Value of cardiac magnetic resonance on the risk stratification of cardiomyopathies
2023-12-02RafaelVidalPerezMarianaBrandWaelZaherRubenCasadoArroyoAlbertoBouzasMosqueraRicardoFontesCarvalhoJoseManuelVazquezRodriguez
Rafael Vidal-Perez,Mariana Brandão,Wael Zaher,Ruben Casado-Arroyo,Alberto Bouzas-Mosquera,Ricardo Fontes-Carvalho,Jose Manuel Vazquez-Rodriguez
Abstract Cardiomyopathies represent a diverse group of heart muscle diseases with varying etiologies,presenting a diagnostic challenge due to their heterogeneous manifestations.Regular evaluation using cardiac imaging techniques is imperative as symptoms can evolve over time.These imaging approaches are pivotal for accurate diagnosis,treatment planning,and optimizing prognostic outcomes.Among these,cardiovascular magnetic resonance (CMR) stands out for its ability to provide precise anatomical and functional assessments.This manuscript explores the significant contributions of CMR in the diagnosis and management of patients with cardiomyopathies,with special attention to risk stratification.CMR’s high spatial resolution and tissue characterization capabilities enable early detection and differentiation of various cardiomyopathy subtypes.Additionally,it offers valuable insights into myocardial fibrosis,tissue viability,and left ventricular function,crucial parameters for risk stratification and predicting adverse cardiac events.By integrating CMR into clinical practice,clinicians can tailor patient-specific treatment plans,implement timely interventions,and optimize long-term prognosis.The noninvasive nature of CMR reduces the need for invasive procedures,minimizing patient discomfort.This review highlights the vital role of CMR in monitoring disease progression,guiding treatment decisions,and identifying potential complications in patients with cardiomyopathies.The utilization of CMR has significantly advanced our understanding and management of these complex cardiac conditions,leading to improved patient outcomes and a more personalized approach to care.
Key Words: Cardiac magnetic resonance;Cardiomyopathies;Prognosis;Dilated cardiomyopathy;Hypertrophic cardiomyopathy;Restrictive cardiomyopathy
INTRODUCTION
Cardiomyopathies encompass a diverse range of diseases affecting the heart muscle,each with varied causes.The European Society of Cardiology (ESC) traditionally categorizes them into hypertrophic,dilated,arrhythmogenic,restrictive,or other forms[1].Moreover,they are further classified as either familial/genetic or non-familial/non-genetic.We must highlight that this classification is highly discussed[2].
Symptoms of cardiomyopathies can manifest differently and change over time,necessitating regular evaluation through cardiac imaging techniques.These approaches play a crucial role in diagnosis,treatment guidance,and prognosis optimization.
Patient evaluation involves gathering medical history,conducting a physical examination,and performing an electrocardiogram (ECG).Transthoracic echocardiography (TTE) can raise suspicions of cardiomyopathy.To enhance the precision of anatomical and functional evaluation and obtain valuable prognostic insights,cardiovascular magnetic resonance (CMR) is typically employed.In some cases,nuclear medicine tests or cardiovascular computed tomography may also be necessary.
This manuscript aims to explore how the CMR contribute to the diagnosis and management of patients with cardiomyopathies.
CARDIOMYOPATHIES WITH DILATED PHENOTYPE
CMR plays a crucial role in the diagnosis and evaluation of dilated cardiomyopathies (DCM).We usually distinguish the DCM on the basis of the etiology between two groups,the non-ischemic DCM (NIDCM) and ischemic DCM (IDCM).
Non-ischemic dilated cardiomyopathy
CMR plays a crucial role in the diagnosis and management of NIDCM.NIDCM is characterized by left ventricular (LV)enlargement,systolic dysfunction,and myocardial fibrosis without significant coronary artery disease[3] and absence of other abnormal loading conditions like hypertension,valvular heart disease or congenital heart disease.CMR provides a noninvasive and accurate assessment of LV morphology,function,and remodeling[3].It allows for the quantification of myocardial fibrosis,which is useful in assessing viability in ischemic cardiomyopathy[4].CMR can also provide detailed and clinically useful information about the type and severity of cardiac damage by characterizing tissue changes in the myocardium[5].
One important application of CMR in NIDCM is the identification and characterization of fibrosis microstructure.Late gadolinium enhancement (LGE) imaging,a technique used in CMR,can detect enhancement patterns associated with fibrosis in NIDCM patients[6].Fibrosis microstructure has been found to modulate reentry in NIDCM,and understanding these variations can improve risk stratification and guide treatment decision[6].Computational modeling based on CMR images has been used to examine variations in fibrosis microstructure and quantify their effect on reentry inducibility and mechanism[6].This information can help identify patients at high risk of sudden cardiac death (SCD)and guide the selection of appropriate interventions[6].CMR is also valuable in differentiating NIDCM from other cardiomyopathies.CMR-derived myocardial parameters,such as total LV myocardial mass index and percentage of noncompacted myocardium,have been found to be discriminators between patients with LV non-compaction cardiomyopathy,other cardiomyopathies,and healthy controls[6].This differentiation is important for accurate diagnosis and appropriate management of patients with NIDCM[7].
