Effect of small interference RNA on expression of the Skp2 in human chondrocytes cell
2023-11-23YUANChangshenLIYanhongLIUJinyiLIAOShuningMEIQijieXUWenfeiDUANKan
YUAN Chang-shen, LI Yan-hong, LIU Jin-yi, LIAO Shu-ning, MEI Qi-jie, XU Wen-fei,DUAN Kan✉
1.Orthopedic Department of the Limbs, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, China
2.Guangxi University of Chinese Medicine, Nanning 530000, China
Keywords:
ABSTRACT Objective: To study the inhibitory effect of siRNA mediated by expression vectors on Skp2 expression in human chondrocytes.Method: Three Skp2 sequences siRNA-59, siRNA-318 and siRNA-504 were designed as target sites using online siRNA design tools.Skp2 siRNA expression vectors were successfully constructed in vitro by gene recombination technology,and the influence of recombinant plasmid transfection on Skp2 mRNA expression was detected.DNA electrophoresis was used to verify the results.Results: The sequence of Skp2 interference was correct by sequence analysis.The expression of Skp2 mRNA in siRNA-59, siRNA-318, siRNA-504 transfection group was significantly lower than that in no-load group and NC group(P<0.05), the inhibition rates of Skp2 mRNA in siRNA-59, siRNA-318 and siRNA-504 were respectively 60%, 41% and 64%, and the siRNA-504 transfection group had the highest inhibition rate.Conclusion: The siRNA eukaryotic expression vector of Skp2 gene was constructed successfully which effectively inhibit Skp2 mRNA expression in human chondrocytes cell, and can provide strong evidence for the treatment of osteoarthritis.
1.Introduction
S-phase kinase associated protein(Skp2) is a protein involved in cancer, mainly in the nucleus and cytoplasm, can widely expressed in bone marrow and other organizations (https://www.ncbi.nlm.nih.gov/gene/6502#gene-expression).Skp2 as the key part of the cell cycle regulator, can promote cell from G1 phase to S phase transformation, and is closely related to the occurrence and development of tumors[1,2]; Targeting Skp2 pathway is even considered to be a key target for cancer therapy[1].However, the mechanism of Skp2 action in non-neoplasms,especially(Osteoarthrit is)OA, remains unclear.
RNA interference(RNAi)can cause homologous double-stranded RNA in the target gene sequence to induce a specific posttranscriptional gene silencing phenomenon[3].RNAi is characterized by sequence specificity, high efficiency and low drug toxicity, which can participate in the regulation of cell proliferation, differentiation and apoptosis, and affect the incidence of non-tumor and tumor[4].RNAi has become an important tool for gene function research,and even cancer gene therapy.The small interfering RNA (siRNA)technology has been used to esophageal cancer[5], pancreatic cancer[6], laryngeal cancer[7], lung cancer[8] research, etc, but few reports on OA have been obtained.
Therefore, in this study, the siRNA transfected human chondrocytes were designed and synthesized to observe the protein expression of Skp2 in human chondrocytes and the effect of Skp2 on human chondrocytes after gene silencing, providing a basis for further gene targeted therapy of OA.
2.Materials and methods
2.1 Materials
Human cartilage cells complete medium(CM-H107, Porcell); IL-1β(CG93, novoprotein); FBS(10099-141, Gibco); Lipofectamine 3000 Transfection Reagent(L3000015, Invitrogen); OPTIMEM medium(31985-062, Gibco); Trypsin-EDTA digestive solution(T1300, Solarbio); 1×PBS(0.01M, pH7.4)(KGB5001, Keygen Biotech); Method of CCK 8 cell proliferation test kit(KGA317,Keygen Biotech); Internal parameters: β-actin; Skp2 siRNA-318, Skp2 siRNA-504, Skp2 siRNA-59(Provided by Hong Boyuan Molecular Laboratory); Trizon Reagent(CW0580S, CWBIO);Ultrapure RNA extraction kit(CW0581M, CWBIO); HiScript II QRT SuperMix for qPCR(+gDNAwiper)(R223-01, Vazyme); ChamQ Universal SYBR qPCR Master Mix(Q711-02, Vazyme); 50×TAE scavenged buffer(T1060, Solarbio); 6×DNA Loading Buffer(GH101-01, TRANS); 50bp DNA Ladder(MD108, TIANGEN); Gsafe Red plus nucleic acid dye(GK20002, GLPBIO); Agarose powder(75510-019, Invitrogen).
