Effectiveness of BNT162b2 and CoronaVac vaccines against omicron in children aged 5 to 11 years
2023-12-02EduardoOliveiraMariaChristinaOliveiraAnaCristinaSimesSilvaEnricoColosimoRobertMakMarianaVasconcelosLudmilaSilvaDaniellaMartelliClaraPinhatiHerclioMartellinior
Eduardo A.Oliveira · Maria Christina L.Oliveira · Ana Cristina Simões e Silva · Enrico A.Colosimo ·Robert H.Mak · Mariana A.Vasconcelos · Ludmila R.Silva · Daniella B.Martelli · Clara C.Pinhati ·Hercílio Martelli-Júnior
Abstract Background This study aimed to estimate vaccine effectiveness (VE) against omicron variant infection and severe corona virus disease 2019 (COVID-19) in children aged 5–11 years hospitalized with acute respiratory syndrome.Methods A test-negative,case–control analysis was conducted from February 2022 to June 2022.We enrolled 6950 eligible children,including 1102 cases and 5848 controls.VE was calculated after immunization with one and two doses of BNT162b2 or CoronaVac.The outcomes were hospitalization with acute respiratory symptoms and detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19.The adjusted odds ratio for the association of prior vaccination and outcomes was used to estimate VE.Results For fully vaccinated children,the overall estimated VE against hospitalization with SARS-CoV-2 infection was 42%[95% confidence interval (CI) 26 to 54].VE peaked at 29–42 days (67%,95% CI 40% to 82%) and then declined to 19%(95% CI,− 20% to 45%) at 57–120 days after the second dose.The BNT162b2 vaccine had a similar VE against hospitalization with SARS-CoV-2 infection (45%,95% CI,20 to 61) compared to the CoronaVac vaccine (40%,95% CI,17% to 56%).Among cases,56 (5%) children died;53 (94.6%) were not fully vaccinated.For cases,the two-dose schedule effectiveness against ICU admission,need for invasive ventilation,severe illness,and death were 10% (95% CI,− 54%–45%),22% (95% CI − 70%–68%),12% (95% CI,− 62%–52%),and 16% (95% CI,− 77%–75%),respectively.Conclusions For hospitalized children aged 5–11 years during the omicron-predominant period in Brazil,two doses of both vaccines had moderate effectiveness against hospitalization with acute respiratory symptoms and SARS-CoV-2 infection and offered limited protection against endpoints of COVID-19 severity.
Keywords Children · COVID-19 · Effectiveness · Omicron · Vaccine
Introduction
Coronavirus disease 2019 (COVID-19) vaccination has substantially altered the course of the pandemic,saving tens of millions of lives globally [1,2].In the preliminary report of trials,messenger RNA vaccines against COVID-19 were found to be safe,immunogenic,and efficacious for children 5–11 years of age [3–5].However,as the pandemic evolved,variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged and raised concerns about the immunogenicity of vaccines [6].Case–control studies reported a substantial reduction in vaccine effectiveness(VE) against symptomatic SARS-CoV-2 infection and severe outcomes (hospitalization or death) in adolescents during the omicron-predominant period [7–11].Recent studies have demonstrated that BNT162b2,in the omicron-predominant period,provided moderate protection against SARS-CoV-2 infection and COVID-19-related hospitalization in children aged 5–11 years [12,13].Nevertheless,all published studies to date were performed in developed countries with messenger RNA vaccines [8,9,14].Estimating the effectiveness of a low-cost vaccine against SARS-CoV-2 variants in young children in developing countries can provide information for implementing public health interventions targeting children in these regions.
The Brazilian National Health Surveillance Agency(ANVISA) issued an emergency use authorization for the BNT162b2 messenger RNA vaccine (Pfizer-BioNTech)for children from 5 to 11 years of age on December 16,2021.In addition,children between 6 and 11 years were also eligible to receive the CoronaVac vaccine.The vaccination campaign started on January 21,2022,for this age group.According to the Brazilian COVID-19 official report,by July 14,2022,among children aged 5–11 years,about 77.8% had received at least one dose of the vaccine,and 49.6% had completed the primary cycle of two doses [15].In this case–control test-negative study,we aimed to estimate the effectiveness of the BNT162b2 and CoronaVac vaccines in children (5 to 11 years) hospitalized with acute respiratory syndrome during the period of omicron dominance in Brazil.The secondary aim of the study was to evaluate vaccine protection against severe illness in children with laboratoryconfirmed SARS-CoV-2 infection.
Methods
Study design
We used a test-negative case–control design to estimate VE against COVID-19 in children aged 5–11 years hospitalized with acute respiratory syndrome enrolled in the Influenza Epidemiological Surveillance Information System (SIVEPGripe) in Brazil.