Furthermore,CMR can provide prognostic information in NIDCM.Global longitudinal strain (GLS) of the left ventricle measured by CMR feature tracking (FT) analysis has revealed enhanced prognostic utility when compared to conventional parameters in NIDCM[8].Moreover,researchers have investigated the prognostic significance of right ventricular(RV) GLS through CMR-FT analysis has been evaluated in a cohort of individuals with NIDCM[8].These investigations collectively highlight the promising ability of CMR to predict significant cardiac events and events related to heart failure in patients with NIDCM[8].
In relation with sequences like T2-STIR,T1,T2 and Extracellular volume (ECV) mapping there is controversial data some experts state that T1 and ECV have limited value that is explained by the reduced precision in NIDCM due to thinning of the myocardium[9].Other authors have claimed some potential value of T1 and ECV,elevated ECV and T1 measurements have demonstrated prognostic significance regardless of LV ejection fraction (LVEF) and the presence of LGE[10].Moreover,an elevated native T2 value suggests the potential existence of myocardial edema,potentially indicating the presence of inflammatory cardiomyopathy[11].These methods present encouraging novel approaches for risk assessment;nevertheless,additional validation remains necessary.
In summary,CMR is playing a crucial role in the diagnosis,risk stratification,and prognostication of NIDCM.It provides valuable information about LV morphology,function,and remodeling,as well as the presence and characteristics of myocardial fibrosis.CMR can differentiate NIDCM from other cardiomyopathies and help guide treatment decisions.Additionally,CMR-derived parameters,such as GLS,have shown prognostic value in NIDCM.Overall,CMR is a valuable tool in the comprehensive evaluation and management of NIDCM patients.
Ischemic dilated cardiomyopathy
CMR plays a crucial role in the diagnosis and evaluation of IDCM.IDCM is a type of DCM that is caused by ischemic heart disease (IHD)[12].Approximately 70% of heart failure cases have been attributed to IHD[13].From the SOLVD study,IHD tended to have a greater impact than NIDCM,with double the risk of hospitalization and quadruple the risk of death[14].
CMR as we shown before this technique can aid in the differentiation of ischemic from non-ischemic cardiomyopathy subtypes.Currently,CMR-derived cardiac imaging is effective for both definition of IHD and for ischemia detection,with important diagnostic and prognostic implications[15].
The “function-perfusion-tissue characterization” triad should be studied in IHD for an adequate evaluation of cardiac viability and ischemic burden.As mentioned,the subendocardial distribution of LGE identifies an ischemic injury as opposed to fibrosis with meso-or subepicardial distribution,typical of non-ischemic alterations[16] CMR is also effective in defining myocardial viability through discrimination of LGE extension and segmental kinesis[4,17].
From the SPINS registry,extensive ischemic burden was related to a higher risk of major cardiac event,including hospitalization for congestive heart failure (HF),and revascularization was associated with a protective effect only in the extensive ischemia subset[18-21].
CARDIOMYOPATHIES WITH HYPERTROPHIC PHENOTYPE
LV hypertrophy (LVH) is most frequently caused by pressure overload.However,in cardiomyopathies,LVH occurs in the absence of abnormal loading conditions– hypertrophic cardiomyopathy (HCM) accounts for the majority of these cases.
CMR imaging has consolidated its role among the multimodality evaluation of myocardial disease,mostly due to high spatial resolution and unique ability for tissue characterization[22,23].Non-invasive tissue characterization is crucial for differential diagnosis of LVH,identification of HCM phenocopies and risk stratification.This distinctive feature of CMR has led to a decrease in the use of endomyocardial biopsy (EMB) in cardiomyopathies with LVH,that is now restricted to few indications[24,25].A recent position statement limited EMB use to patients with LVH in whom non-invasive evaluation produces inconclusive or discordant results,and there is clinical suspicion of phenocopies,particularly infiltrative or storage disease for which target treatment is available[25].
An Integrative CMR approach,Incorporating morphofunctional assessment with tissue characterization,including identification of the presence,location,and pattern of LGE,and combined with parametric mapping findings (particularly,native T1 and ECV),can be of value for differential diagnosis of hypertrophic phenotypes of cardiomyopathy(Table 1).
