Advances in the mechanism of action of metformin in pituitary tumors
2023-11-18LongYaoZhangYuHuaYinXueJianWang
Long-Yao Zhang,Yu-Hua Yin, Xue-Jian Wang
Abstract Pituitary tumors are common intracranial tumors, but when faced with drugresistant or aggressive tumors, existing medical measures may not provide good control, leading to progression and deterioration.Metformin, a traditional hypoglycemic drug, has recently been discovered to have multiple functions including antitumor effects.There have been studies on the mechanism of metformin for the treatment of pituitary tumors, but it is uncertain whether it will provide new adjuvant or alternative therapies for the treatment of these tumors.We analyzed the potential mechanisms of action of metformin with respect to the inhibition of pituitary tumor growth and hormone secretion by reviewing the available literature.
Key Words: Metformin; Pituitary tumor; Mechanism; Treatment; Study; Review
INTRODUCTION
Pituitary adenoma is a common intracranial tumor, accounting for approximately 10% to 15% of neurological tumors, and its incidence is second only to glioma and meningioma[1-5].Pituitary tumors originate in the anterior pituitary gland and are usually benign lesions with slow growth.They are classified according to their size: Pituitary microadenomas (< 1 cm in diameter), macroadenomas (≥ 1 cm in diameter) and giant adenomas (> 4 cm in diameter)[6,7].According to their different growth sites,they can secrete different hormones such as growth hormone (GH), prolactin (PRL), adrenocorticotropic hormone (ACTH), and thyrotropin, or they can be nonfunctional adenomas that do not secrete hormones.Clinical manifestations mainly include the mass effect of the tumor and endocrine symptoms due to hyper- or hypofunction of the pituitary or target gland[8-10].Although most pituitary tumors can be controlled by drug therapy, surgery, and radiation therapy, some of these tumors may become drug resistant or recurrent, or even invade surrounding tissue structures, which may make treatment more difficult or prevent effective control of the tumor to achieve the desired therapeutic goals.A such,it is critical to find alternative therapies or new technologies to control the growth and hormone secretion of resistant or invasive pituitary tumors.
Metformin is a drug widely used in the treatment of diabetes mellitus, given its ability to reduce liver damage, promote insulin production, and increase insulin sensitivity and peripheral glucose utilization.In recent years, a number ofin vitroandin vivostudies and reviews have shown that metformin has the effect of inhibiting the growth of various types of tumors or cancers, including neuroendocrine tumors,through various mechanisms[11-19].This indicates that metformin may help to reduce the possibility of tumor or cancer occurrence and provide treatment benefits in patients.Although there are some epidemiological data demonstrating the relationship between metformin and risk reduction in patients suffering from multiple tumors or cancers, the role of metformin in cancer treatment is not yet fully clear[3,11,17,20-23].Here, we review the available literature on the role of metformin in pituitary tumors and discuss the potential mechanisms of action of this drug with respect to the treatment of these tumors (Figure 1).
MECHANISM OF ACTION STUDY
Mitochondria-mediated pathways
The B-cell lymphoma 2 (Bcl-2) family is a key regulatory member of the mitochondrial-mediated apoptotic pathway, activating the downstream death program, which in turn leads to caspase-3 enzyme cleavage and ultimately apoptosis, characterized by a decrease in mitochondrial membrane potential(MMP).
In one study, decreased MMP, increased expression of pro-apoptotic proteins, and decreased expression of anti-apoptotic proteins were observed in GH3 cells treated with metformin.This finding suggests the involvement of the mitochondria-mediated apoptotic pathway, indicating that metformin may induce apoptosis in GH3 cells by downregulating the Bcl-2/BAX ratio and inducing caspase-3 cleavage activation and thus achieve anti-tumor effects[24].In another study, metformin was observed to inhibit the proliferation of MMQ, cells and similar mitochondria-mediated apoptosis and experimental results were observed[25].
