Molecular mechanisms of noncoding RNA and epigenetic regulation in obesity with consequent diabetes mellitus development
2023-11-26YiChenGuoHaoDiCaoXiaoFenLianPeiXianWuFanZhangHuaZhangDongHuiLu
Yi-Chen Guo,Hao-Di Cao,Xiao-Fen Lian,Pei-Xian Wu,Fan Zhang,Hua Zhang,Dong-Hui Lu
Abstract Diabetes mellitus (DM) and obesity have become two of the most prevalent and challenging diseases worldwide,with increasing incidence and serious complications.Recent studies have shown that noncoding RNA (ncRNA) and epigenetic regulation play crucial roles in the pathogenesis of DM complicated by obesity.Identification of the involvement of ncRNA and epigenetic regulation in the pathogenesis of diabetes with obesity has opened new avenues of investigation.Targeting these mechanisms with small molecules or RNA-based therapies may provide a more precise and effective approach to diabetes treatment than traditional therapies.In this review,we discuss the molecular mechanisms of ncRNA and epigenetic regulation and their potential therapeutic targets,and the research prospects for DM complicated with obesity.
Key Words: Diabetes mellitus;Obesity;Noncoding RNA;Epigenetic regulation;Insulin resistance
lNTRODUCTlON
The combination of diabetes mellitus (DM) and obesity has become a global health concern due to the high prevalence and serious consequences of these conditions.The pathogenesis of DM combined with obesity is complex and involves multiple mechanisms,including insulin resistance (IR),chronic inflammation,and adipokine dysregulation[1,2].
IR is a key factor in the development of both obesity and type 2 DM (T2DM)[3,4].Adipose tissue,particularly visceral adipose tissue,produces a range of hormones,cytokines,and chemokines,collectively known as adipokines.In obesity,adipose tissue expands and produces increased amounts of proinflammatory adipokines,such as leptin,as well as decreased amounts of anti-inflammatory adipokines,such as adiponectin[5].This leads to chronic inflammation,which exacerbates IR.Obesity and diabetes are associated with alterations in the gut microbiome,which can contribute to the pathogenesis of both conditions[6,7].The gut microbiota of obese and diabetic individuals is distinct from that of healthy individuals,with reduced microbial diversity and altered microbial composition.
While the exact mechanisms underlying the development of DM complicated with obesity are still not fully understood,emerging evidence suggests that epigenetic modifications and noncoding RNA (ncRNA) play a critical role in its pathogenesis[8].Epigenetic regulation refers to the modification of gene expression without changes to the underlying DNA sequence[9].These modified activities can have a significant impact on gene expression and cellular function.Additionally,several genes are linked to an increased risk of developing these conditions,including genes involved in adipogenesis,lipid metabolism,and insulin signaling.Compounded obesity in DM is a multifactorial disorder that involves complicated interplay among genetic,environmental and lifestyle factors.It is vital to establish effective strategies for the prevention and treatment of these disorders by understanding these mechanisms.
ncRNAs,particularly microRNA (miRNA) and long noncoding RNA (lncRNA),have been shown to play critical roles in the development and progression of DM.Dysregulation of miRNA expression can lead to impaired glucose metabolism and IR[10].For example,miRNA-29 regulates insulin signaling by targeting the insulin receptor substrate-1 (IRS-1) gene[11].In obese mice,miRNA-29 expression is decreased,leading to increasedIRS-1expression and improved insulin sensitivity[11].Similarly,miRNA-223 has been shown to regulate glucose uptake by targeting GLUT4,a glucose transporter protein.lncRNA has also been implicated in the pathogenesis of DM[12].In addition,lncRNA MEG3 controls insulin secretion by modulating gene expression involved in insulin synthesis and secretion[13].lncRNA taurineupregulated gene 1 regulates the proliferation and differentiation of pancreatic beta cells,which are responsible for insulin production[14].
DNA methylation can alter gene expression patterns.The promoter region of the insulin gene is hypermethylated in patients with T2DM,leading to decreased insulin production[15].Similarly,the promoter region of the adiponectin gene is hypomethylated in obese individuals,leading to increased adiponectin expression and improved insulin sensitivity[16].The augmentation of gene expression is linked to histone acetylation,whereas histone methylation may either stimulate or hinder gene expression,contingent on the location and extent of methylation[17].