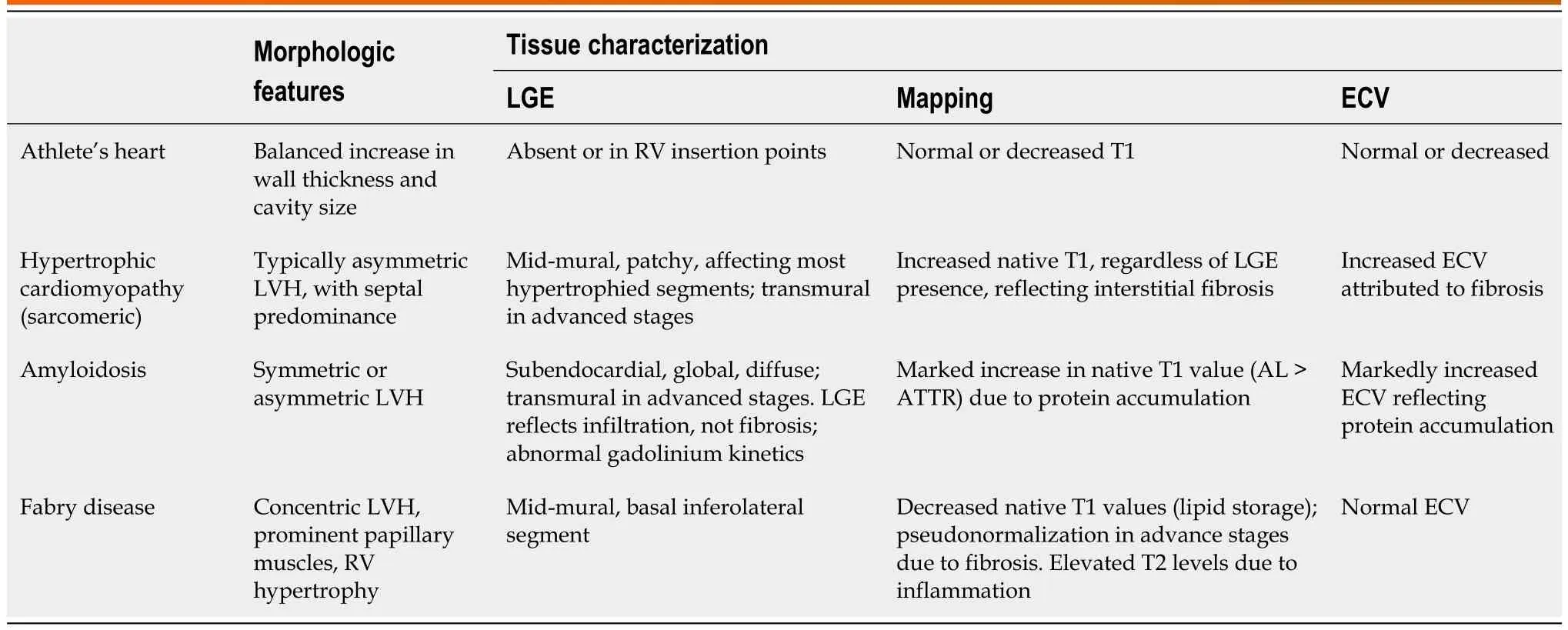
Table 1 Cardiac magnetic resonance findings in hypertrophic cardiomyopathy and phenocopies
Athlete’s heart
CMR is also useful for distinguishing pathological LVH from physiological adaption to exercise.The “athlete’s heart” is characterized by biventricular,symmetrical remodelling,and is associated to a concomitant and proportional increase in cavity size[26,27].Specific reference values of ventricular size and function for athletes have been reported by D’Ascenzietal[27] In athletes,the degree of hypertrophy is usually mild,and LV wall thickness rarely exceeds 12 mm[28].Moreover,reversal of adaptative LVH can be achieved with detraining[29].When present,in athletes,LGE is usually confined to the RV insertion points (mostly the inferior),and its presence has been correlated with training load and intensity[30].This pattern of fibrosis does not affect prognosis nor requires further evaluation in otherwise healthy athletes[30].Mapping data may further differentiate “athlete’s heart” from HCM: While the latter is usually associated with increased native T1 and ECV (reflecting interstitial fibrosis)[28,30],these parameters are normal,or even decreased,in athletes.Although echocardiography remains the major imaging tool for athlete pre-participation screening,CMR can be paramount in situations where suspicious of myocardial disease persists based on symptoms,family history,electrocardiographic or echocardiographic data.
HCM
HCM is defined by unexplained LVH in a non-dilated LV with wall thickness ≥ 15 mm or,alternatively,≥ 13 mm,in the presence of positive family history or a disease-causing gene variant[22].HCM,the most common genetic cardiovascular disease,with a prevalence of 1:200-1:500,is caused by sarcomeric gene mutations,that are inherited as an autosomal dominant trait[31].There is marked phenotypic heterogeneity among HCM probands,even among individuals from the same family,that occasionally poses a challenge in terms of diagnosis and risk stratification.
Morphofunctional evaluation:The “classical” HCM phenotype consists of asymmetrical,septal-predominant hypertrophy,that may be associated to dynamic LV tract obstruction (LVOTO)[31].In hypertrophied hearts,CMR enables precise measurement of maximal wall thickness and an accurate portrayal of LVH pattern,extent and distribution[28].This is of particular importance in the presence of midventricular or apical variants of HCM,in which echocardiographic evaluation encounters limitations[23].