Another study observed that metformin inhibited the proliferation of ACTH-secreting mouse pituitary cortical dystrophoma cells AtT20, promoted apoptosis, and reduced ACTH secretion, but did not prevent progression of the cell cycle.Metformin-induced apoptosis was accompanied by an increase in caspase-3 activity, while metformin downregulated the anti-apoptotic protein Bcl-2 but upregulated the pro-apoptotic protein BAX, suggesting the involvement of a mitochondria-mediated apoptotic pathway[26].However, a different study suggested that metformin does not increase apoptosis in GH3 pituitary tumor cells, possibly due to the experimental design or the nutritional environment used; this effect of metformin needs further investigation[27].
AMPK-mediated related pathways
In one study, adenosine monophosphate activated protein kinase (AMPK) was found to mediate growth inhibition or apoptosis of many types of tumor cells[2,28-30].As metformin is an activator of the AMPK pathway[2], it has been suggested that it activates AMPK by restricting complex I in the mitochondrial respiratory chain, generating cellular energy stress, and thus activating AMPK[27,31-33] and indirectly by increasing the [AMP]:[ADP] ratio[28].However, it is not clear what the role of metformin may be in pituitary tumors, raising concerns about its mechanism of action in pituitary tumor cells.
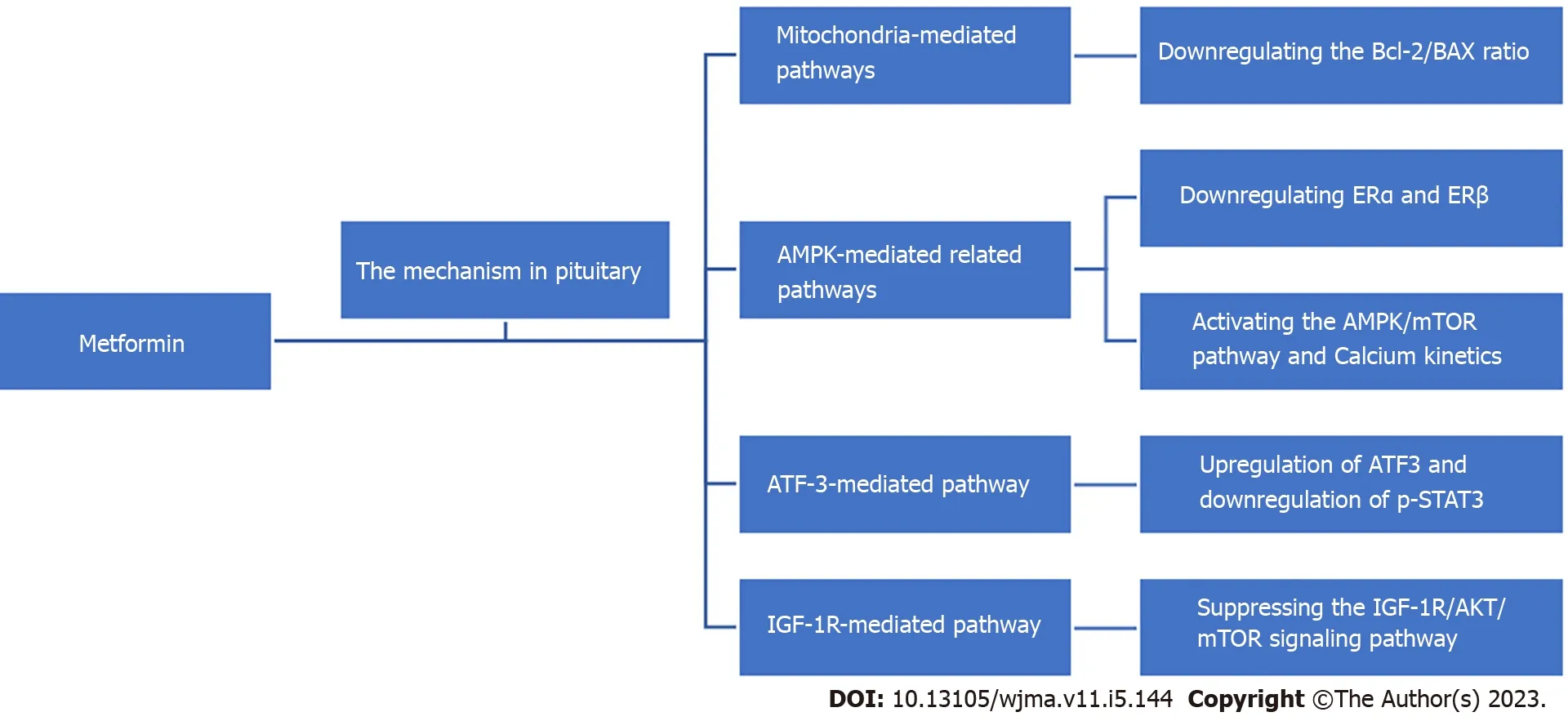
Figure 1 Potential mechanisms of metformin in the treatment of pituitary tumors.AMPK: Adenosine monophosphate activated protein kinase; ATF:Activating transcription factor; AKT: Protein kinase B; BAX: Bcl-associated X, IGF: Insulin-like growth factor; mTOR: Mammalian target of rapamycin.
Previous work has noted sex-dependent effects of mesenchymal epithelial transition (MET) on serum PRL levels, suggesting that the hypothalamic-pituitary-gonadal axis may be a target of metformin.One study investigated the AMPK agonist by measuring AMPK phosphorylation in human primary prolactinoma samples using bromocriptine (BC)-sensitive MMQ cells and BC-resistant GH3 cells and their xenografts as models.The role of MET in prolactinoma and the downstream effectors were investigated.It