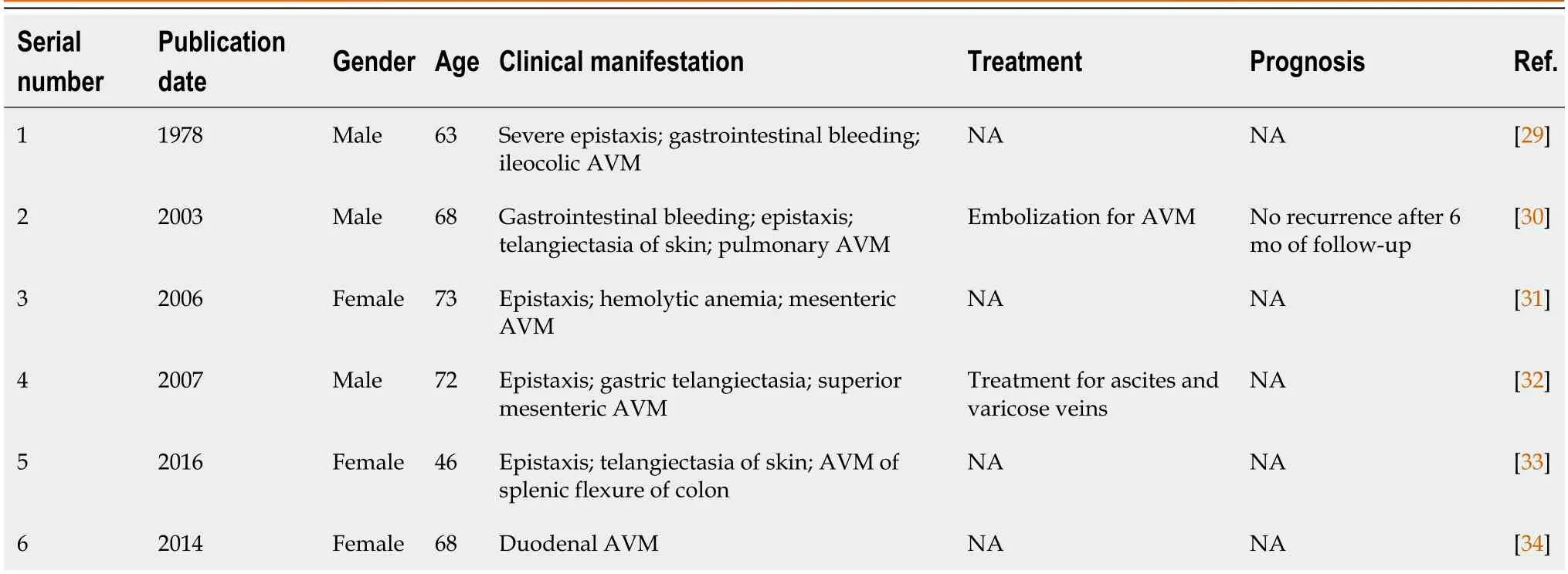
Table 1 Publications that describe similar cases
Internationally,the clinical diagnosis of HHT is mainly based on the Curacao criteria[3].These criteria have a broad application,especially for the identification of patients and non-patients[1].The development of genetic technology enables us to diagnose HHT more accurately,particularly for adolescents and children who have no clinical manifestations of epistaxis or visible telangiectasia but do have unknown vascular malformations[4].HHT can be divided into six common types based on different genotypes (Table 2).Primarily,sequencing methods of theENGandACVRL1genes are currently used[5-8].Although,a negative result in genetic testing cannot rule out the diagnosis of HHT,since many sporadic cases have unique gene mutations that are not one of the common variations.Many studies have found new variations in the above two gene loci.For example,in a study of a Canadian family,nine new mutation loci were found in theENGandACVRL1genes[5].In another investigation of a Chinese family,no significant mutation was found in theENG,ACVRL1,orSMDHgenes.After whole exome sequencing,new and unreported variations were found in the Nethylmaleimide-sensitive factor attachment protein gamma gene[9].In a genomic investigation conducted in the United Kingdom,comprehensive whole-genome sequencing uncovered a previously unknown heterozygous GDF2 sequence variation in all three affected individuals within an HHT family.Notably,gene screening results for ACVRL1,ENG,and mothers against decapentaplegic homolog 4 (SMAD4) yielded negative findings.In vitroexperiments provided compelling evidence that the identified mutation interfered with the normal protein cleavage process,subsequently contributing to the pathogenesis of HHT[10].Consequently,the pathogenic genes associated with HHT may extend beyond our current scope of comprehension.Further analysis is required to discover novel mutations after performing whole exomesequencing.In this case,the existing database only contains commonly reported gene mutations,making it easy to exclude new gene mutations through comparative analysis and ultimately leading to false negative results.To solve this problem,it is necessary to compare the gene sequencing data of multiple similar patients to better screen for meaningful variations.