Data sources
We performed an analysis of all children 5–11 years of age registered in the SIVEP-Gripe from February 2022 to June 2022.Data were extracted from the SIVEP-Gripe database on June 27,2022.The SIVEP-Gripe database was established by the Brazilian Ministry of Health (MS) in 2009 to monitor severe acute respiratory syndrome (SARS) cases.In 2020,the surveillance of COVID-19 was incorporated into the system network.Case notification is mandatory for the entire country,including private and public hospitals,and the system is updated weekly.To enter the SIVEP-Gripe database,the case must have a flu-like syndrome and at least one of the following criteria: dyspnea or respiratory distress or oxygen saturation less than 95% in room air or cyanosis or symptoms specific for infants (intercostal retractions,nasal flaring,dehydration,and inappetence).In 2021,the vaccination status of registered cases was incorporated into the SIVEP-Gripe database.In Brazil,the MS is the only provider of COVID-19 vaccines,and private and public healthcare providers must notify suspected COVID-19 cases and hospitalizations;therefore,all Brazilians treated in a health system and hospitalized with COVID-19 must be registered in the database.Detailed information regarding this database,including reporting form and data dictionary,codes,and all deidentified data,are publicly available at https:// opend atasus.saude.gov.br/ datas et.Additional information regarding SIVEP-Gripe and the data retrieval steps are provided in Supplementary material 1.
Study population
Cases were defined as those with positive quantitative real-time quantitative PCR (RT-qPCR) or antigen tests for SARS-CoV-2 infection.The controls were defined as children hospitalized with an acute respiratory syndrome in the same period and with a negative SARS-CoV-2 test.Detailed information about the selection of the cases and controls is shown in Fig.1.
Exposure of interest
The primary exposure of interest was vaccination status.Vaccination status was categorized at the time of the onset of the symptoms as unvaccinated (no vaccine dose or symptom onset 0–13 days after the first dose),partially vaccinated (symptom onset 14 days or more after the first dose or 0–13 days after the second dose),or fully vaccinated(14 days or more after the second dose).
Covariates and definitions
Clinical and demographic data recorded in SIVEP-Gripe are described in detail elsewhere [16,17].The first case of COVID-19 attributed to the omicron variant in Brazil was reported on December 1,2021.By the week ending December 25,2021,Omicron was already the predominant circulating variant in the country (https:// outbr eak.info/ locat ion-repor ts?loc=BRA).According to genotype surveillance data in Brazil,during the time frame of our study,the prevalence of the omicron variant ranged from 95% to 100% from the last week of February 2022 to June 2022 [18].Additional information about data preparation,covariate definitions,and codification is provided in Supplementary material 3.
Outcomes
We evaluated two outcomes among children hospitalized with acute respiratory symptoms: detection of SARSCoV-2 infection and the incidence of severe COVID-19 in cases with proven SARS-CoV-2 infection.The following endpoints were considered for the analysis of VE against severe outcomes: admission to the intensive care unit (ICU),need for respiratory support,and in-hospital mortality.Additionally,we created a composite outcome,combining the same indicators above into a single index.For this composite outcome,cases were classified as mild (no need for oxygen support and no ICU stay),moderate (need for noninvasive oxygen support and no ICU admission),and severe (admission to ICU,mechanical ventilation,or death).Information regarding the definitions of the endpoints is shown in the Supplementary material 3.
Statistical analysis
For the descriptive statistical analysis,we used medians and interquartile ranges to summarize continuous variables and calculated frequencies and proportions for categorical variables.The Chi-square and the Mann–WhitneyUtest were used for the comparisons of proportions and medians.
The analysis of VE was carried out in two steps.First,the odds of vaccination in children with laboratory-proven SARS-CoV-2 infection were compared with the odds in children who tested negative for SARS-CoV-2.VE against COVID-19–associated hospitalization was estimated using binary logistic regression,comparing odds ratios of vaccination status in cases compared with controls using the following equation: vaccine effectiveness=100×(1 − odds ratio).Vaccination status was included as an independent variable (fully vaccinated vs.partially vaccinated vs.unvaccinated).All models were adjusted by age,sex,ethnic group,geographic macroregion,and presence of comorbidities [19].First,we evaluated the overall VE and then also the VE stratified according to the age group(5–7 years vs.8–11 years) and immunization schedule(BNT162b2 or CoronaVac).Using the same multivariable regression model,we also assessed the possible loss of immunity provided by the pediatric vaccination against SARS-CoV-2 infection at 14-day intervals from the first dose of vaccine (15–28 days,29–42 days,and 43–84 days).Using the one minus the Kaplan–Meier survival function estimate,we assessed the possible loss of immunity provided by the vaccination by estimating the cumulative incidence of cases over time after the first dose of the vaccine [20].
In the second step of the analysis,we assessed the VE against severe illness in cases with laboratoryproven COVID-19.For this analysis,life-supporting interventions,disease severity,and in-hospital mortality were included as dependent variables in separately constructed models using the binary regression logistic method.Vaccination status was included as an independent variable.For this analysis,we considered the vaccination status as fully vaccinated versus partially vaccinated/unvaccinated cases due to the smaller sample size.All models were also adjusted by age,sex,ethnic group,geographic macroregion,and presence of comorbidities.The results are expressed as vaccine protection (%) using the same formula previously described=100×(1 − adjusted odds ratio) and their 95% confidence intervals (CIs).
Ethical aspects
We accessed data in SIVEP-Gripe,which are already deidentified and publicly available.Therefore,following ethically agreed principles on open data,this analysis did not require ethical approval in Brazil.