was proposed that AMPK signaling is inhibited in D2R-positive BC-resistant human prolactinomas.The AMPK activator MET inhibited the proliferation of BC-sensitive (MMQ) and drug-resistant (GH3)prolactinoma cells.It has also been shown that bromocriptine resistance is associated with downregulation of AMPK activity and high estrogen receptor (ER) expression, and that MET downregulates ERα and ERβ by activating the AMPK signaling pathway and inhibits prolactinoma growth and PRL secretion[34].Overall, MET inhibits prolactinoma growth and PRL secretion by activating the AMPK signaling pathway.
It has been shown that metformin enhances phosphorylated AMPK expression and decreases phosphorylation levels of mammalian target of rapamycin (p-mTOR) expression in MMQ cells.Additionally, compound C, an AMPK inhibitor, reduces the inhibitory effect of metformin on p-mTOR expression.It has been suggested that metformin activates the AMPK/mTOR pathway, which may be part of the mechanism to inhibit MMQ cell proliferation and induce apoptosis and G0/G1 phase block[25].Meanwhile, metformin significantly increased the levels of phosphorylated AMPK, phosphorylated protein kinase B, and phosphorylated mTOR in AtT20 cells in a dose-dependent manner,demonstrating that metformin activated AMPK and inhibited mTOR in AtT20 cells, suggesting that the activation of AMPK/mTOR signaling pathway may be related to metformin-induced proliferation inhibition and apoptosis promotion in AtT20 cells.However, it remains to be verified whether the activation of AMPK is related to the reduction of hormone secretion[26].
However, another study found that in GH-secreting PitNET cells, metformin induced GH3 cells to inhibit the target of epidermal growth factor (EGF) -induced mTOR-p70S6 6 kinase signaling pathway.As a potential mechanism, it was suggested that downstream EGF receptors were incorporated into AMPK substrates, indicating that membrane receptors are direct targets and may be involved in mediating their inhibitory effects on cell growth.In this study, the presence of AMPK targets, including cell surface receptors in GH3 cell membranes, was demonstrated using protein fractions[27].
