Engineering of Sodium-Ion Batteries: Opportunities and Challenges
2023-11-14LinaZhaoTengZhangWeiLiTaoLiLongZhangXiaoguangZhangZhiyiWang
Lina Zhao*, Teng Zhang, Wei Li, Tao Li, Long Zhang, Xiaoguang Zhang,Zhiyi Wang
a Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
b School of Materials Science and Engineering, Peking University, Beijing 100871, China
c Beijing Innovation Center for Engineering Science and Advanced Technology (BIC-ESAT), Beijing 100871, China
d Beijing Key Laboratory for Magnetoelectric Materials and Devices (BKL-MMD), Beijing 100871, China
Keywords:Electrical energy storage Sodium-ion batteries Commercialization Next generation
ARTICLEINFO The recent proliferation of sustainable and eco-friendly renewable energy engineering is a hot topic of worldwide significance with regard to combatting the global environmental crisis.To curb renewable energy intermittency and integrate renewables into the grid with stable electricity generation,secondary battery-based electrical energy storage (EES) technologies are regarded as the most promising solution,due to their prominent capability to store and harvest green energy in a safe and cost-effective way.Due to the wide availability and low cost of sodium resources, sodium-ion batteries (SIBs) are regarded as a promising alternative for next-generation large-scale EES systems.This review discusses in detail the key differences between lithium-ion batteries (LIBs) and SIBs for different application requirements and describes the current understanding of SIBs.By comparing technological evolutions among LIBs, leadacid batteries (LABs), and SIBs, the advantages of SIBs are unraveled.This review also offers highlights on commercial achievements that have been realized based on current SIB technology, focusing on an introduction of five major SIB companies,each with SIB chemistry and technology,as well as commercialized SIB products.Last but not least,it discusses outlooks and key challenges for the commercialization of next-generation SIBs.
1.Introduction
1.1.Renewable energy penetration
Energy is the engine that sustains the economy and modern life.Primary energy resources have different forms, such as fossil fuels(i.e., coal, oil, and natural gas), nuclear energy, and renewable energy (i.e., solar, wind, geothermal, and hydropower).These primary energy sources can be converted into electricity,a secondary energy source, which flows though transmission infrastructures and power lines into our homes and businesses.
Electricity dominates the market in energy use.Ongoing needs for electricity supply are growing at a faster rate than overall energy consumption.The total global electricity generation capacity by source in 2018 was reported to be ~25 000 TW·h.Currently,approximately 64% of the total electricity generation is obtained from fossil energy (coal 38%, natural gas 23%, and oil 3%), while 10%comes from nuclear energy and the remaining 26%comes from renewable energy (Fig.1).Coal burning is the dominant form of power generation and is predicted to remain as the largest energy source for power generation in the next several decades.However,the extensive use of fossil fuels causes severe issues related to climate change, the energy crisis, and environmental pollution.Nearly 25% of global greenhouse gas emissions are from fossilfuel-based electricity production, particularly coal burning.Every kilowatt-hour of electricity capacity obtained from coal burning can produce almost 1 kg of life-cycle carbon dioxide (CO2) emissions;thus,coal burning is commonly considered to be the largest contributor to global warming [1].The amount of CO2emissions from coal burning is estimated to linearly increase during 2015–2040.Therefore, users of fossil fuels for electricity production,which has long been indispensable for power generation,are facing multiple social, political, and environmental pressures to aim for carbonless emission.
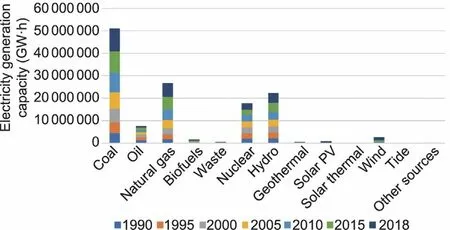
Fig.1.Illustration of global electricity generation by energy source from 1990 to 2018.PV: photovoltaic.Credit: Courtesy of the International Energy Agency, with permission.