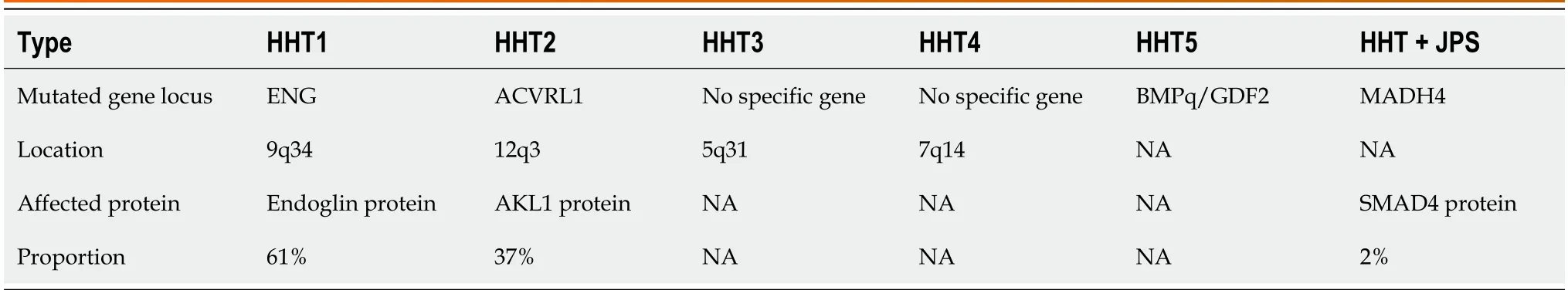
Table 2 Common genetic variants in hereditary hemorrhagic telangiectasia patients
The different genotypes of HHT can also lead to different clinical manifestations in patients.Studies have found that HHT1 is more prone to cerebral arteriovenous malformations,while HHT2 is more prone to hepatic arteriovenous malformations[11],and the age at diagnosis of HHT1 patients is generally young[12].A specific type of HHT caused by MADH4 mutations is usually accompanied by juvenile polyposis syndrome[13].Prior studies have suggested that theENG,ACVRL1,andMADH4genes all play a role in regulating cell signaling through the transforming growth factor beta(TGF-β) signaling pathway.The TGF-β family activates the ENG glycoprotein and ALK1 on the cell membrane,leading to changes in gene transcription levels within the cell nucleus that regulate cell proliferation,differentiation,migration,apoptosis,and secretion,ultimately affecting vascular structure and function[14].Furthermore,studies have found that clinical manifestations differ amongst patients within families,with vascular malformations only appearing in specific organs,suggesting that interpretation solely from a genetic variation perspective cannot fully explain the occurrence of HHT.Therefore,another hypothesis known as the “second strike” theory has been suggested[15].Firstly,heterozygous variations in the HHT gene lead to the loss of a single allele that encodes for endothelium,which is the first strike.Secondly,environmental or genetic factors provide the second strike.These two factors can also have a synergistic effect.The appearance of HHT clinical symptoms may be the result of multiple factors working together,controlled by complex molecular mechanisms.The cause of the mesenteric AVFs in this patient is currently unknown and requires further study.The “second-strike” hypothesis has been proposed for many years but previously lacked convincing evidence.However,with the advancement of sequencing technologies,Snellingset al[16] have successfully validated three hypotheses pertaining to the second-strike theory.These hypotheses involve the presence of telangiectasia containing a somatic mutation in the same gene as a germline mutation responsible for HHT,bi-allelic somatic and germline mutations,with both mutations leading to loss of function.This strong evidence has substantiated the plausibility of the two-hit hypothesis and provided a new direction for the development of HHT therapies.
Common clinical manifestations of HHT include epistaxis,cerebral vascular malformations,pulmonary arteriovenous malformations (PAVM),gastrointestinal bleeding,and hepatic arteriovenous malformations (Figure 4).HHT patients typically exhibit lower social index values for EuroQol-visual analogue scale scores,indicating a reduced quality of life compared to the general population.Notably,they attribute a substantial impact on their quality of life to epistaxis (nosebleeds),particularly among older patients[17].AVMs are present in 42.2% of patients,most of which are small.However,one-fifth of these AVMs rupture,and nearly half of these patients have related symptoms including headache,epileptic seizures,and focal neurological deficits[18].PAVM is present in as many as 49% of HHT patients.PAVM can cause lifethreatening events including massive bleeding from a ruptured PAVM,pulmonary hypertension,paradoxical embolism with stroke,and brain abscess[19].Approximately 30% of HHT patients experience gastrointestinal bleeding due to mucosal telangiectasias in the stomach,small intestine,or colon,with the main consequence being anemia,and the incidence increases with age[20].The probability of hepatic vascular malformations in HHT patients is 41%-78%[21].Among these patients,only 8% had clinical symptoms such as high-output heart failure,portal hypertension,or bile duct necrosis,which are all known clinical manifestations of HHT.Currently,the proportion of patients with mesenteric AVFs is unknown,which is a clinical manifestation that doctors may easily overlook.It is concerning that patients with this manifestation have poor gastrointestinal function and may suffer from serious gastrointestinal ischemia and hypoxia.If left untreated,mesenteric AVFs may seriously affect patient quality of life and even result in death.