The presence of apical aneurysms is associated to higher rates of ventricular arrhythmias (VA),SCD,thromboembolic events,and heart failure in patients with HCM[32,33].CMR has enabled more frequent identification of this high-risk subset of patients,by detecting small aneurysms that may remain unnoticed during non-contrast echocardiographic evaluation[23] but are still relevant for risk stratification.Accordingly,the presence of an apical aneurysm,regardless of size,has been considered a major risk factor by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines,assigning it a class IIa (level of evidence B) recommendation for implantable cardioverter-defibrillator(ICD) implantation for primary prevention of SCD[34].The ESC guidelines on SCD prevention have recently included LV apical aneurysm as an additional factor for consideration of an ICD (class IIb recommendation,level of evidence B)[35],even in patients with a low estimated risk according to the HCM Risk-SCD score[36].
Detection of thrombi within the scared LV apex also carries meaningful management considerations.In a recent study,Leeetal[37] found a linear relationship between aneurysm size and the risk of adverse events,including apical thrombus formation and thromboembolic stroke.Moreover,patients with an aneurysm size ≥ 2 cm showed a significant increase in 5-year SCD risk (9.7%vs2.9%,P=0.037)[37].
Other morphologic abnormalities related to HCM can be further demonstrated by CMR,including mitral subvalvular apparatus abnormalities or myocardial crypts.Maronetalreported the presence of myocardial crypts– narrow,deep blood-filled invaginations within LV myocardium– in 61% of genotype positive/phenotype negative (G+P-) patients without overt LV hypertrophy,suggesting this morphologic feature to be part of the phenotypic expression of HCM[38].Contrastingly,in a large Danish cohort assessed by computed tomography,LV crypts were frequent among the general population,and were not associated with a composite endpoint of death,myocardial infarction,heart failure,or stroke[39].However,among family members of patients with HCM,the presence of crypts may prompt careful follow-up to monitor progression to an overt phenotype.
Another subclinical marker of HCM observed by CMR has been proposed by the same group– LV apical-basal muscle bundles.LV muscle bundles were suggested as a latent marker in G+/P-individuals,and were related to HCM phenotypic expression,irrespective of LV wall thickness[40].
CMR is useful for depicting papillary muscle (PM) architecture and functional abnormalities.PM hypertrophy (minor axis diameter >11 mm or combined mass >7 g/m2) is present in more than half of HCM cases,and my contribute to midventricular obstruction[41,42].Additional abnormalities that contribute to LVOTO,such as accessory,bifid or displaced PM,can be adequately demonstrated by CMR[41].
Tissue characterization:The presence of LGE in HCM reflects replacement fibrosis,and its prognostic value is wellestablished[43].LGE is found in more than half of HCM patients,usually presenting an mid-mural pattern within the most hypertrophied segments[43,44].In advanced stages of the disease,LGE with transmural extension can be observed and carries a worse prognosis[44].
LGE has been consistently associated increased SCD incidence,and its presence and extent was included as a major risk factor in the ACC/AHA risk stratification algorithm[34] and,more recently,in the 2022 ESC Guidelines for prevention of SCD[35].In a landmark multicenter study,LGE exceeding 15% of the LV mass was associated with a >2-fold risk of SCD in patients who were deemed low risk by conventional tools,compared with patients in whom LGE was absent[43].Therefore,presence of “extensive LGE” (≥ 15% of total LV mass) is regarded as a high-risk parameter,and in HCM patients without a defibrillator,CMR should be repeated every 3-5 years to monitor LGE progression and reconsider SCD prevention strategies[23,34].
T1 mapping and ECV (derived from native and post-contrast T1) allow for identification of diffuse,interstitial fibrosis[23].Native T1 and ECV may be elevated in segments without LGE,and even in variant carriers without overt LVH[28].Mapping techniques allow differentiation of HCM from phenocopies (Table 1).
Edema with abnormal T2 findings (T2-Stir) could be observed in HCM patients often indicative of an acute myocardial injury (i.e.,ischemic extravascular damage) and associated with electrical instability[45].
Perfusion:Microvascular dysfunction is part of the pathophysiology of HCM and can be evaluated by means of CMR perfusion imaging.In HCM,reduced myocardial blood flow correlates with increased LV wall thickness and mass,presence of LGE,and increased ECV[23,28].Aguiar Rosaetal[46] showed that increased ischemia severity,assessed by CMR,was associated with higher values of native T1 and greater LGE extension.Patients with severe ischemia demonstrated higher incidence of atrial arrhythmias and performed poorer in cardiopulmonary stress testing[46].
HCM phenocopies
CMR has an increasing role in the evaluation of rare forms of myocardial disease that also manifest with LVH,otherwise known as phenocopies of HCM.In such cases,family history,electrocardiographic patterns and extracardiac manifestations may raise diagnostic suspicion,that may be corroborated by imaging findings.