The global energy system is currently undergoing a major transition toward a more sustainable and eco-friendly energy layout.Renewable energy is receiving a great deal of attention and increasing market interest due to significant concerns regarding the overuse of fossil-fuel energy and climate change [2,3].Solar power and wind power are the richest and most easily available renewable energy sources [4,5].Receiving just 1 h of solar energy from sun’s radiation on the earth would be enough to meet the whole world’s electrical energy requirements for one year.Capturing just a small portion of the world’s wind energy could also fulfill the world’s demand for electricity.Aside from its advantages of being environmentally friendly and decarbonizing, another merit of renewable energy is its modular nature;this offers the possibility and feasibility of distributed power stations, which would be beneficial in decreasing the economic cost and environmental hazard of electricity distribution and transmission from centralized power-plant locations.It is notable that solar photovoltaic (PV)and wind technologies lead to a sharp reduction in electricity cost;therefore,there has been strong growth in deploying renewables in the past decade.In 2018,about 7%(450 TW·h)more electricity production occurred from renewable energy resources than in 2017,such that renewable sources now exceed a quarter of the world’s total power generation.As shown in Fig.2, renewable-energybased electricity generation continues to increase progressively.The share of total renewable electricity generation is predicted to increase by 31% by 2035.
1.2.Battery-based electrical energy storage
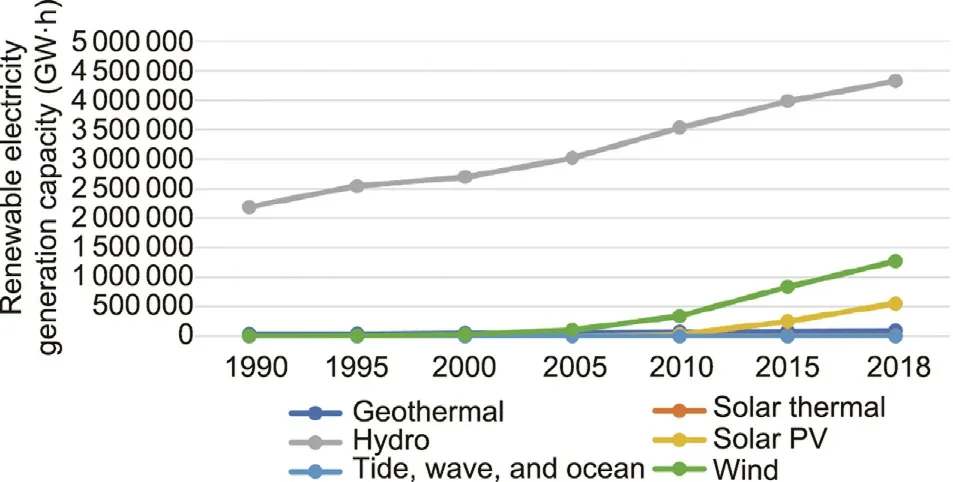
Fig.2.Illustration of renewable electricity generation by source.Credit:Courtesy of the International Energy Agency, with permission.
Cost reduction and the advantages of using renewable energy for developing a low carbon economy provide huge opportunities for energy storage and conversion.There is an urgent need for the development and utilization of renewable energy for the electricity supply.However,the major obstruction for most renewable energy sources lies in their intermittent properties[3].A grid needs a continuous and stable electricity supply for normal operation;however, renewable energy sources are hindered by various environmental limitations such as season,weather,and location,which are commonly regarded as unfavorable factors for power grids.Solar power generation can only occur when there is sunshine;almost none can occur during the nighttime.Similarly,wind power sometimes yields gigawatts and at other times produces just a few megawatts;of course,when there is no wind,then no wind power can be generated.As renewable energy sources begin to supply an increasing percentage of power generation and supply to the grid,integrating them into the current grid operations will become difficult.
At present,the current global energy systems are facing a range of challenges, including an increase of renewable energy penetration and the electric vehicle (EV) market, ongoing growth in the demand for carbonless emissions, aging facilitates, and energy safety.Smart grids offer ways to not only solve these challenges,but also transition the energy industry into a new era of more reliable, available, and efficient systems that will contribute to both environmental and economic health.Fig.3 shows a roadmap for the transition of the global electricity systems from the current electricity operation model toward a smarter grid in future.In short, a smart grid is an electricity network that enables a twoway flow of electricity and data, with digital communications and other advanced technologies making it possible to detect,react,and‘‘pro-act”(i.e.,proactively respond based on predictions)to changes in usage and multiple issues.The benefits of a smart grid include enhanced efficiency and reliability of electricity transmission and supply, reduced operational and managemental costs for utilities and consumer terminals,increased integration of largescale renewable energy sources into the modern grid, support for the deployment of large-scale EVs, improved energy safety, and lower carbon emissions [6].