Results
Study population
In the time frame of the study,193,425 individuals met the criteria to be included in the SIVEP-Gripe database.Of them,11,346 children aged 5–11 years were initially eligible for analysis.We excluded 4055 children without available results of the tests for SARS-CoV-2 infection and 341 children without information regarding vaccination status.Therefore,6950 were included in the analysis.Of them,1102 (16%) had laboratory-confirmed SARS-CoV-2 infection and were categorized as cases,and 5848 (84%)had negative tests for SARS-CoV-2 infection and were assigned as controls.All 5848 controls had a negative test for SARS-CoV-2,but 795 (13.6%) had another virus detected by RT-PCR or antigen testing.The clinical and demographic characteristics of the participants,stratified as cases and controls are shown in Table 1.
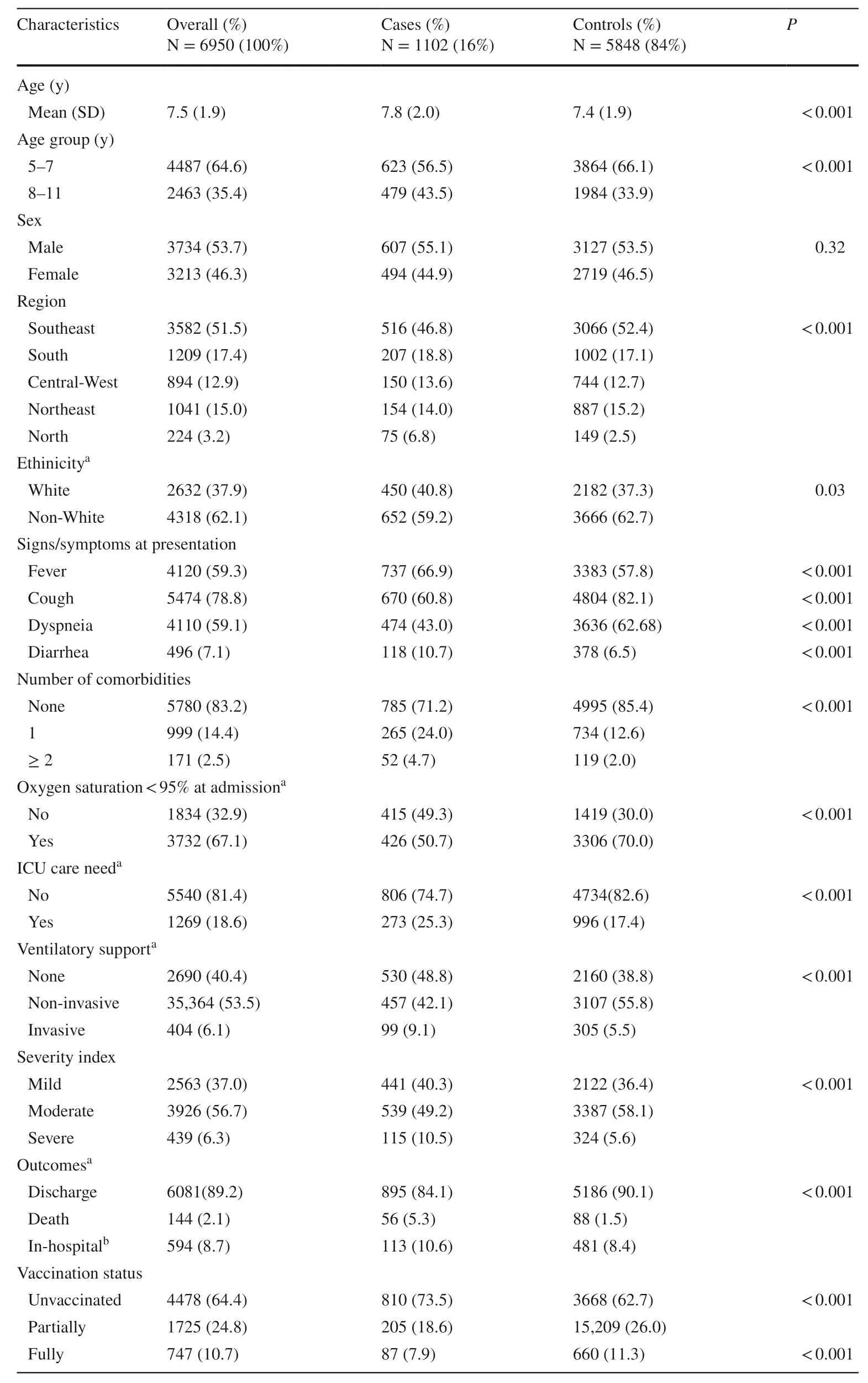
Table 1 Demographic and clinical characteristics of case and controls hospitalized with acute respiratory symptoms from February to June 2022 in Brazil
Vaccination status
Two vaccine schedules were authorized in Brazil for this age group: mRNA vaccine (pediatric Pfizer-BioNTech)and virus-inactivated vaccine (Sinovac;CoronaVac).Among 2472 vaccinated children,1353 (54.7%) received an mRNA vaccine (Pfizer-BioNTech),1054 (42.6%) were given a virus-inactivated vaccine (Sinovac;CoronaVac),and for 65 (2.6%) individuals,the type of vaccine administered was unknown.Additional information about vaccination status and schedules used in Brazil is provided in Supplementary material 2.