Calcium has been reported to be a relevant second messenger for pituitary cell physiology.It has been shown that the effect of metformin on PitNET may involve AMP-activated protein kinasedependent calcium kinetics, thereby altering cell viability.However, the altered calcium kinetics induced in different pituitary tumor cells are variable, suggesting that metformin inhibits different types of pituitary tumor cells differently, and that the observed altered calcium kinetics appear to be related to hormone secretion[35].
Activating transcription factor-3-mediated pathway
Activating transcription factor 3 (ATF3) is a stress response transcription factor belonging to the ATF/CREB family.In one study, ATF3 was found to be upregulated by metformin, and its knockdown significantly reduced metformin-induced apoptosis, suggesting that ATF-3 may mediate the proapoptotic effect of metformin.The inhibitory effect of compound C on AMPK did not alter the inhibitory effect of metformin on STAT3 activity, suggesting that metformin may reduce GH secretion by inhibiting non-AMPK-dependent STAT3 activity.Metformin also significantly inhibited cell proliferation and GH secretion in primary human growth hormone-secreting pituitary adenoma (GH-PA)cells.Upregulation of ATF3 and downregulation of p-STAT3 were also demonstrated in xenografts.It was revealed that metformin inhibited the growth of somatic dystrophic adenoma cells bothin vitroandin vivothrough ATF-3-mediated pro-apoptotic effects.These findings suggest that metformin is a potentially promising therapeutic agent for the treatment of GH-PA[24].
Insulin-like growth factor -1R-mediated pathway
Insulin-like growth factor (IGF) -1R is an important growth factor receptor that activates the downstream phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mTOR pathway upon binding to IGF-1.The overactivation of this pathway is associated with tumor development.It has been observed that IGF-1R, p-AKT (S473), and p-ERK levels decreased with increasing metformin concentration after treatment.The IGF-1R inhibitor PPP inhibited MMQ cell proliferation, suggesting that metformin may inhibit cell proliferation by inhibiting the IGF-1R pathway in MMQ cells[25].These results suggest that metformin may inhibit cell proliferation by inhibiting the IGF-1R pathway in MMQ cells.
In another study, metformin decreased IGF-1R expression, AKT (S473) phosphorylation, and mTOR(Ser2448) phosphorylation, which inhibited AtT20 cell proliferation, Moreover, PPP (an IGF-1R inhibitor) significantly inhibited AtT20 cell proliferation in a dose-dependent manner, suggesting that IGF-1R plays a role in tumor progression.Taken together, these findings suggest that metformin may inhibit AtT20 cell proliferation by suppressing the IGF-1R/AKT/mTOR signaling pathway[26].
DISCUSSION
It is known from the above studies that there may be multiple pathways for the effect of metformin on pituitary tumors, but the complete mechanisms of these different pathways are not entirely clear.Moreover, the findings and opinions on the same pathway are not consistent.The effects of metformin have been attributed to its actions on different cells or in different environments.Indeed, it has been pointed out that the effect of metformin at the cellular level depends on the metabolic characteristics and metabolic demands of the cells, and the tumor microenvironment may influence this response.Pyruvate metabolism branching points are likely to play a major role in the variability of the cellular response to metformin, a role supported by significant differences in pyruvate dehydrogenase complex expression levels between myogenic cells and pituitary tumor cells[36].Research conductedin vitroand in and clinical trials are still limited or unavailable; as such, more evidence is needed to verify the accuracy of these ideas.
There isin vitroevidence suggesting that it may not be feasible to achieve high concentrations of metformin in humans[37,38].The observation that the prevalence of various tumor types is lower in patients with type 2 diabetes on regular metformin doses and that serum concentrations of metformin are much lower than those that inhibit cancer cellsin vitroraises the possibility that the mechanism of tumor preventionin vivowith regular therapeutic doses of metformin may be largely indirect and related to metformin ameliorating such metabolic or hormonal abnormalities such as obesity,hyperglycemia, and hypertension.It is also important to consider that there may be physiological metabolic differences between rat pituitary tumor cell lines and human pituitary tumor cell lines,among others.