Amyloidosis:Cardiac amyloidosis (CA) produces LV “pseudo-hypertrophy”,resulting from interstitial expansion due to amyloid fibrils deposition,rather than from myocyte proliferation[47].Transthyretin (ATTR),both hereditary and wildtype,and immunoglobulin-derived light chain amyloidosis are responsible for most cases of amyloid-related myocardial disease[48].Extracellular expansion in CA is depicted in CMR parametric mapping findings by a marked increase in native T1 Levels and ECV[28].Patients with CA show global,diffuse subendocardial LGE,that may become transmural in more advanced stages of the disease.This pattern of LGE,in the adequate setting,is very specific for CA[49].LGE not rarely extends to the RV and the atria,particularly in ATTR[28].Another characteristic CMR feature in CA is the abnormal gadolinium kinetics,with myocardial nulling preceding with the blood pool,or an equalization of these points[48,49].More detailed information will be provided in the section entitled cardiomyopathies with restrictive phenotype.
Fabry disease:Fabry disease (FD),an X-linked lysosomal storage disorder,usually leads to concentric LVH,due to both glycosphingolipid accumulation and myocyte hypertrophy[50].Prominent PM s are a typical feature of FD,as is concomitant RV hypertrophy[28,44].
Parametric mapping is of particular utility for the differential diagnosis of FD.Native T1 decreases with lipid deposition[22];accordingly,in early stages of FD,native T1 values are low,when compared to normal reference values and other forms of LVH[28,50].However,as disease progresses and replacement fibrosis becomes evident,pseudo-normalization of native T1 relaxation times occurs[28,50].ECV remains within normal range in LGE-free areas[50],since FD leads to intracellular storage of glycosphingolipids.T2 values can be elevated due inflammatory response triggered by lipid accumulation.
LGE is present in >50% of FD patients,and is usually located in the LV basal inferolateral segment,with a mid-mural or subepicardial pattern[22,44,49].Presence of LGE has been reported in female mutation carriers without LVH[49].Similar to HCM,presence of LGE in FD is associated to adverse outcomes and poor response to replacement therapy[50].
CARDIOMYOPATHIES WITH RESTRICTIVE PHENOTYPE
Less than 5% of all cardiomyopathy cases are attributed to restrictive cardiomyopathies (RCM),which have a diverse range of causes[51].
RCM is characterized by a significant alteration in myocardial compliance,presenting severe diastolic dysfunction while maintaining preserved systolic function,especially in the early stages.The initial diagnosis typically involves a TTE that reveals normal or increased LV wall thickness,often with a concentric or symmetric distribution,along with a restrictive pattern observed through Doppler analysis.It also shows the absence of LV dilation,preserved LVEF,and significant biatrial enlargement[52].However,while TTE plays a crucial role in the initial assessment and raising diagnostic suspicions,its utility becomes limited when establishing a differential diagnosis.In such cases,CMR imaging becomes highly relevant.
Two of the most common entities where CMR is essential are the endomyocardial fibrosis (EMF) and CA.
EMF
EMF represents a rare subtype of RCM.It is characterized by an unusual thickening of the endocardium,resulting from the deposition of fibrous tissue[53].This condition is typically secondary to various factors,including infections (often found in tropical regions),inflammation,exposure to toxic agents,among others.Echocardiographic observations in EMF include apical obliteration due to thickening of the endocardium,a reduction in ventricular cavity size,and a pronounced restrictive diastolic pattern.EMF can primarily affect the left ventricle,both the left and right ventricles (in approximately 50% of cases),or predominantly the right ventricle[54].The presence of apical thrombus is also a frequently encountered feature.
CMR is the gold standard for EMF evaluation and specifically for localization,characterization,and quantification of fibrous tissue by LGE sequences.LGE strongly correlates with histopathological findings and its extension is associated with increased mortality risk[55].CMR may also identify apical thrombus or calcifications.
CA
In patients with CA,cine sequences or functional assessment methods provide a means to observe the structural characteristics of the infiltrated myocardium.These characteristics encompass biventricular hypertrophy,thickening of cardiac valves,interatrial septum,pericardial effusion,and biatrial dilation.Additionally,these techniques enable the precise evaluation of both systolic and diastolic function[56].It is essential to not only focus on the assessment of the LV but also on the other cardiac chambers.Notably,the involvement of RV has been identified as a predictor of mortality in CMR,consistent with findings from TTE[57].As the disease advances into later stages,there is a notable increase in atrial volume and dysfunction.This phenomenon is attributed to the direct infiltration of amyloid fibrils into the atria and indirectly to elevated filling pressures due to diastolic dysfunction.
The Table 2 summarizes the main cardiac magnetic resonance (CMR) findings in CA with an explanation of the prognostic and diagnostic implications.