To achieve the purposes of smart grids, the development and deployment of low-cost large-scale electrical energy storage(EES) technologies are critically important for realizing a cleaner and more sustainable energy future [7,8].Batteries stand out as an important clean energy technology due to their ability to produce electricity from chemical energy and vice versa [9,10].Battery-based EES systems are highly valued as a way to meet various grid functions by providing a number of accessorial services,including ① frequency regluing and load following; ② coldstarting;③acting as emergency reserves;④permitting an energy balance to be maintained between energy peaks and off-peaks;and⑤offering more localized power to resolve quality issues and providing reactive power support.
1.3.Lithium-ion batteries and the current market
Rechargeable lithium-ion batteries (LIBs) are a state-of-the-art EES system with various advantages, including high energy density, high volume density, and a long service lifetime [11].In the past three decades since their first commercialization in 1991,LIBs have revolutionized people’s living styles [12].The Nobel Prize in Chemistry in 2019 was awarded to John B.Goodenough,M.Stanley Whittingham, and Akira Yoshino for their contributions to LIB development.This lightweight,rechargeable,and powerful battery has been extensively used in a wide range of applications in smallscale consumer electronics,from the mobile phones to the laptops that we use to communicate,study,work,entertain ourselves,and search for knowledge.LIBs have also enabled the development of the next generation of EVs, plug-in hybrid electric vehicles(PHEVs),as well as large-scale grid storage from renewable energy sources such as solar and wind power,opening up the possibility of a fossil-fuel-free society.
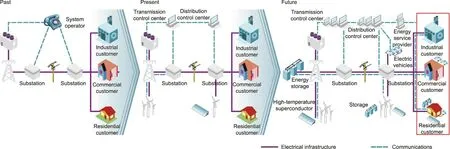
Fig.3.Roadmap of the transition of current energy systems toward a smarter grid.Credit: Courtesy of the International Energy Agency, with permission.
With the wide spread of LIBs, especially after their entry into the transportation-sector market, the manifold consumption of lithium (Li) and lithium-based chemistries has resulted in a steep rise in price and deep concerns over future geopolitical tension due to poor lithium source reserves and nonuniform geographic distribution [13,14].The price of LIBs depends almost entirely on the scarcity of lithium, which can be only found in compounds in nature due to its high reactivity.Lithium constitutes about 0.0017 wt%of the Earth’s crust.According to the US Geological Survey (USGS), the global lithium reserves in the years from 2017 to 2020 were estimated to be 14 million, 16 million, 14 million, and 17 million tonnes, respectively.The average consumption of lithium carbonate (Li2CO3), an important industrial chemical that is widely used in the manufacturing of most LIB cathodes, is projected to grow annually by 16.7% within the next six years.Without recycling, the world’s lithium reserves can sustain economically viable production runs for only 28 years.Furthermore, the current status of lithium shortages will threaten the EV market supply,since the most directly available resources are geographically concentrated.As the country with the largest lithium reserves in 2019, Chile has around 8.6 million tonnes of lithium reserves (Fig.4(a)).The second to fifth countries with the largest lithium reserves in 2019 were Australia (2.8 million tonnes),Argentina (1.7 million tonnes), China (1.0 million tonnes), and the United States (0.63 million tonnes), respectively.The gradual expansion of the EV market will put pressure on lithium supplies,driving an ongoing increase in the cost of lithium worldwide.
The global LIB market is expected to increase sustainably in the coming decade, from less than 30 billion USD in 2017 to over 90 billion USD by 2025 (Fig.4(b)).Since the demand for PHEVs and EVs powered by LIBs is continually increasing, the EV sector will also push forward the growth of the LIB market.In 2019, Asia-Pacific(APAC)dominated the global LIB markets, and China is still in the world’s leading position of electronic device producer.The top LIB producers in these regions were estimated in 2019 to be Panasonic Sanyo (Japan), Sony (Japan), Contemporary Amperex Technology Co., Ltd.(CATL; China), Samsung (Republic of Korea),and LG Chem (Republic of Korea).The expanding LIB market generate tension regarding a potential shortage in lithium reserves and further price increases.Given their resource depletion and rising costs,it appears that the world’s lithium reserves cannot simultaneously satisfy the ongoing and increasing demands of industrial development in the applications of both transportation and the grid [15].With the aforementioned resource constraints and cost concerns, there is a great need to achieve the challenging task of finding an alternative, installation-flexible and -scalable, costeffective, energy-efficient, and environmentally friendly battery system that can match the performance and commercial achievement of LIBs.