Vaccine effectiveness against hospitalization with SARS-CoV-2 infection
Fig.2 shows the VE against hospitalization with acute respiratory symptoms and SARS-CoV-2 infection in children according to the subgroups.The overall VE for fully vaccinated children was 42% (95% CI 26%–54%),and for those partially vaccinated,it was 40% (95% CI 30%–50%).Vaccine effectiveness against infection peaked at 29–42 days after the second dose (67%,95% CI 42%–82%) and declined to only 19% (95% CI − 20%–45%) at 57–84 days.Notably,the protection vanished about 3 months after the second dose.The effectiveness for children fully vaccinated aged 8 to 11 years reached 58% (95% CI 40%–71%),whereas for children aged 5–7 years,the VE was only 29% (95% CI 11%–43%).The comparative analysis of vaccine schedules showed that for fully vaccinated children,the BNT162b2 vaccine had a similar VE against hospitalization with SARSCoV-2 infection (45%,95% CI 20%–61%) compared to the CoronaVac vaccine (40%,95% CI 17%–56%).
Figure 3 a shows the cumulative incidence of COVID-19 cases from 14 days after the first dose of the vaccine.Notably,between 70 and 84 days after the first dose of the vaccine,protection against SARS-CoV-2 infection began to wane for the fully vaccinated children.Figure 3 b shows that the BNT162b2 and CoronaVac vaccines provide a similar period of immunity (P=0.21).There was also no difference in the durability of the immunity period when comparing age groups,sex,ethnicity,and presence of comorbidities(Supplementary Fig.1.a–d).
Vaccine effectiveness against severe outcomes