2.2 Methods
2.2.1 siRNA carrier design and build
According to the human Skp2(NM_001243120, Gene ID: 6502)sequence from Genebank, through https://www.ncbi.nlm.nih.gov/online siRNA tool design three siRNA.The designed gene targets was 59, 318, 504 in NS gene, target sequence were: Skp2(human)siRNA-59: CAAAAAACUCAAAUUUAGUTTACUAAAUUUGA GUUUUUUGTT; Skp2(human)siRNA-318: CCAUCUAGACUU AAGUGAUTTAUCACUUAAGUCUAGAUGGTT; Skp2(human)siRNA-504: CCUUCAACUGUUAAAGGAATTUUCCUUUAA CAGUUGAAGGTT; And set the negative control siRNA: UUCU CCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT and light group.There was no homology with any gene sequence,and the above sequences were by Jiangxi Zhonghong Boyuan Biotechnology Co., LTD.
SacI and XhoI cleavage sites were designed at both ends of the template chain, and the vector construction name was YCS-WTSkp2-Pmi-rGLO, the binding site was mutated, the mutant target gene fragment was biosynthesized, and the vector was connected to the pmirGLO vector, which was constructed with the name of YCS-UMT-Skp2-pmirGLO.SacI and XhoI were used to mutate the YC-UMt-Skp2-Pmirglo plasmid and attach the target fragment to the pmirGLO vector.The bands were separated by 1% agarose gel electrophoresis after 30min digestion of the two polynucleotide fragments at 37°C.The linearized bands were cut under the UV lamp, and the agarose DNA recovery kit was applied to recover the linearized carrier and the linkage reaction system was as follows:Target plasmid(50 ng)1 μL, pmirGLO-F 0.5 μL, pmirGLO-R 0.5 μL, 2× ES Taq Master Mix(Dye)12.5 μL, and deionized water 10.5 μL.The junction products were named as siRNA-59, siRNA-318,siRNA-504 and NC(negative control plasmid), respectively.
2.2.2 Cell transfection
The experiment was divided into 5 groups: no-load group, NC group, Skp2 siRNA-59 group, Skp2 siRNA-318 group and Skp2 siRNA-504 group.the Skp2 siRNA-504 group.First of all, on 6 orifice implanted cells, each hole of about 2×105~10×105cells, 6cm dish every hole about 5×105~2×106cells.When the cell density reached 70%, it was ready for transfection; 1ml serum-free medium was used instead of cell medium.Secondly, two sterilized EP tubes were taken, 125 μL Opti-MEM was added to each tube, 5 μL lipofectamine 3000 was added to one tube, and 12.5 μL siRNA or miRNA was added to the other tube (siRNA or miRNA dry powder was dissolved in DEPC water; 125 μL/1OD), mixed and incubated at room temperature for 5min; Finally, the above 2 EP test tubes were mixed and incubated at room temperature for 15 min.After that, the mixed solution was dropped into the corresponding holes in the 6-well plate, and the cells were moved back to room temperature for incubation.4-6 h after transfection, 1ml complete medium containing 20% serum was added to 6-well plates, and follow-up experiments were performed 48 h later.