Despite these studies, metformin has not been formally used as a clinical treatment for pituitary tumors.There have been case reports of reduced prolactin levels and tumor size in 2 patients treated with a combination of bromocriptine and metformin, whereas bromocriptine alone was not sufficient to reduce prolactin levels or slow tumor growth[39].In another case report, the combination of bromocriptine and metformin reduced prolactin levels and tumor size.In a third case report, the combination of metformin and capsaicin did not show consistent inhibition of serum prolactin levels in either the short- or long-term in 10 patients with prolactinoma resistant to capsaicin[40].Additional studies have evaluated the effects of metformin on cell viability and hormone secretion when combined with other agents; for example, metformin/somatostatin (SSA) analog combination therapy did not increase the effectiveness of SSA monotherapy[34].Metformin/SSA combination therapy did not increase the effectiveness of SSA monotherapy, but did appear to enhance the role of octreotide in GHomas, and MET + BC significantly inhibited PRL secretion, further reducing tumor growth and serum PRL levels in xenografts when compared to BC treatment alone[35].However, in the face of metformin treatment, the tumor growth and serum PRL levels in xenografts were further reduced.
The heterogeneity among patients with pituitary tumors and the diversity of drug treatment options add to the complexity of disease treatment, and further studies are needed to demonstrate whether treatment with metformin alters the risk of pituitary tumor morbidity and mortality and to determine the dose and duration of treatment and the effect when combined with other drugs.It is also important to consider whether it is reasonable to use metformin to treat pituitary tumors in patients without diabetes and to pay mind to the potential side effects or complications of using different concentrations of metformin in humans.Attention to these possible issues could help to improve the management of pituitary tumor patients in a more individualized manner.Given the available data, the use of metformin may be a promising and clinically relevant option for patients with pituitary tumors.Further studies are needed to confirm metformin’s clinical relevance as an adjuvant or novel therapy and to further develop a comprehensive understanding of the potential antitumor mechanisms of this drug in the treatment of pituitary tumors.
CONCLUSION
Metformin, a traditional hypoglycemic drug, has recently been discovered to have multiple functions including antitumor effects.There have been several studies on the mechanism of metformin for the treatment of pituitary tumors, but it remains to be investigated whether it will be incorporated as an alternative therapy for the treatment of these tumors.
FOOTNOTES
Author contributions:Wang XJ and Zhang LY contributed to conceptualization; Zhang LY contributed to writing the original draft preparation; Yin YH contributed to review and editing of the manuscript; All authors have read and agreed to the final version of the manuscript.
Supported bythe Science and Technology Program of Nantong Health Committee, No.MA2019003, No.MA2021017,No.Key003; Science and Technology Program of Nantong City, NO.MS12015016, and No.JCZ2022040; and Kangda College of Nanjing Medical University, No.KD2021JYYJYB025, No.KD2022KYJJZD019, and No.KD2022KJJZZD022.
Conflict-of-interest statement:All the authors declare no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Long-Yao Zhang 0000-0001-7460-7903; Yu-Hua Yin 0000-0003-3760-0264; Xue-Jian Wang 0000-0003-0389-5674.
S-Editor:Liu JH
L-Editor:Filipodia
P-Editor:Yu HG
杂志排行
World Journal of Meta-Analysis的其它文章
- Exploratory systematic review and meta-analysis on period poverty
- Vitamin D deficiency among outpatients and hospitalized patients with diabetic foot ulcers: A systematic review and meta-analysis
- Pulmonary cytomegalovirus infection: A case report and systematic review
- Real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the Delta and Omicron variants: Systematic review
- Haploidentical hematopoietic stem cell transplantation as promising therapy in the improved survival of pediatric patients with leukemias and myelodysplasias
- Diabetes mellitus: An overview of the types, prevalence, comorbidity, complication, genetics, economic implication, and treatment