Emerging evidence suggests that epigenetic modifications and ncRNA play a critical role in the development and progression of DM complicated with obesity (Figure 1).Dysregulation of miRNA and lncRNA expression,as well as altered DNA methylation and histone modifications,can lead to impaired glucose metabolism and IR[18].Although much is still unknown about the mechanisms underlying these epigenetic changes,identification of these modifications as potential therapeutic targets offers new hope for the prevention and treatment of DM.Future research should elucidate the role of epigenetic regulation and ncRNA in diabetes pathogenesis and develop effective therapies targeting these pathways.The aim of this review is to explore the molecular mechanisms of ncRNAs and epigenetic regulation in the pathogenesis of DM complicated by obesity.We intend to discuss the potential therapeutic targets associated with these mechanisms and highlight the research prospects for DM complicated with obesity.
Figure 1 Epigenetic modifications and noncoding RNA play a critical role in the development and progression of diabetes mellitus
MOLECULAR MECHANlSMS OF NCRNA lN THE PATHOGENESlS OF DM COMPLlCATED WlTH OBESlTY
Role of lncRNAs
lncRNAs in obesity and DM:The utilization of cutting-edge bioinformatic techniques has facilitated the identification of lncRNAs associated with obesity and adipocyte differentiation[19].Investigations of gain-of-function and loss-of-function have both strongly pointed to the pivotal participation of lncRNAs in adipogenesis.To date,various lncRNAs have been examined in a range of models and they are potent modulators of diverse genetic pathways linked to white adipose tissue (WAT) compartmentalization and activity[20].
The first adipogenesis-related lncRNA was a steroid receptor RNA activator (SRA),which acts as a coactivator of peroxisome proliferator-activated receptor (PPAR)γ[21].Among the lncRNAs involved in adipogenesis,ASMER-1 and ASMER-2 are upregulated in subcutaneous adipose tissue (ScAT) and are linked to adipocyte-specific metabolism and IR[20].Several lncRNAs have roles in adipogenesis (the formation of fat cells),lipolysis (the breakdown of fat),and adiponectin secretion in human adipocytes (fat cells).ADNCR is an endogenous competitive RNA for miR-204,and overexpression of SIRT-1 inhibits adipocyte differentiation and impairs the PPARγ pathwayin vitro.Finally,HOTAIR is implicated in preadipocyte differentiation[20,22-26].
Brown adipose tissue (BAT) is a specialized form of adipose tissue that is mainly responsible for thermogenesis and energy expenditure.It is characterized by the presence of uncoupling protein 1 (UCP1),leading to increased energy expenditure and weight loss[27,28].Recent studies have identified several lncRNAs that are involved in BAT regulation,including brown fat lncRNA1 (Blnc1) and H19[25,29].Research has indicated that Blnc1 plays a role in regulating thermogenic genes,resulting in an increase in the expression of UCP1 and mitochondrial genes[30].Conversely,H19 has been found to have an inverse correlation with body mass index (BMI) and a positive correlation with browning markers.H19 is involved in modulating adipogenesis,oxidative metabolism,and mitochondrial respiration in BAT.Thus,the manipulation of lncRNA expression shows promise as a therapeutic approach for metabolic diseases.This could involve enhancing BAT activity or inducing browning in WAT[31].Various studies have suggested the potential of different lncRNAs as biomarkers for diagnosing and managing obesity.For example,Sunet al[32] found reduced expression of three lncRNAs in obese but not in lean subjects.The expression of these lncRNAs was inversely correlated with waist-tohip ratio,BMI and fasting plasma insulin levels.lncRNA-p19461 was upregulated following weight loss due to a 12-wk diet,suggesting that bariatric interventions could manage expressed lncRNA profiles.Alterations in the expression levels of lncRNAs were found following bariatric surgery in animals,particularly those engaged in digestive,absorptive and inflammatory pathways.
While the potential of lncRNAs as therapeutic targets for obesity management is promising,several challenges need to be addressed before their clinical application.One major challenge is the lack of understanding of the precise molecular mechanisms underlying the regulation of lncRNA expression in different tissues and under different physiological conditions[33].The delivery of lncRNA-based therapeutics to specific tissues remains a major hurdle due to their large size and potential off-target effects[33,34].Therefore,additional investigation is required to uncover the molecular pathways involved in the regulation of lncRNA and to develop delivery methods that can specifically target tissues while minimizing off-target effects.
lncRNAs in DM:In animal models and human islets,dysregulation of lncRNAs is engaged in various stages of insulin secretion and is implicated in the progression of IR[35,36] (Table 1).In addition,the genes that encode them are located near islet-specific chromatin domains that contain genes involved in β-cell function modulation[37].The specific functions and action mechanisms of these lncRNAs are still not fully understood[36].