2.2.3 RT-PCR detection of Skp2 mRNA expression levelTotal RNA of human chondrocytes was detected by RT-PCR, and total RNA was extracted according to TRIzol operating instructions.1.3 μL of total RNA solution of 38 μL system was extracted by UV spectrophotometer, and the data were measured and recorded under OD 260 and OD 280.RNA concentration and purity were calculated by OD 260×40=RNAng/ uL formula and 1.8≤OD 260/OD 2802.2 formula, respectively.Total RNA was extracted for agarose gel electrophoresis, and the RNA was run gel, the voltage was 200 V, the current was about 180 mA, and the time was about 13 min.The gel was removed, and the image was collected by the chemiluminescence imaging system after development.Single strand cDNA was obtained by HiScript II QRT SuperMix for qPCR reverse transcription kit.PCR reaction system: 2×miRNA Universal SYBR qPCR Master Mix 10 μL, cDNA template 1ul, primers 0.4ul, DEPC water 8.2 μL total volume 20 μL.PCR conditions were as follows:predenaturation at 95 ℃ for 10 min; Denaturation at 95 ℃ for 10 s;Annealing at 58 ℃(β-actin F and β-actin R at 58 ℃, Skp2 F and Skp2 R at 60.5 ℃) for 30s, extension at 72 ℃ for 30 s, 40 cycles.Images obtained by agarose gel electrophoresis of PCR products,an ultra-high sensitivity chemiluminescence imaging system, using β-actin as the internal reference, primer names and sequences are shown in Table 1.
2.2.4 Cell proliferation inhibition rate detection
After the cells were attached to the wall for 24 h, the 96-well plate cells were tested using the same medium, 100 μL per well;10 μL CCK8 reagent was added to each well, and incubated in the incubator for 2 h.The absorbance value(IOD value)of each well at 450 nm wavelength was detected by enzymoscope.After the cells were attached to the wall for 24 h, the 96-well plate cells were replaced with the same medium, 100 μL/ well medium; Skp2 mRNA expression inhibition rate(%)was calculated as follows: 1-siRNA groups of Skp2 mRNA/NC group of Skp2 mRNA×100%.

Tab 1 Names and sequences of primers
2.2.5 Statistical processing
SPSS 18.0 software was used for statistical analysis, and the mean values between groups were compared by T-test and ordinary oneway analysis of variance.Experimental data were expressed as mean± standard deviation(±s), andP<0.05 indicated that the differences were statistically significant.
3.The results
3.1 Rebuild plasmid enzyme identification and sequencing identification
YCS-WT-Skp2-pmirGLO and YCS-MUT-Skp2-pmirGLO carrier by PCR amplification bands of 578bp, as shown in figure 1.The electrophoresis results coincide with the theoretical values, the sequencing results and design the same pieces of DNA sequences,confirm all are correct plasmid, the purpose of the restructuring of the interference plasmid pieces perfectly accurate forecasts.
3.2 Recombinant plasmid transfection of Skp2 mRNA expression
Recombinant plasmid after transfection, siRNA-59, siRNA-318 and siRNA-504 transfection group Skp2 mRNA expression was lower than that in group NC, with significant difference(P<0.05), and the Skp2 mRNA expression in siRNA-504 was the lowest; And no-load and NC groups, statistically significant difference(P<0.05), after siRNA interference experiment groups of Skp2 mRNA expression,as shown in Table 2.Recombinant plasmid transfection of Skp2 mRNA expression of as shown in Figure 2.At the same time, further using DNA electrophoresis to validate the results, the results with each group after recombinant plasmid transfection Skp2 mRNA consistent results.The expression levels of no-load group, NC group,siRNA-59 group, siRNA-318 group and siRNA-504 group were from left to right, and the grouping order was consistent with the concentration determination.β-actin and Skp2 bands were normal in brightness and in correct position, as the target products, see Figure 3.