The clinical and demographic characteristics of the cases stratified by vaccination status are shown in Table 2.Vaccine protection against clinical outcomes of disease severity and death in patients with proven COVID-19 according to vaccination status is shown in Fig.4.All models are adjusted by age,sex,ethnicity,macroregion of the country,and presence of comorbidities.There was limited protection against all endpoints of severe disease for fully vaccinated cases,but the difference was not statistically significant.The VE against ICU admission,need for invasive ventilation,severe illness,and death were 10%,(95% CI,− 54%–45%),22%,(95% CI,− 70%–68%),12%,(95% CI,− 62%–52%),and 16% (95% CI,− 77%–75%),respectively (Fig.4).
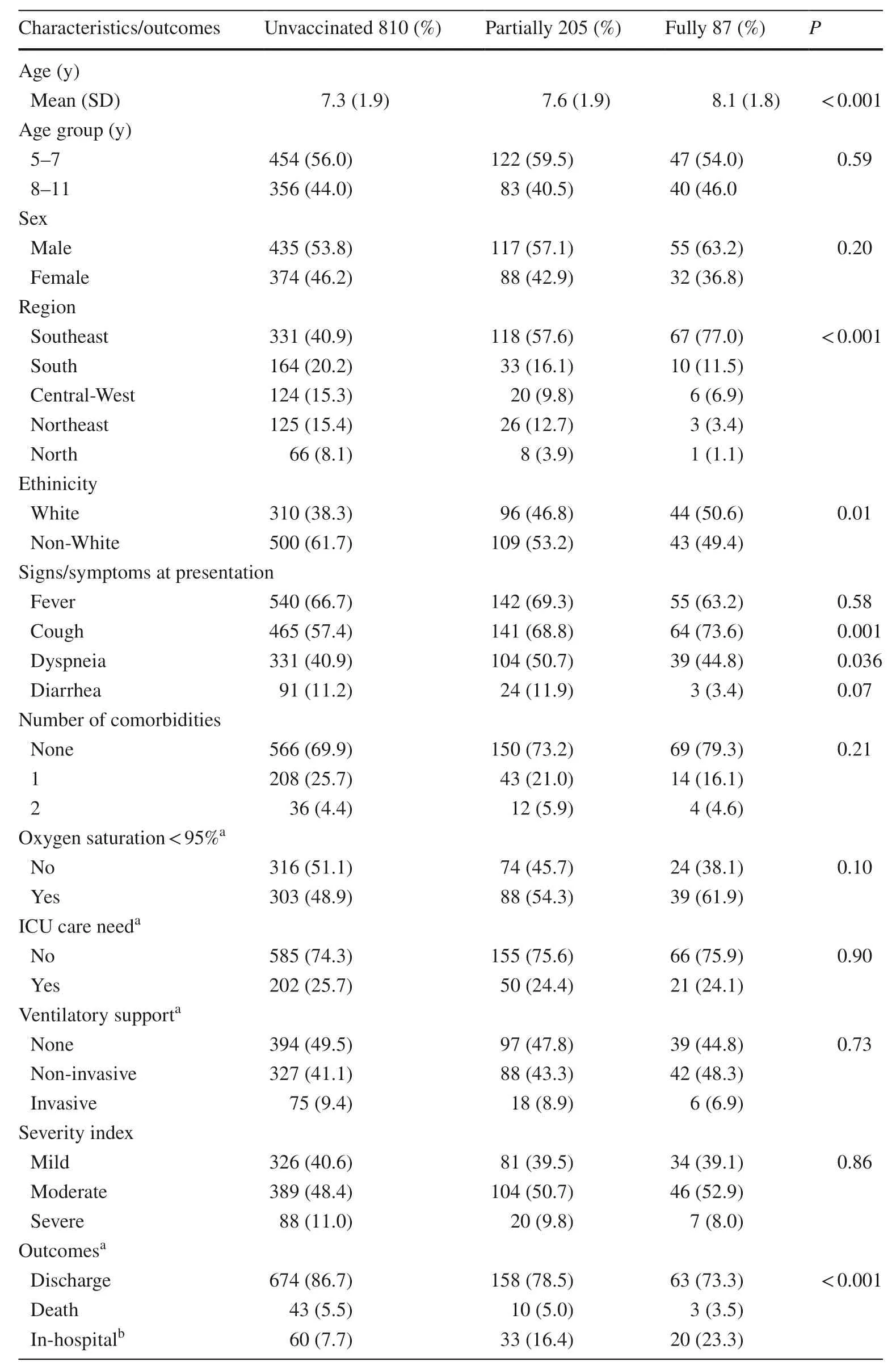
Table 2 Clinical characteristics and outcomes of hospitalized cases according to vaccination status (n =1102)
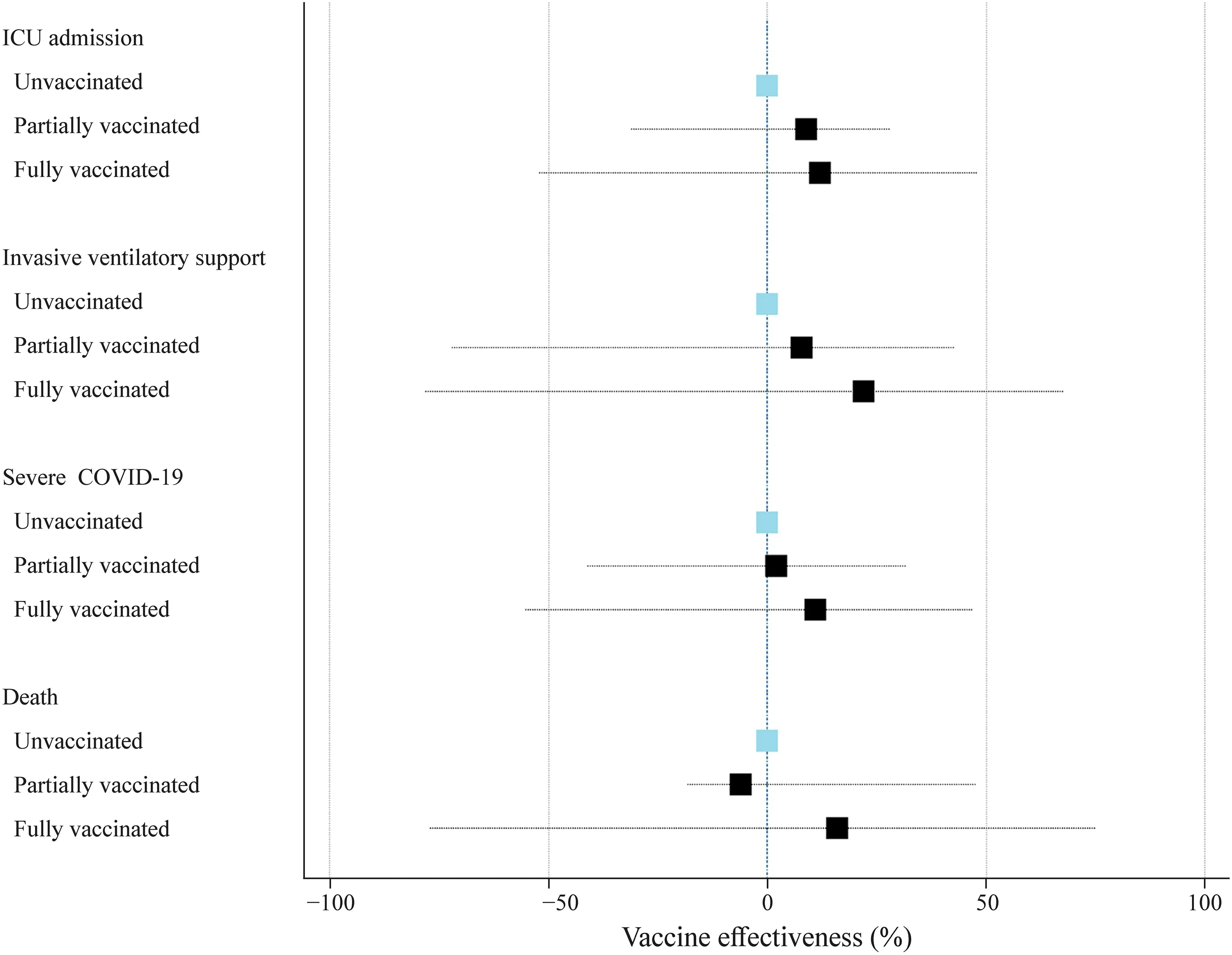
Fig.4 Effectiveness of the vaccine against severe outcomes of COVID-19 in children,including all endpoints.The reference category is unvaccinated individuals.ICU intensive care unit,COVID-19 corona virus disease 2019
Among the 56 children with proven SARS-CoV-2 infection who died,53 (94.6%) were not fully vaccinated.All three fully vaccinated children who died had comorbidities(chromosomal abnormality,unspecified genetic syndrome,and asthma),and all had received the BNT162b2 vaccine.According to the competing-risk survival analysis,the estimated probabilities of fatal outcomes during the first 20 days for unvaccinated,partially vaccinated,and fully vaccinated patients were 4.7% (95% CI 3.2%–6.4%),4.1% (95% CI 1.7%–8.0%),and 2.7% (95% CI 1.1%–6.3%),respectively.