2.Sodium-ion batteries: A potential alternative to LIBs
2.1.The revival of room-temperature sodium-ion batteries
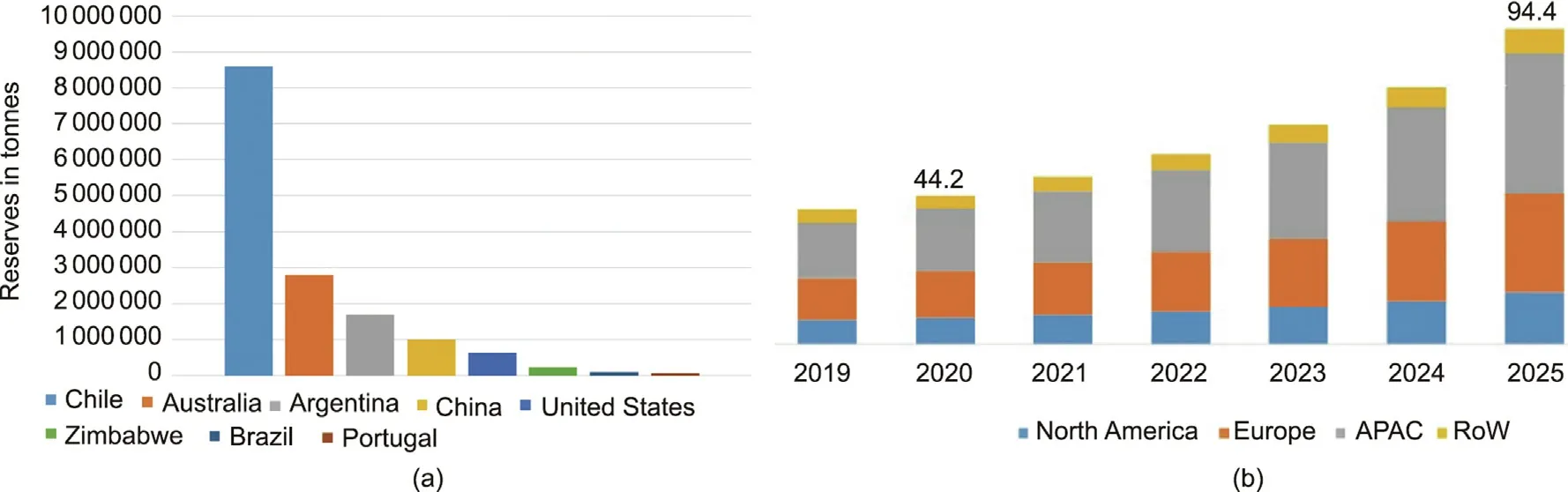
Fig.4.(a)Countries with the largest lithium reserves in 2019.Credit:US Geological Survey,with permission.(b)LIB market by region(billion USD).APAC:Asia-Pacific;RoW:rest of the world.Credit: Courtesy of the International Energy Agency, with permission.

Fig.5.(a) Abundance of elements in the Earth’s crust; (b) schematic illustration of an SIB; (c) comparison of the physicochemical properties of Li+ and Na+ for secondary batteries.SHE: standard hydrogen electrode; PC: propylene carbonate.
Due to the abundant sodium (Na) reserves in the Earth’s crust(Fig.5(a))and to the similar physicochemical properties of sodium and lithium, sodium-based electrochemical energy storage holds significant promise for large-scale energy storage and grid development.For example, high-temperature zero emission battery research activity (ZEBRA) cells based on Na/NiCl2systems [16]and high-temperature Na–S cells [17], which are successful commercial cases of stationary and mobile applications [18], have already demonstrated the potential of sodium-based rechargeable batteries.However, their high operating temperature of around 300 °C causes security issues and decreases the round-trip efficiency of sodium-ion batteries (SIBs) [7].Room-temperature (RT)SIBs are therefore widely regarded as the most promising alternative technology to LIBs [19–21].
Over the history of batteries in the past 200 years, research on SIBs was fervently carried out side-by-side with LIB development[22–24].The electrochemical activity of TiS2for lithium and its feasibility for energy storage was first put forward in the 1970s.Following this discovery, the capability of Na+ions to be inserted into TiS2was realized in the early 1980s.With the discovery of graphite as a low-cost and moderate-capacity anode material for LIBs and the failure to intercalate sodium ions, rapid LIB development occurred in the 1990s, superseding the growth in sodium chemistry[19].Then,in 2000,the availability for sodium storage in hard carbon (HC), which would deliver an energy capacity similar to that of Li+in graphite, rejuvenated research interest in SIBs.