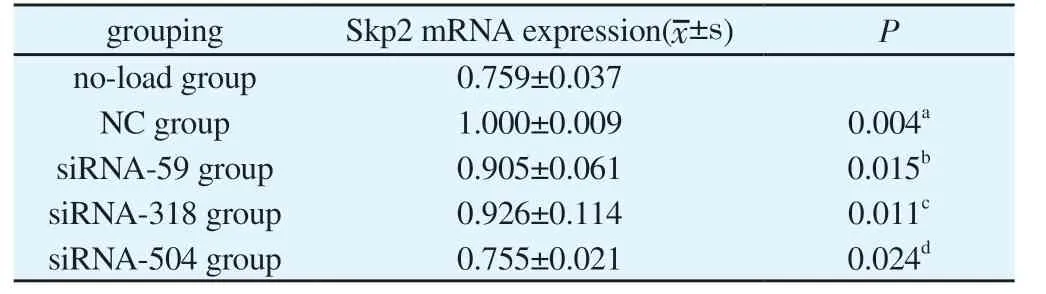
Tab 2 Skp2 mRNA expression quantity after siRNA interference in all experimental groups(n=3)

Fig 2 Effect of recombinant plasmid transfection on Skp2 mRNA expression
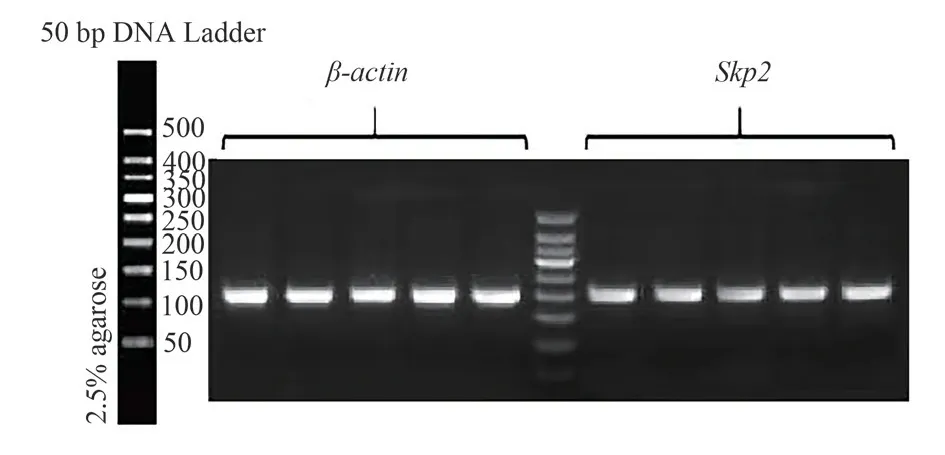
Fig 3 DNA electrophoresis results
3.3 Cell proliferation inhibition rate detection
Cell proliferation inhibition rate detection: 1-siRNA groups of Skp2 mRNA/NC group of Skp2 mRNA×100%, calculate the group of siRNA-59, siRNA-318, siRNA-504 cell proliferation inhibition rate,the results were: 60%, 41% and o64%, of which siRNA-504 group of cell proliferation inhibition rate was the most significant.
4.Discuss
In 1998, Fire A et al[9], first discovered that Double-stranded RNA(dsRNA)was the pathogenic factor for posttranscriptologic gene silencing in C.elegans, and called this phenomenon RNAi.RNAi as a highly conserved during evolution of dsRNA molecules,one of the adjustable mRNA stability and translation nearly all human cells[3].In RNAi, there is an important endonuclease Dicer, it can be combined with dsRNA molecules, and from the dsRNA head or 3 side have small dsRNA for cutting of the suspension, to cut it into 2l-23nt long and 3’ end of fragments, namely siRNA[10].Has highly specific siRNA in the nucleotide sequences, can effectively to specific RNAi gene silence[3], because siRNA inside cells are attached to the RNA polymerase 1, 2 and 6 further amplification,and bind to AGO1 protein in a single-stranded form, Thus forming RNA-induced silencing complex(RISC), leading partial or complete complementary mrnas to interact with them, leading to the degradation of specific mrnas.Result in Post-transcriptional gene silencing(PTGS)[11].PTGS exist in natural biological cells and are a defense means for cells to resist the invasion of foreign nucleic acids and maintain the integrity of their genome[12].PTGS can introduce specific homologous dsRNAs into cells, so that target genes are not expressed or the expression level is decreased.Therefore, RNAi is also a simple and effective alternative to gene knockout[11].With the continuous development of RNAi technology, because of its high stability, high specificity and efficiency, it has been widely used in gene function and tumor treatment[13], but it is relatively rare in the field of OA.Therefore, the discovery and application of RNAi provide a unique and adaptable tool for biomedical research and become an important direction for the development of life science and drug research and development[14].RNAi is also expected to be an effective method for the treatment of OA, opening up a new way for the prevention and treatment of OA.