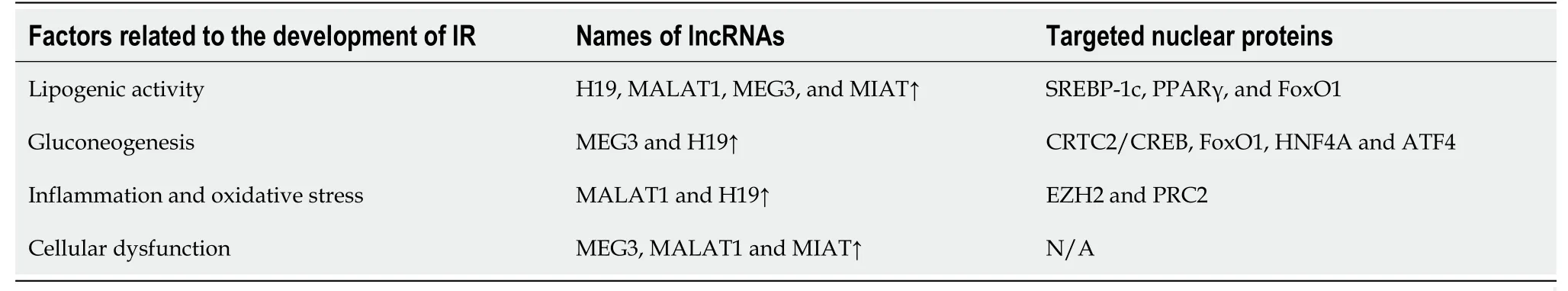
Table 1 Transcription factors and long noncoding RNAs in insulin resistance
In T2DM,metabolic syndrome and low-level high-density lipoprotein,a decline in MALAT1 expression was found,along with overexpressed H19 in patients with worse glycemic control than those with glycated hemoglobin concentration < 7%[38].Additionally,MALAT1 is related to angiogenesis in diabetic eyes and kidneys.A few dysregulated lncRNAs in diabetic subjects are positively correlated with transcriptional markers of IR,impaired glucose control,and aging.These lncRNAs were apparently relevant to DM,even after correction[39].Newly diagnosed diabetic patients exhibited similar results,indicating that dysregulated lncRNAs control IR and inflammation,ultimately resulting in disrupted glucose homeostasis[40].
The role of lncRNAs in both microvascular and macrovascular complications of DM has been investigated.A widely studied lncRNA associated with diabetic complications is ANRIL,which is considered a potential biomarker[41,42].Another is MALAT1 in association with elevated production of reactive oxygen species and proinflammatory cytokines,contributing to endothelial lesions in the microvasculature[35,41].
Dysregulation of specific genes has been identified in renal biopsies affected by diabetic nephropathy.Additionally,a study of diabetic patients with chronic complications found downregulation of CASC2 in the serum and renal tissue of DM patients with chronic kidney disease when compared to healthy controls[36,43].Both MIAT and MALAT1 were found to be over-regulated in renal specimens from diabetic subjects and in animal models[36].The effect of lncRNAs in diabetic patients with peripheral neuropathy has also been investigated.Specifically,NONRATT021972 was shown to be increased in T2DM subjects with exacerbated symptoms connected to neuralgia,together with an increase in tumor necrosis factor (TNF)-α levels.Furthermore,siRNA-NONRATT021972 alleviated neuropathic pain by decreasing TNF-α in rats,resulting in decreased blood glucose and inflammation,which paved the way for potential therapies of neuropathic pain[44].MALAT1 is over-expressed in gastrointestinal spasms and in T2DM sufferers with signs related to gastric spasms,and its impact is likely associated with smooth muscle cells.
Function of miRNAs in DM with obesity:miRNAs prevent the translation of mRNA into protein,leading to mRNA degradation or translational repression.miRNAs have been shown to regulate various cellular processes.Dysregulated miRNA has been implicated in metabolic disorders,such as in obesity (Table 2).