2.2.A comparison of sodium and lithium
The revival of SIBs—coupled with the ever-increasing pressure from the lack of availability of lithium reserves and the corresponding escalation in cost—provides a complementary strategy to LIBs.SIBs have gained increasing research attention, combined with fundamental achievements in materials science, in the drive to satisfy the increasing penetration of renewable energy technologies[25,26].The cell components and the electrochemical reaction mechanisms of SIBs are basically identical to those of LIBs, except for the charge carrier,which is Na+in one and Li+in the other.The major reason for the rapid expansion in SIB materials chemistry is ascribed to the parallels in physicochemical properties between the two alkali metals.
First, the operating principles and cell construction of SIBs are similar to those of commercial LIBs, albeit with Na+serving as the charge carrier[27–31].As shown in Fig.5(b),four main components exists in a typical SIB: a cathode material (usually a Nacontaining compound); an anode material (not necessarily containing Na);an electrolyte(in a liquid or solid state);and a separator.During the charge process,sodium ions are extracted from the cathodes,which are typically layered metal oxides and polyanionic compounds, and are then inserted into the anodes [32–34], while the current travels via an external circuit in the opposite direction.When discharging,Na+leaves the anodes and returns into the cathodes in a process referred to as‘‘the rocking-chair principle.”These similarities have enabled the preliminary understanding of and rapid growth in SIB technology.
Furthermore, material synthesis methods and routes can easily be borrowed from lithium compounds and adapted for sodium compounds.Thus, the existing infrastructures used for manufacturing LIBs, such as pouches and prismatic and cylindrical cells,can be directly employed with minor modifications to make SIBs.This similarity is promoting the foundation of SIB companies,such as Faradion, Ltd.(UK) and HiNa Battery Technology Co., Ltd.(China), leading to rapid growth in the commercialization of SIBs.
In terms of the inherent nature of the two elements, there are something in common between sodium and lithium.A detailed comparison of the physicochemical characteristics of sodium and lithium indicates why Na+was once thought to be equally important as Li+for energy storage.Both lithium and sodium are located in Group 1 of the periodic table, and are thus referred to as alkali metal elements.Both possess one outermost electron belonging to the s orbit,which results in high reactivity[27–31].Fig.5(c)displays some of the physicochemical parameters that are of great importance in SIB development for energy storage.
Na+has a larger ionic radius(1.02 vs 0.67 Å,1 Å=10-10m)and heavier atom weight (23 vs 6.94 g·mol-1) than Li+[35], indicating that the electrochemical equivalent in SIBs is more than three times heavier than that in LIBs.It can be expected that this increase will result in Na+exhibiting worse transport kinetics, lower phase stability with interphase formation, low solubility in solids, unfavorable gravimetric and volumetric energy density,and low Madelung energies, resulting in lower operation voltage [36].
Moreover, one of the important parameters in a comparison of lithium and sodium comparison is their redox potential.Sodium has a higher standard electrode potential than lithium (-2.71 vs-3.02 V), thus setting a thermodynamic minimum limit for anode materials in most instances, which results in SIBs having a lower energy density than LIBs.However, the mass of the charge carrier represents a small percentage of the overall weight of the active component;thus,the difference in the theoretical specific capacity of the electrode materials becomes smaller.Assuming that the LiCoO2and NaCoO2cathodes have the same crystallographic structure for one electron transition reaction based on the Co3+/Co4+redox, the reduction of the calculated theoretical capacity (274 and 235 mA·h·g-1) is small.A compensation for this capacity sacrifice can potentially be made through innovation in structural modification of the material.Furthermore,the larger mole volume of sodium (39.3 Å per Na atom vs 21.3 Å per Li atom) leads to a much lower volumetric energy of sodium metal than of lithium metal.Similarly, the volumetric capacity between LiCoO2and NaCoO2cathodes is quite close due to the small difference in mole volume for LiCoO2and NaCoO2[19].The power/energy loss in SIBs could potentially be resolved if the final realization of SIB technology depends on Na+in the future, rather than on sodium metal;therefore, SIBs have been regarded as one of the best potential alternative strategies for LIBs.