Skp2as Skp1-Cullin-F-box(SCF)in the E3 ligase an F-box protein,can be mediated by ubiquitin G1 checkpoint CDK inhibitors-p21 and p27, p57 and fork transcription factor 1 degradation to targeted cell cycle progression.Because theSkp2has the function of regulating the cell cycle[4], so its expression level influence tumor occurrence, development and prognosis, etc[2,15,16,17].At present,Skp2 has been widely studied in the field of tumor, but it is rarely studied in OA.Wang X et al[18], found thatSkp2exists in OA through comprehensive meta-analysis of GSE48556, GSE46750 and GSE32317 data sets.It is also believed thatSkp2overexpression can promote chondrocyte proliferation under high glucose condition[19].At the same time, the study found that[20], under the condition of rich nutrition, theSkp2SCFE3 ubiquitin ligase can maintain the nucleus of arginine methyl transferase coactivator 1(CARM1); Under starvation,Skp2activates AMP-dependent protein kinase(AMPK) in the nucleus.Activated AMPK phosphorylates fork-head transcription factor O3, resulting inSkp2downregulation and increased CARM1 protein level in the nucleus.Stable CARM1 acts as a necessary activator of the transcription factor EB to regulate the expression of autophagy and lysosome genes.Skp2plays a key role in the AMPK-Skp2-CARM1 signal axis[21], soSkp2is considered as a potential therapeutic target for autophagy related diseases[19].Shengxin prediction found[18] thatSkp2is a key gene in OA, and autophagy can eliminate damaged and dysfunctional organelle and macromolecule, and maintain the homeostasis in OA cartilage[22],soSkp2may play an important role in OA autophagy.Therefore, the observation ofSkp2expression in human chondrocytes provides a basis for the study ofSkp2targeted therapy in OA.
In this study,Skp2siRNA expression vector was successfully constructed in vitro by using an online siRNA design tool,Skp2sequence was used as the target site, three sequences were designed,and theSkP2siRNA expression vector was successfully transfected into human chondrocytes by using gene recombination technology.The results showed thatSkp2mRNA expression in siRNA-59,siRNA-318 and siRNA-504 transfection groups was significantly lower than that in NC group (P<0.05).The results were further verified by DNA electrophoresis, and the results were consistent with theSkp2mRNA results after transfection of recombinant plasmid in each group.Furthermore, the inhibition rate ofSkp2mRNA expression was observed.The experiment showed that the inhibition rate ofSkp2mRNA in siRNA-59, siRNA-318 and siRNA-504 were 60%, 41% and 64%, respectively, and the siRNA-504 transfection group had the highest inhibition rate.Skp2siRNA can effectively reduceSkp2mRNA expression and produce gene silencing effect,suggesting thatSkp2may be a target gene for OA therapy, andSkp2site 504 May be an important target for its research.
In this study, the inhibition effect of siRNA onSkp2expression in human chondrocytes was successfully mediated through the construction of a vector, indicating that it is feasible in the field of OA gene therapy.With the improvement of siRNA technology, we have reason to believe that this technology may become an important part of OA gene therapy.
Contribution: Yuan Changshen.The content of this paper was revised by Li Yanhong, Liu Jinyi, Liao Shuning and Xu Wenfei.The article is designed by Duan Kan.The article was evaluated as Meiqijie.Yuan Changshen was the later proofreader of the article.siRNA sequence synthesis, cell transfection and RT-PCR detection were completed in collaboration with Jiangxi Zhonghongboyuan Biotechnology Co., LTD.
Conflict of interest: All authors declare that there was no conflict of interest during the research and writing of the paper.
杂志排行
Journal of Hainan Medical College的其它文章
- Research progress in mechanism of traditional Chinese medicine in the treatment of Knee Osteoarthritis
- Research progress and prospects of arenavirus
- Application of OCTA in diabetic retinal microangiopathy
- Meta-analysis effects of levosimendan on mortality and hemodynamics in patients with sepsis
- Meta-analysis of influencing factors associating with treatment outcome of multidrug resistant tuberculosis
- Association of novel and legacy PFAS with reproductive hormones in women of child-bearing age