Figure 4 Common clinical manifestations of hereditary hemorrhagic telangiectasia patients. AVM: Arteriovenous malformation;HHT: Hereditary hemorrhagic telangiectasia.
The most common treatment for anemia caused by gastrointestinal bleeding in HHT patients is iron supplementation or blood transfusion,which is not sustainable for long-term treatment.Currently,the treatment options for chronic gastrointestinal bleeding in HHT patients include medication and endoscopic treatment,but they cannot cure AVFs.Al-Samkari and Eng[22] presented several cases of drug therapy for complex HHT-related vascular malformations,all of which achieved favorable treatment outcomes.However,these cases involved more severe manifestations of common symptoms rather than rare clinical presentations similar to the present case.Therefore,the treatment has limited relevance for this particularly rare clinical scenario.The main problem with the patient described in this study was the SMAVF,as conventional treatments were not an option.AVFs are abnormal direct connections between high-pressure arteries and low-pressure veins[23].While AVFs are commonly found in the extremities,superior and inferior mesenteric AVFs are extremely rare[24,25].The incidence of a SMAVF is 0.09%,and the mortality rate is 39%-77%[25].Treatment methods for AVFs include interventional closure and surgery[26,27].If vascular interventional treatment is ineffective,the second-line treatment generally involves surgical ligation of the AVF or the removal of the portion of the jejunum most affected by the fistula[28].Although surgery was previously considered the most effective treatment method for SMAVF[27],with the development of interventional techniques and improvements in embolization materials,embolization has become the preferred treatment for SMAVF[28].Due to the broad range of AVFs in this patient and the many adverse consequences,such as short bowel syndrome and anastomotic stenosis,superior mesenteric artery embolization was chosen as the treatment for the SMAVFs in this case.After the procedure,the patient’s abdominal pain,bloating,and diarrhea improved.Unfortunately,the patient eventually died.Therefore,we believe that selective mesenteric artery embolization can lead to short-term benefits,but the long-term benefits require further.From this case,we found that clinical symptoms in HHT patients also include SMAVFs,which carries a high risk and should be treated appropriately.Furthermore,the gene mutations associated with this symptom do not appear to be the common mutations identified in previous studies.
CONCLUSION
The patient in this case did not exhibit a genetic mutation commonly associated with HHT.Furthermore,for patients with diffuse SMAVF,selective mesenteric arterial embolization may produce some positive outcomes.However,it is still unclear whether selective superior mesenteric arterial embolization is always advantageous in the long term or whether immediate surgery would be more beneficial in cases of intestinal necrosis.Researchers need to continue exploring new pathogenic mutations and the most appropriate treatment methods for rare HHT symptoms,to provide a basis for future prevention and diagnosis,and to deepen our understanding of HHT.
FOOTNOTES
Author contributions:Wu JL wrote the initial draft of the manuscript;Sun G and Wang SF provided critical analysis and review of the manuscript;Chen J,Zhang HW,Zhao ZZ,and Luan Z analyzed the patient’s whole genome sequencing data;Zhao YM,Li CY,and Jing YJ collected the clinical data;and all authors read and approved the manuscript.
Supported bythe Youth Independent Innovation Science Project of PLA General Hospital,No.22QNFC058.
Informed consent statement:Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jun-Ling Wu 0000-0002-6714-4549;Zhi-Zhuang Zhao 0000-0001-7759-0457;Jun Chen 0000-0001-6093-7207;Han-Wen Zhang 0000-0002-4554-1914;Zhe Luan 0000-0002-0003-1195;Cong-Yong Li 0000-0002-6820-9957;Yi-Ming Zhao 0000-0002-9441-7258;Yu-Jia Jing 0000-0002-7708-1510;Shu-Fang Wang 0000-0001-7032-3540;Gang Sun 0000-0001-8594-557X.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Gastric adenosquamous carcinoma with an elevated serum level of alpha-fetoprotein: A case report
- Giant dedifferentiated liposarcoma of the gastrocolic ligament: A case report
- Mucocutaneous ulcer positive for Epstein-Barr virus,misdiagnosed as a small bowel adenocarcinoma: A case report
- Three-dimensional computed tomography reconstruction diagnosed digestive tract perforation and acute peritonitis caused by Monopterus albus: A case report
- Postpolypectomy syndrome without abdominal pain led to sepsis/septic shock and gastrointestinal bleeding: A case report
- Bariatric surgery reduces colorectal cancer incidence in obese individuals: Systematic review and meta-analysis