Furthermore, the larger ionic radius of Na+brings its own advantages: increased flexibility of electrochemical positivity and decreased de-solvation energy in polar solvents.The greater gap in the ionic radius between Li+and the transition metal ions(Mx+) usually leads to failure of the flexibility of material design.In contrast, a sodium-based system enables more flexible solid structures than a lithium-based system, and possesses enormous ionic conductivity.A typical example is β-Al2O3, for which Na+intercalationhas the perfect size and high conductivity.More layered transition metal oxides with different Mx+stacking manners can be easily realized in a sodium-based system.Similarly, the wide variety of crystal structures that are known for sodium ionic conductor (NaSICON) family is much more complicated than that of the lithium analogs.More importantly,a much higher ionic conductivity can be allowed in NaSICON compounds, which by far exceeds the ionic conductivity in lithium ionic conductor(LiSICON)compounds.
On the other hand, systematic investigations with different aprotic polar solvents have demonstrated that the larger ionic radius of Na+causes a weaker desolvation energy[37].The smaller Li+has a higher surface charge density around the core than Na+when both possess the same valence.Li+is therefore thermodynamically stabilized by sharing more electrons with the polar solvent molecules.That is,Li+can be classified as a type of Lewis acid.As a result, a relatively high desolvation energy is needed for the highly polarized Li+,leading to a relatively large transfer resistance being induced by the transport of Li+from the liquid state (electrolyte) to the solid state (electrode).Since the desolvation energy is closely related to the transfer kinetics occurring at the liquid/solid interface [38,39], the relatively low desolvation energy is a significant advantage for designing high-power SIBs.
2.3.Advantages of SIBs
As a cost-effective replacement to LIBs, RT SIBs exhibit several merits compared with the current battery-based technologies.Fig.6 shows a comparison of three established rechargeable battery technologies in the current market: SIBs, LIBs, and lead-acid batteries (LABs).
2.3.1.Cost-saving
The Earth’s crust contains 2.27%sodium, making it the seventh most abundant element on Earth and the fifth most abundant metal.Furthermore,the oceanic abundance of sodium is estimated to be 1.08×104mg·L-1[40],presenting a seemingly unlimited global distribution.Sodium can be extracted from seawater, which indicates that Earth’s sodium reserves are effectively infinite.Moreover, many natural sodium-containing minerals have been discovered, along with the corresponding crystal information recorded.The reserves of sodium compounds are vast,and are relatively cheaper than those of lithium-containing chemicals.Take carbonate materials as an example:The cost of trona,the precursor for the production of sodium carbonate,is about 135–165 USD per tonne, in comparison with lithium carbonate at about 5000 USD per tonne in 2010.Thus, the cost of SIBs can be expected to be low if the materials for the electrode and electrolyte do not include rare earth elements.

Fig.6.A comparison of three established rechargeable battery technologies in the current market.
In addition,aluminum(Al)foil can be used as the current collector for both the cathodes and anodes of SIBs.In LIBs, the collector for the anode side must be made from copper foil, which is more expensive and much heavier than aluminum foil; this is because aluminum reacts with lithium via alloying at a low potential, but does not react with sodium.Battery-grade aluminum foil costs about 70 USD per meter, which is much cheaper than copper foil,at 210 USD per meter for battery grade; therefore, its use greatly decreases the overall cost of SIBs.
As mentioned above, since SIBs use the same producing protocols and manufacturing methodologies as LIBs due to the similarly operating ‘‘rocking-chair principle,” SIBs are a promising replacement for LIBs—not only for commercial applications, but also in terms of their producing/manufacturing processes.As a result, no additional capital cost is needed for the technology transition from LIBs to SIBs.However,a battery cost analysis must address comprehensive considerations,as battery cost is not a simple issue of material costs alone.Auxiliary features such as electrode design, cell structure, cell depreciation, and manufacturing and processing costs are also important factors.A cost-energy analysis was performed by Berg et al.[41] to compare the costs of LIBs and SIBs.Despite the cheap material costs of SIBs, an HC||NVPF (NVPF:Na1.5VPO4.8F0.7)sodium-ion full cell was calculated as having a total cost greater than that of a graphite||lithium iron phosphate (LFP)lithium-ion full cell,at 320 USD·(kW·h)-1versus 280 USD·(kW·h)-1.Peters et al.[42]applied the Battery Performance and Cost(BatPac)model to determine the final battery price for three different battery chemistries of the 18 650 battery type.The LFP battery showed the highest cell price (230 EUR·(kW·h)-1), which was followed by Faradion’s HC||sodium nickel manganese magnesium titanate oxide(NMMT)SIB cell(223 EUR·(kW·h)-1);the lithium nickel manganese cobalt oxide(NMC)-based LIB,at 168 EUR·(kW·h)-1,was the cheapest.Both results reveal that the energy density of the active materials is a significant cost factor determining the total price per kilowatt-hour of storage capacity.In this regard,identifying active materials with a high energy density would be highly beneficial for further developing cost-effective SIBs.