Discussion
The first trial of the BNT162b2 vaccine in recipients 5 to 11 years of age reported a favorable safety profile and high effectiveness against COVID-19.For instance,Walter and colleagues [4] reported a vaccine efficacy against COVID-19 of 90.7% (95% CI 67.7%–98.3%) at 7 days or more after the second dose.Similarly,Creech and colleagues[3] reported that the estimated efficacy of the mRNA-1273 vaccine was 88.0% (95% CI 70.0%–95.8%) against COVID-19 occurring 14 days or more after the first dose.In our analysis,the overall estimated VE of full vaccination against hospitalization with acute respiratory symptoms and SARS-CoV-2 infection was substantially lower than those reported in these clinical trials.Among the diverse factors that could explain the reduction of the VE,the time frame of the study is possibly the most important one.Both trials were conducted before the emergence of the omicron variant.In contrast,our study was conducted during a period in which the omicron variant accounted for about 95% to 100% of infections in Brazil.Several studies of the effectiveness of the vaccines against COVID-19 have consistently shown a substantial reduction in the VE against the omicron variant in adults and adolescents [7,21,22].We found nine studies,including children 5 to 11 years of age that reported VE against various endpoints of COVID-19 (Table 3) [8–10,12–14,22–24].Interestingly,all studies of VE in this age group were conducted during the period of omicron predominance since the vaccines for these age groups were authorized later in 2021.In addition,all studies,except one,evaluated the VE of the BNT162b2 vaccine(Table 3).The results of these studies have shown a VE against symptomatic infection ranging from 28.9% [9] to 83.4% [24].Regarding the protection against hospitalization,the VE ranged from 41.1% [14] to 87.0% [13].In fact,the study by Sacco et al.[14] reported a VE of 41.1%(95% CI 22.2%–55.4%) against severe outcomes defined as SARS-CoV-2 infection resulting in hospital admission or death within 28 days.Taken together,these data from diverse pediatric populations,mostly from developed countries,highlighted the moderate effectiveness of the BNT162b2 vaccine against omicron infection in children 5–11 years of age.In agreement with the study by Sacco et al.[14],our findings from a developing country showed an overall adjusted VE against hospitalization with acute respiratory symptoms and SARS-CoV-2 infection in fully vaccinated children of about 42%.Interestingly,our results regarding the duration of the immunity showed that the VE peaked around 29–42 days after the first dose,reaching 67% and drastically reducing after around 2 months to only about 3%.In the study by Sacco et al.[14],the VE against infection peaked at 38.7% (37.7%–39.7%) at 0–14 days and decreased to 21.2% (19.7%–22.7%) at 43–84 days after full vaccination.From Israel,Cohen-Stavi et al.[12] reported that the estimated VE against documented infection was 51% (95% CI 39%–61%) at 7 to 21 days after the second dose.