Table 2 Circulating microRNA in obesity
The pathogenesis of metabolic diseases has been linked to the expression of various miRNAs.Kunejet al[45] found that 221 of the 1736 Loci associated with obesity coincided with miRNAs.It has been reported that miRNAs can modulate pathways that control adipogenesis[46,47],which is impaired in obesity.Consequently,miRNA dysregulation could be involved in metabolic processes that contribute to obesity[48,49].
miRNA-375:The islet-specific miRNA-375 is expressed at high levels in pancreatic islets and regulates insulin secretion by modulating gene expression.The impact of miRNA-375 on glucose-stimulated insulin secretion (GSIS) and insulin gene transcription was investigated by Poyet al[50],who found that its overexpression suppressed GSIS and reduced insulin gene transcription,whereas its downregulation resulted in increased insulin secretion.This study confirmed the crucial role of miRNA-375 in the development of T2DM,as demonstrated by its higher expression in the pancreas of T2DM patients compared to healthy individuals.Dysregulation of miRNA-375 was observed 5 years prior to the start of T2DM and in prediabetes,indicating its potential use in the prediction and prevention of high-risk populations[51].
miRNA-130b:In prepubertal obesity,some miRNAs may become deregulated,as evidenced by a study which showed that the expression of miRNA-130b in plasma was upregulated and directly correlated with BMI and other indicators of obesity in children.
miRNA-200 family:The miRNA-200 family can contribute to protection against beta-cell apoptosis and dedifferentiationin vitro[52].miRNA-200c is one of the most highly expressed miRNAs in beta cells,and partially protects against oxidative stress-induced beta-cell apoptosis,suggesting that the miRNA-200 family is essential in diabetes pathophysiology[53].
miRNA-7:Human islets are enriched in another miRNA named miRNA-7,which adversely modulates GSIS by restricting the expression of genes participating in the integration of insulin granules within the plasma membrane and the SNARE proteins[54].The levels of hsa-miRNA-7-1-3p were reduced in pancreatic islets of individuals with T2DM compared to nondiabetic donors.The expression levels of hsa-miRNA-7-3-5p were increased in T2DM pancreatic islets[55].
miRNA-184:miRNA-184 is one of the miRNAs predominantly expressed in beta cells of pancreatic islets,regulating insulin secretion and beta-cell proliferation during IR[56].Knockout of miRNA-184 in beta cells has been shown to increase their proliferation,resulting in improved insulin secretion following glucose stimulation.Blocking miRNA-184 in rat and human islets has been demonstrated to protect beta cells from apoptosis induced by prolonged exposure to proinflammatory cytokines and/or fatty acids.
Circular RNAs in obesity and DM
Role of circular RNAs:Adipose tissue is a complex and metabolically active organ,playing an essential role in energy storage and homeostasis.Adipocytes are the primary cell type in adipose tissue,and their differentiation and function are tightly regulated by multiple molecular mechanisms.In recent years,the role of circular RNAs (circRNAs) in adipose tissue has gained significant attention.
circRNA expression in carboxy-terminal region,prediabetic and T2DM patients showed 411 downregulated and 78 upregulated circRNAs[57].Notably,220 circRNAs demonstrated differential expression,including 107 upregulated and 113 downregulated circRNAs[58].Of particular interest were the ci-INS and ci-Ins2 Lariats,derived from human INS and mouse Ins2,respectively in beta cells[59].
EPlGENETlC REGULATlON AND lTS ROLE lN THE PATHOGENESlS OF DM COMPLlCATED WlTH OBESlTY
Genetic variation is a crucial factor in the regulation of DNA methylation[60].As methylated DNA predominantly arises on cytosine nucleotides after a guanine,it is evident additions or deletions of variants of cytosine-guanine dinucleotides(CG dinucleotides) affect the likelihood of methylated DNA at the loci[61].Remarkably,roughly one-fourth of single nucleotide polymorphisms (SNPs) add or delete CpG site[62].
The presence of an SNP in NDUFB6 Led to the emergence of a CpG site that in turn affected DNA methylation and gene expression in human skeletal muscle,particularly age-related gene expression[63].Although genetic variations can directly impact DNA methylation,it remains unclear whether they can affect methylation in more remote sites and,if so,what the underlying mechanism would be.The extent to which this phenomenon is widespread throughout the genome and its potential contribution to clinical phenotypes remain uncertain.Another study identified that nearly half of the genetic variations associated with diabetes introduce or remove a CpG site[64].
In 2014,a study extended previous research and provided a whole-genome description of genetic and epigenetically variations in human pancreatic islets[63].Numerouscis-andtrans-SNP-CpG pairs were determined,even though the machinery of the latter is still unclear[65].Additionally,causal inference test established a catalytic interaction between SNPs,DNA methylation and genetic expression of annotated HLA regions highly correlated with type 1 DM[66].More than 100000 DNA metylation quantitative trait loci (mQTLs) were identified by GWASs,which were linked to adiposetissue gene expression,BMI,and insulin levels[67,68].