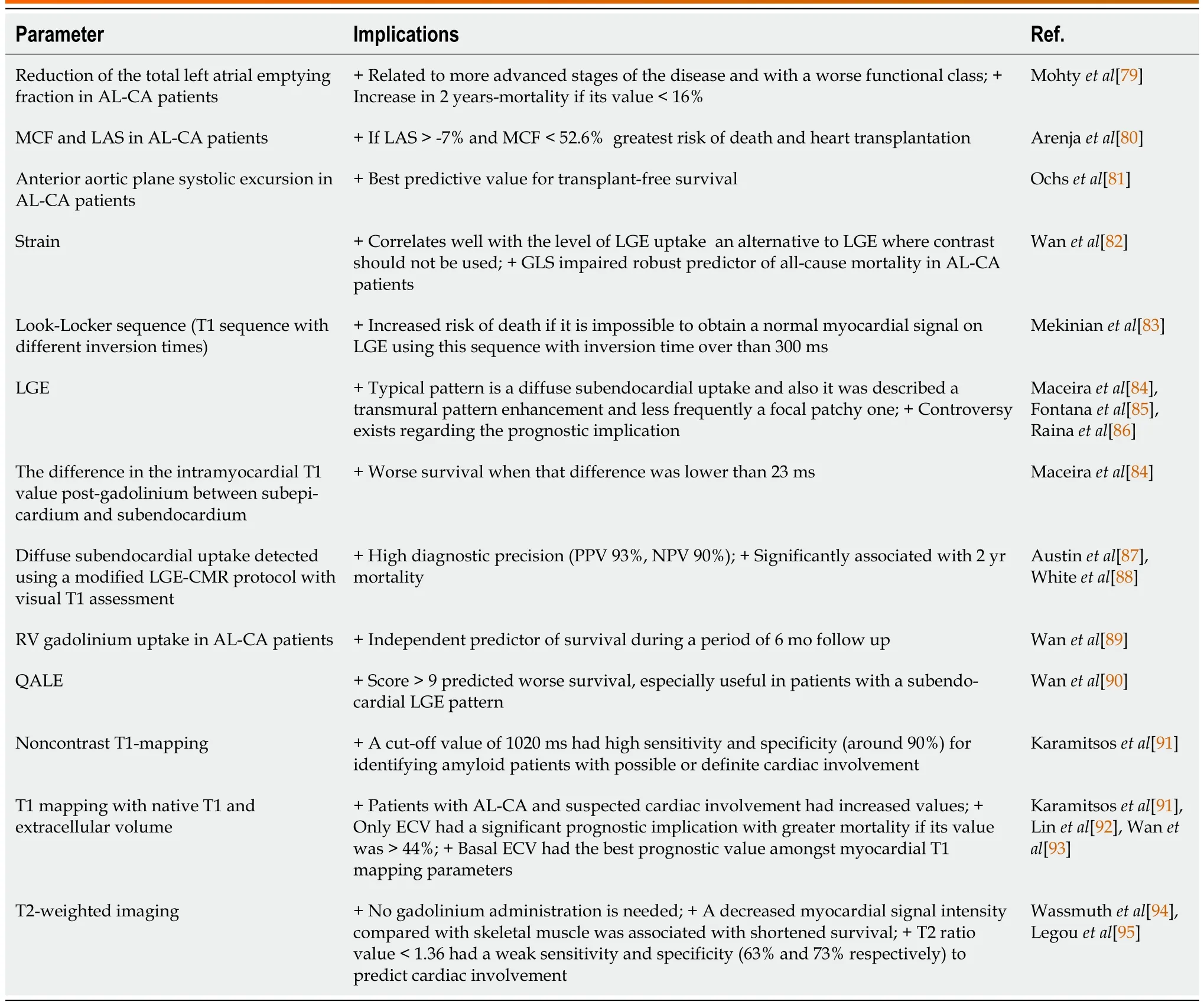
Table 2 Cardiac magnetic resonance findings in cardiac amyloidosis.Prognostic and diagnostic implications
To sum up,CMR represents a complementary diagnostic step in the evaluation of patients suspected of having CA.This imaging method is not widely accessible in numerous medical facilities,and its lengthy duration per study restricts the total number of examinations feasible in a day.
ARRHYTHMOGENIC CARDIOMYOPATHY
Arrhythmogenic cardiomyopathy (ACM) is an inherited cardiomyopathy characterized by replacement of myocardium by fatty and fibrous tissue.Historically named Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) because of the RV involvement,the late recognition of left and biventricular forms led to the new term ACM,encompassing both phenotypes.VA through macro-reentry mechanism related to the fibrofatty involvement is one of the main clinical presentations,manifesting in the worst case by SCD.In the advanced stages,the disease is characterized by heart failure[58,59].
Diagnosis criteria established by the International Task Force (ITF) included morphological (dysfunction and structural alteration) and anatomopathological characterization,ECG abnormalities,history of arrhythmias and family history[60].CMR role was limited to evaluation of regional RV motion wall,RV ejection fraction (RVEF) and RV end-diastolic volume.Critics have been raised about the absence of LV involvement and the limited role of CMR.In 2020,the Padua Criteria was proposed,revisiting the ITF criteria by including tissue characterization provided by CMR.Functional or structural abnormality is enough for the diagnosis[61].Pathogenic mutations,ECG abnormalities,or VA are no longer sufficient.This highlights the role of CMR,which has become one of the preferred non-invasive imaging techniques,allowing an increase of diagnostic sensitivity for ACM.CMR offers an advanced evaluation of the heart,including ventricular morphology,volume,thickness,ejection fraction,regional motion,myocardial fibrous,adipose content,edema,flow,LGE,as well as new emerging and promising feature as global longitudinal and circumferential strain(assessed by using feature-tracking CMR).Despite its complete evaluation,CMR has some limitations: fatty infiltration is nonspecific and does not preclude the diagnosis.