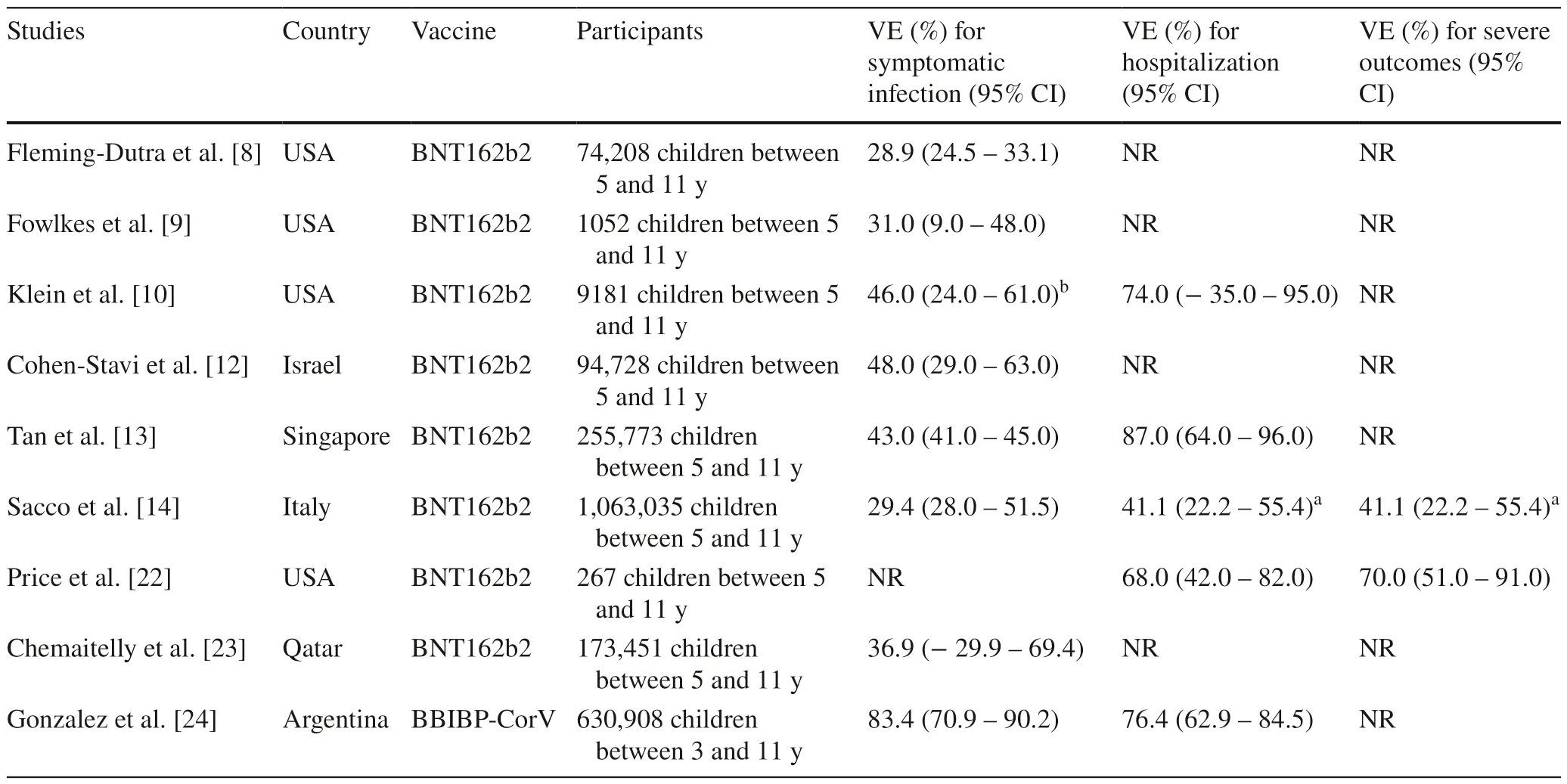
Table 3 Summary of studies that evaluated the efficacy of two doses of the BNT162b2 and BBIBP-CorV (similar to CoronaVac) vaccines for SARS-CoV-2 in children 5–11 years old
To our knowledge,our study was the first to compare the effectiveness of mRNA and virus-inactivated vaccines for this age group.The effectiveness of the BNT162b2 and CoronaVac vaccines against hospitalization with SARSCoV-2 infection was similar in children 5 to 11 years of age (45% vs.41%),with overlapping confidence intervals.However,the VE of effectiveness of the CoronaVac vaccine in our study was lower than the figure reported by Gonzalez and colleagues [24] (Table 3).In our analysis,effectiveness against hospitalization with SARS-CoV-2 infection was apparently higher for fully vaccinated children aged 8 to 11 years than for children aged 5 to 7 years (58% vs.29%).We also did not find any study in the literature comparing the two age groups.The reason why the vaccine's effectiveness may be lower in 5-to 7-year-olds than in 8 to 11-yearolds remains to be determined.However,once more,these findings overlap at the 95% CI levels.Therefore,we cannot rule out that different sample sizes may have influenced these results.The variability in reported VE is probably due to several factors in the studies,including the country,population size of the study,age groups,and the SARSCoV-2 variants circulating during the study period.In fact,the relevant decreases in the effectiveness of vaccines for omicron infections are consistent with genomic data that predicted the potential immune evasion of the omicron variant due to multiple mutations to the spike protein,including its receptor-binding domain (RBD) and N-terminal domain [25–28].Moreover,recent evidence shows that omicron sublineages BA.2.12.1,BA.4,and BA.5 may evolve mutations to evade the humoral immunity elicited by BA.1 infection [29].
In the second part of our analysis,we assessed the VE against severe COVID-19 and COVID-19-related death in children.Early studies in the preomicron period showed the high effectiveness of the BNT162b2 vaccine against serious illness in adolescents.For instance,in a case–control study,Olson et al.[30] reported an effectiveness of 98% against ICU admission and the need for life-support interventions.On the other hand,Price et al.[22] reported that for adolescents from 12 to 18 years of age,the vaccine effectiveness was 40% (95% CI 9%–60%) against hospitalization for COVID-19 and 79% (95% CI 51%–91%) against critical COVID-19 during the omicron-predominant period.Few studies have assessed vaccine effectiveness against severe COVID-19 in children.We observed limited protection with the two-dose schedule,ranging from only about 11% to 30%,against all endpoints of the severity of the disease.In addition,our estimates of VE against severe COVID-19 in 5-to 11-year-old children were significantly lower than estimates in previous studies.For example,Sacco et al.[14] reported an adjusted VE against severe COVID-19 of 41.1% (22.2%–55.4%) for the fully vaccinated group of Italian children.However,we believe that comparing our findings concerning the VE against severe COVID-19 with these studies is somewhat misleading,as the endpoints used as markers of disease severity were quite different across the studies.For example,Sacco et al.[14] defined severe COVID-19 as a SARS-CoV-2 infection resulting in hospital admission or death within 28 days.In addition,in their study,the most severe adverse events were hospital admissions,with very few patients being admitted to the ICU or dying.Nonetheless,despite the low precision of estimates (with wide 95% CIs) derived from the relatively small size of the sample,with few fully vaccinated children and a small number of severe events,our findings highlight the importance of evaluating vaccine performance in many different subgroups and regions [31–33].Moreover,a cost-effective vaccination program for children from poor regions to prevent COVID-19 and severe illness is an urgent public health need since the reported clinical outcomes of COVID-19 in children from developing countries are quite diverse from those of developed countries [34].While studies from developed regions have reported an overall mortality rate of 1% or lower in hospitalized children [35],data from low-income regions showed a death rate of about 7% in this age group [16,17,36].