RESEARCH PROSPECTS
ncRNAs as early diagnostic markers
ncRNAs are involved in the development of both diabetes and obesity and may be potential early diagnostic markers for these conditions.miRNAs are small ncRNAs that play important roles in post-transcriptional gene regulation.These miRNAs are dysregulated in both DM and obesity and may serve as potential early diagnostic markers for these conditions[69].For example,miRNA-126 has been shown to be downregulated in obese individuals and may serve as a potential early diagnostic marker in obesity[70].Similarly,miRNA-375 has been shown to be upregulated in individuals with T2DM and may serve as a potential early diagnostic marker for this condition[71].
lncRNAs are longer ncRNAs that also play important roles in gene regulation,suggesting that lncRNAs are involved in the development of both diabetes and obesity and may serve as potential early diagnostic markers[19].The lncRNAs HOTAIR and H19 have been shown to be upregulated in individuals with T2DM and may be early diagnostic markers[72,73].
circRNAs are a class of ncRNAs that form covalently closed circular RNA molecules,which have recently been observed in the dysregulation in both DM and obesity and may be early diagnostic markers[74].For example,circRNA-000911 has been shown to be downregulated in individuals with T2DM,and may serve as a potential early diagnostic marker[75].Similarly,serum and exosome circRNA-0000907 and circRNA-0057362 have been shown to be upregulated in patients with diabetic foot ulcer (DFU),indicating that they may have a potential role as early diagnostic markers for DFU[11].
ncRNAs have emerged as potential early diagnostic markers for both DM and obesity.Early diagnosis and management of DM and obesity are crucial to prevent complications and improve outcomes.Therefore,the identification of novel early diagnostic markers for these conditions is of utmost importance.ncRNAs may serve as valuable tools in this regard and may help improve patient outcomes.Therefore,further research is needed to validate the potential of ncRNAs in early diagnosis.
Possible treatment targets for DM with obesity
miRNAs are one of the best-studied classes of ncRNAs,and they have been implicated in the regulation of glucose homeostasis and insulin sensitivity.It was shown that miRNA-29a regulates insulin signaling by targeting IRS1 in adipocytes[76].Additionally,miRNA-103 and miRNA-107 have been shown to promote IR by targeting the insulin receptor and GLUT4,respectively[77].Another lncRNA,NEAT1,has been shown to regulate the expression of genes involved in the inhibition of high glucose-induced diabetic retinopathy[78].Furthermore,S961-treated mouse sera reproduced beta-cell replication in pancreatic islets in an E2F1-dependent way,indicating that IR-induced adipocyte proliferation signaling activates E2F1 and is a potential target for promoting beta-cell compensation[79].