Prognosis in ACM is mostly related to VA.ICD being the only intervention improving survival[62],risk stratification is vital to identify the high-risk patients benefiting the most from primary prevention ICD implantation.RV dysfunction and syncope history are strong predictor for arrhythmia event and have been both included in the guidelines.CMR is not included in the risk stratification recommendations,neither in the 2019 Heart Rhythm Society guidelines[63],nor the 2022ESC guidelines for the management of patients with VA and the prevention of SCD[35].In this context,CMR role is only limited to diagnosis.A 2019 consensus expert developed the ARVC 5-year Risk-VAs calculator: A prediction model for VA risk to guide decision regarding primary prevention ICD (www.arvcrisk.com)[64].CMR is included only to assess RVEF.CMR parameters of tissue characterization and regional wall motion of both ventricles were not included.The role of CMR in risk stratification remains to be determined.Lack of consistent studies explains the absence of CMR from the risk stratification recommendation,even though some emerging data shows the potential prognosis information provided by CMR.
Different CMR phenotype of ACM are associated with different prognoses.Normal CMR has an excellent negative predictive value for major clinical events[65-67].Tandrietal[68] showed that delayed gadolinium enhancement of RV correlates with inducible VT during electrophysiology testing.Lieetal[69] confirm that CMR findings,as low RVEF,RV wall-contraction abnormalities,or RV aneurysms are predictors of life-threatening ventricular arrhythmia.Evaluation of longitudinal strain by feature-tracking CMR could also bring risk stratification information,as reduced strain seems to be associated with sustained VA[70].
Regarding the LV involvement,its association with adverse outcomes is inconsistent[71].Presence of LV dysfunction is associated with arrhythmic adverse outcomes as reported by a small European registry[72].Some studies have suggested that CMR imaging features of LV phenotypes,as fat infiltration and LGE,in ACM may be associated with adverse outcomes.Aquaroetal[66] highlight that the different CMR presentations of ACM are associated with different prognoses,LV involvement (LV dominant and biventricular) being the worst prognosis.Zhangetal[73] confirm the bad prognosis of LV LGE,being associated with an increased risk of ICD therapy and cardiac death,independently of LVEF.LV myocardial assessment by CMR could also predict the HF-related event risk as reported by Chunetal[74].On the contrary LV dysfunction was not a predictor of arrhythmic risk in two meta-analysis[75,76].Zghaibetal[77] showed that LV fibrofatty infiltration in CMR was not associated with arrhythmic outcomes.The role of identification of LV involvement by CMR and its prognosis significance remains to be established.
There is some potential speculation on some cases of ACM that could be explained by an inflammatory activity or hot phase,if the diagnosis it is in a very early-stage sequences like T2-STIR for oedema detection could help[78],this hot phase could be related with arrhythmias during the disease.
To finalise,CMR role in diagnosis is well established (Figure 1).Regarding the risk stratification,only RV function is validated in international guidelines and risk calculator.Lack of consistent data about correlation between CMR characterization and adverse outcomes could explain the absence of CMR role from guidelines,but a few emerging studies show new evidence about CMR imaging,Indicating that the presence of structural abnormalities in the RV as observed through CMR plays a crucial role in evaluating the risk of arrhythmias.: RV dilatation,dysfunction and LGE are among the strongest predictors.Regarding the LV involvement,the few data are contradictory,but may trend towards an association with high-risk event.CMR may have a promising role in association with classical clinical feature,but further studies are needed to better define CMR place in risk stratification.