The strength of our study is the size of the populationbased cohort,which allows a real-world analysis of VE among children hospitalized during the omicron-predominant period in a middle-income country.However,our study has several limitations.First,in an effort to provide results of public health importance as early as possible,data from SIVEP-gripe were extracted on June 27,2022.However,at this point,we had to exclude 36% of the potentially eligible children (4055/11,346) since the COVID-19 tests were not available.Second,another relevant limitation is that the cohort included only hospitalized children,which was not representative of the entire pediatric population.Therefore,the characteristics of our sample may underestimate the effectiveness of the vaccines.For example,due to the inherent limitations of the database,we cannot exclude that an underlying debilitating condition,such as immunosuppression,was responsible for the severity of the disease but not COVID-19 itself.Regarding the design of our study,the test-negative design(TND) has been routinely used to estimate VE against seasonal influenza,but its application in studies of COVID-19 is relatively new [37].However,some important methodological points about the design of this study must be considered.First,the issue of case–control misclassification bias is relevant in TND studies.For example,our analysis cannot rule out this issue,especially given the reduced sensitivity of routinely used SARS-CoV-2 antigen assays.However,for most individuals (80%) included in the study,the test used was RT-PCR.Second,we were unable to include previous SARS-CoV-2 infection status as a confounder.Prior infection may provide protective immunity,especially when associated with vaccination,and therefore may modify the effectiveness of the vaccine[38].Third,a relevant point of this study design is whether there are differences between vaccinated and unvaccinated people that could influence the occurrence of the disease [37].To partially overcome this issue,we used various regression models to adjust for several measured confounders,including demographic and clinical covariates.Finally,the results could have been influenced by the care seeking and severity perception among the families of the hospitalized children.However,due to its characteristics,the TND is a study design that minimizes this issue since the cases and controls recruited are individuals who undergo the same exams for the same reasons in the same health unit and who test positive or negative for a particular illness [39].
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-023-00699-6.
AcknowledgementsWe are profoundly grateful and in debt to all frontline healthcare workers for their impressive efforts to tackle the COVID-19 pandemic in Brazil.These frontline healthcare workers systematically provided all data in the SIVEP-Gripe (Influenza Epidemiological Surveillance Information System).We want to thank the participation of the student from Faculty of Medicine (UFMG),Pedro Alves Soares Vaz de Castro,who helped to compile the articles regarding the vaccine effectiveness in children.
Author contributionsEAO: conceptualization,data curation,formal analysis,funding acquisition,investigation,methodology,project administration,resources,supervision,validation,visualization,writing—original draft,writing—review and editing.MCO:supervision,conceptualization,data curation,investigation,methodology,project administration,writing—original draft,writing—review and editing.EAC: formal analysis,investigation,methodology,validation,visualization,writing—original draft,writing—review and editing.ACS,RHM: investigation,writing—original draft,writing—review and editing.CCP: investigation.MAV,LRS,DM,HM:investigation,writing—review and editing.
FundingThis study was supported by the CNPq (National Council for Scientific and Technological Development) and FAPEMIG (Research Support Foundation of Minas Gerais).
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interestThe authors declare that they have no competing interests.No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.Author RH Mak is a member of the Editorial Board for Word Journal of Pediatrics.The paper was handled by the other Editor and has undergone rigorous peer review process.Author RH Mak was not involved in the journal's review of,or decisions related to this manuscript.
Ethical approvalWe accessed data in SIVEP-Gripe.All data on this database are publicly available and are anonymous.Data collection and analysis for this investigation were carried out under ethically agreed principles on open data in Brazil,for which ethics review is exempted.
杂志排行
World Journal of Pediatrics的其它文章
- Sepsis heterogeneity
- How are children with medical complexity being identified in epidemiological studies? A systematic review
- Consensus for criteria of running a pediatric inflammatory bowel disease center using a modified Delphi approach
- Maternal weight,blood lipids,and the offspring weight trajectories during infancy and early childhood in twin pregnancies
- Association between maternal gestational diabetes and allergic diseases in offspring: a birth cohort study
- Determinants of infant behavior and growth in breastfed late preterm and early term infants: a secondary data analysis