Epigenetic regulation has also emerged as an important contributor to the pathogenesis of DM with obesity.DNA methylation regulates motifs involved in glucose homeostasis and insulin signaling[80].Histone modifications regulate the expression of key genes in the insulin signaling pathway[81].
In addition,lncRNAs are newly emerging and promising biomarkers,so we summarize the shared lncRNAs in both obesity and DM in order to provide further information (Table 3).
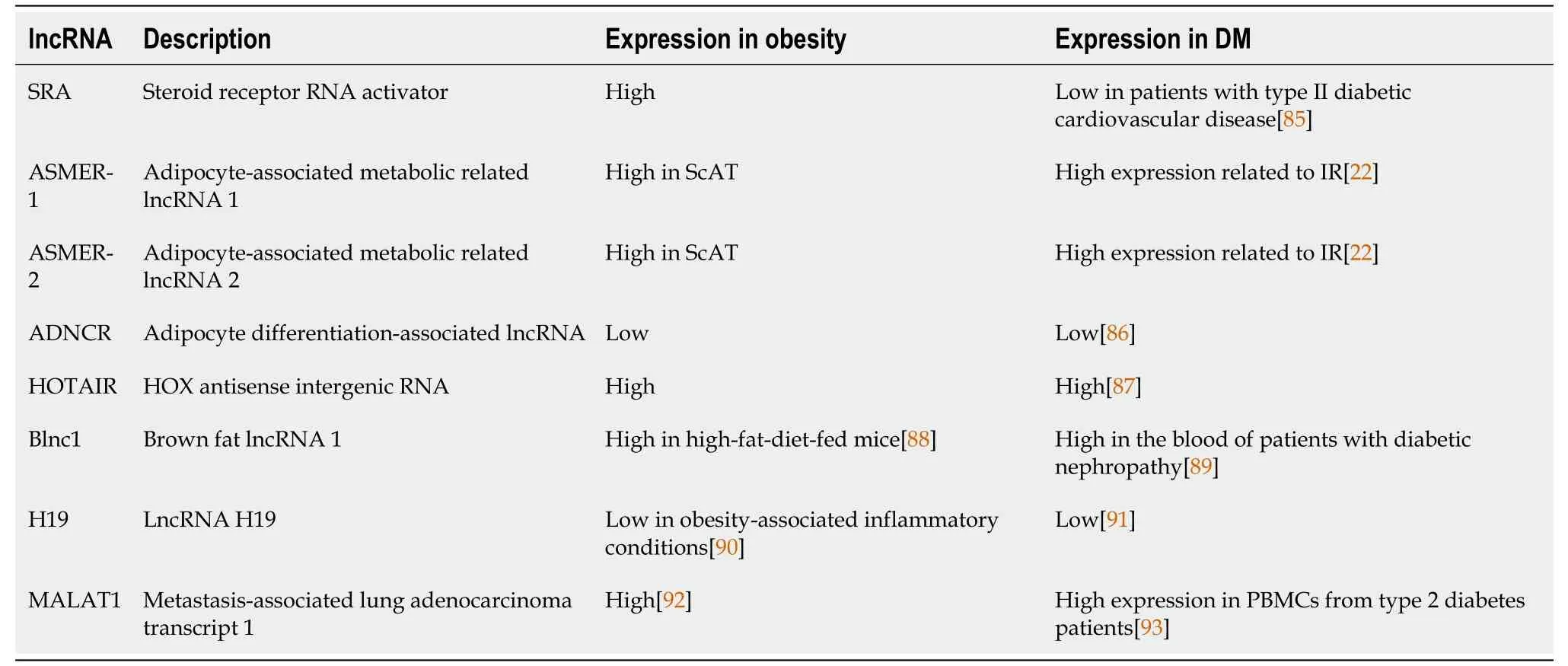
Table 3 The profiles of shared long noncoding RNAs in obesity and diabetes mellitus
Identification of the pathogenesis of DM with obesity has opened new avenues.Targeting these mechanisms with small molecules or RNA-based therapies may provide a more precise and effective approach to DM treatment than traditional therapies.For example,miRNA-based therapies have already been tested in preclinical models of DM,with promising results.
CONCLUSlON
The pathogenesis of DM complicated with obesity involves complex molecular mechanisms,including ncRNA and epigenetic regulation.Understanding the roles of ncRNA and epigenetic regulation in the pathogenesis of DM complicated with obesity provides new insights into the development of novel therapeutic targets and strategies.Future research should focus on exploring the potential of ncRNA and epigenetic regulation as biomarkers for diagnosis and prognosis,as well as precision medicine and personalized treatment strategies.
FOOTNOTES
Author contributions:Guo YC and Cao HD contributed equally to this work;Guo YC,Cao HD,Lian XF,Wu PX,Zhang F,Zhang H,and Lu DH designed the research;Cao HD and Lian XF contributed analytic tools and specifically visualization;Guo YC analyzed the data and wrote the manuscript;Wu PX,Zhang F,Zhang H,and Lu DH edited and reviewed the manuscript;and all authors have read and approve the final manuscript.
Supported bythe Shenzhen Science and Technology Innovation Committee Projects,No.JCYJ20170816105 416349;Shenzhen High-level Hospital Construction Fund;and Shenzhen Key Medical Discipline Construction Fund,No.SZXK010.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yi-Chen Guo 0000-0003-2502-3893;Hao-Di Cao 0009-0007-6347-3162;Xiao-Fen Lian 0000-0001-7950-8867;Fan Zhang 0000-0001-5147-663X;Hua Zhang 0000-0002-4627-7529;Dong-Hui Lu 0000-0001-9172-2989.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YX
杂志排行
World Journal of Diabetes的其它文章
- Cellular and molecular overview of gestational diabetes mellitus: ls it predictable and preventable?
- Exploring the targets and molecular mechanism of glycyrrhetinic acid against diabetic nephropathy based on network pharmacology and molecular docking
- Molecular targets and mechanisms of Jiawei Jiaotai Pill on diabetic cardiomyopathy based on network pharmacology
- Vascular endothelial growth factor B improves impaired glucose tolerance through insulin-mediated inhibition of glucagon secretion
- Reduced risk of dementia in patients with type 2 diabetes mellitus using Chinese herbal medicine: A nested case-control study
- Mechanisms of action of natural products on type 2 diabetes