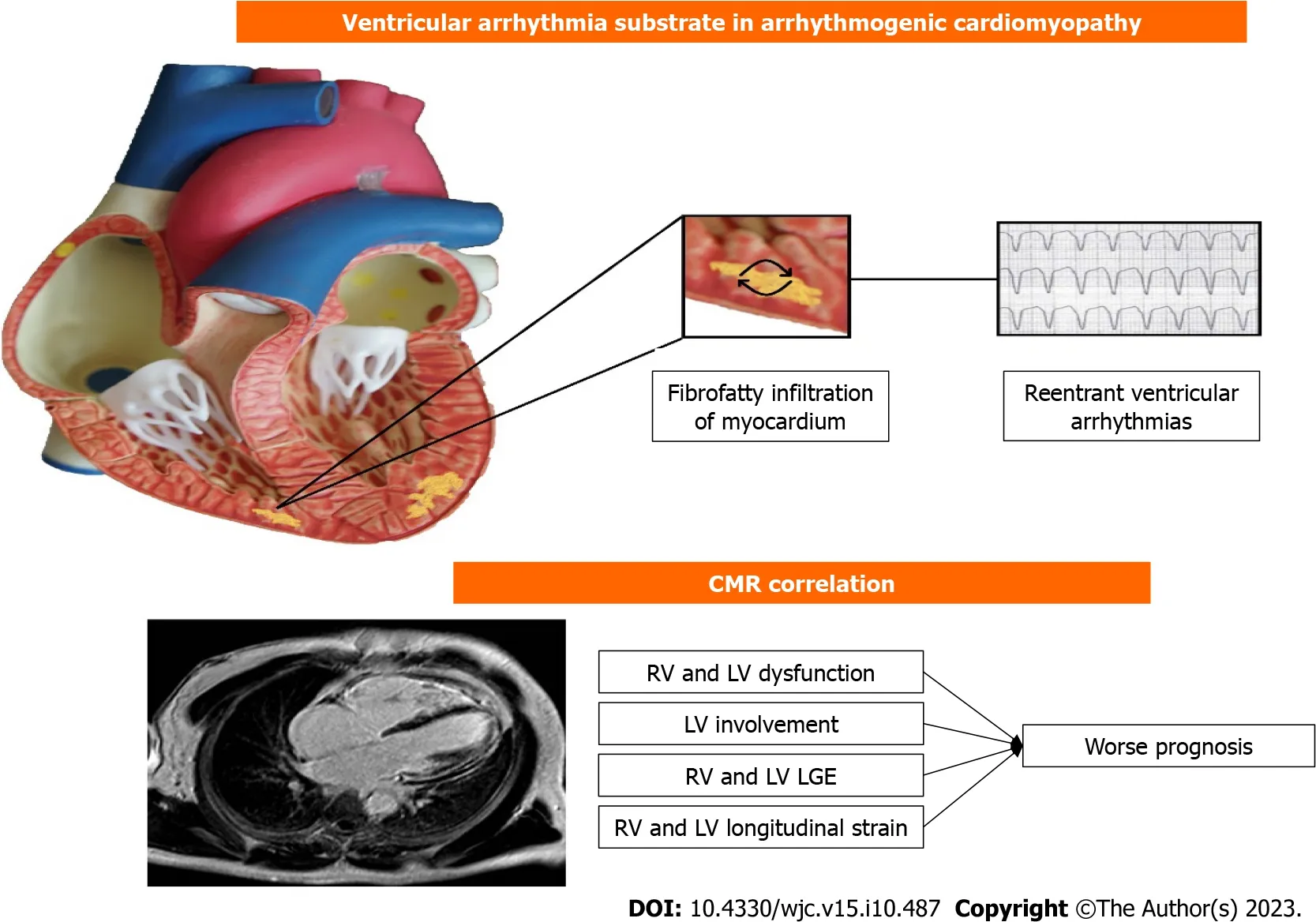
Figure 1 Correlation between cardiac magnetic resonance and ventricular arrythmia in arrhythmogenic cardiomyopathy. CMR: Cardiac magnetic resonance;RV: Right ventricle;LV: Left ventricle;LGE: Late gadolinium enhancement.
CONCLUSION
Cardiovascular imaging methods play a vital role in investigating cardiomyopathies,furnishing valuable diagnostic and prognostic insights.The inclusion of CMR in the evaluation of all patients is highly recommended,owing to its ability to offer comprehensive anatomical,functional,and tissue-specific data,which holds significant prognostic value.While other imaging techniques might be employed selectively,the integration of multiple modalities of cardiac imaging assumes a crucial role in clinical decision-making,leading to enhanced patient management and care outcomes.
FOOTNOTES
Author contributions:Vidal-Perez R designed,edited and wrote the final paper;Brandão M,Zaher W,Casado-Arroyo R,Bouzas-Mosquera A,Fontes-Carvalho R performed the collection of the data and helped in writing the original draft;Vidal-Perez R and Vazquez-Rodriguez JM contributed to the critical revision of the paper.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Spain
ORCID number:Rafael Vidal-Perez 0000-0001-9944-8363;Mariana Brandão 0000-0001-9913-0435;Wael Zaher 0000-0002-0537-9262;Ruben Casado-Arroyo 0000-0002-3876-6074;Alberto Bouzas-Mosquera 0000-0002-2741-732X;Ricardo Fontes-Carvalho 0000-0003-2306-8393;Jose Manuel Vazquez-Rodriguez 0000-0003-0888-6937.
S-Editor:Lin C
L-Editor:A
P-Editor:Yuan YY
杂志排行
World Journal of Cardiology的其它文章
- Candida endocarditis: Update on management considerations
- Systemic right ventricle complications in levo-transposition of the great arteries: A case report and review of literature
- Do cardiopulmonary resuscitation real-time audiovisual feedback devices improve patient outcomes? A systematic review and metaanalysis
- Cardiovascular complications following medical termination of pregnancy: An updated review
- Establishment of a prediction model for prehospital return of spontaneous circulation in out-of-hospital patients with cardiac arrest
- Integrated analysis of comorbidity,pregnant outcomes,and amniotic fluid cytogenetics of fetuses with persistent left superior vena cava