2.3.2.Energy density
The energy density of SIBs can be 1–5 times higher than that of LABs, depending on the material chemistry and technology used for the SIBs.However, it has commonly been assumed that SIBs would never exceed or reach the same level of energy density as their LIB analogs.This understanding was misled, as it was based on simply taking into account the heavier atomic mass of sodium to lithium and the higher standard electrode potential for Na+/Na redox in comparison with the Li+/Li couple.Such a theory is rational only for metal batteries in which lithium metal or sodium metal serves as the anode material.In rechargeable ion batteries,an anode can be made of any substance with electrochemical activity other than the alkali metal itself.It is important to realize that the energy density of rechargeable ion batteries is determined by the capacity of each individual anode and cathode material, along with the output voltage of the whole metal-ion battery [43,44].Strictly speaking,the output voltage of a full cell is simply dictated by the Gibbs energy change of the cell reaction.The intercalation potential of lithium and sodium is highly dependent on the host structure.In general, the higher the operating potential interval of the individual anode and cathode is,the higher the working voltage of a cell will be.According to these facts, there is no reason to believe that SIBs will be inferior to LIBs with respect to energy density.
In principle, high-energy-density SIBs are not out of reach.Recent developments have already resulted in several potential electrode materials exhibiting similar or even better electrochemical performance in comparison with those of LIBs [45,46].The working voltage is usually found to be lower in an SIB system because the cathode materials involve a lower redox couple in most cases.This is because the insertion of Na+into a host structure is energetically less favorable than Li+intercalation.Encouragingly, several high-voltage NaSICON-type cathodes with a redox activity of over 4.0 V have been reported,indicating the possibility of realizing high-working-voltage SIBs [47,48].Although the challenge remains, this advantage may be gained in future by relying on the exploitation of new electrode materials with high performance for SIBs.It should be noted that the usage of light aluminum foil as the current collector helps to further enhance the energy density of SIBs.
2.3.3.Safety
It is well known that some lead compounds are strongly toxic and harmful for human health.Although LABs themselves have been demonstrated to be safe during operation, their corrosive acid-based electrolytes cause environmental concern to some extent.Furthermore, while lead-acid battery recycling is a wellestablished program around the world, lead release caused by improper disposal and by the lead mining and manufacturing industry is unavoidable.
LIBs are quite stable if treated properly,but are susceptible to a thermal runaway (TR) under specific circumstances, including physical damage,electrical abuse such as overcharging or short circuits,and exposure to elevated temperature.LIBs’TR incidents are closely associated with their high-energy-density characteristics coupled with the use of a flammable organic electrolyte.Since many of the same solvents in the electrolyte used in LIBs can also be used in SIBs, the higher compatibility between HC and the propylene carbonate (PC) solvent is one of the direct advantages of SIBs over LIBs.Therefore, electrolytes with a higher PC content can be designed for SIBs by avoiding the use of the highly flammable diethyl carbonate(DEC)and dimethyl carbonate(DMC)that are preferred for LIBs, which will help to significantly enhance the safety of SIBs.
Aside from the consideration of electrolytes, the zero energy storage and transportation realized in SIB systems are another direct advantage in terms of safety issues.To minimize the possibility of copper foil dissolution when the voltage drops too low,LIBs are required to be transported under a particular state of charge (SOC)—typically about 30% [49].This requirement not only imposes a high level of restriction and significant safety risks, but also results in additional costs during transportation.SIBs that use aluminum foil as the current collector do not suffer from these issues.In fact,SIBs can be stored and transported under a fully discharged state of 0 V;that is,zero energy storage and transportation is possible for SIBs.Studies have demonstrated that keeping SIBs at 0 V for prolonged periods of time hardly affects their energy capacity and cell performance.Zero energy storage and transportation can be regarded as the ultimate safety condition and is a major advantage of SIBs in comparison with the well-established LIBs.
3.Global commercialization and strategy
SIBs have been touted as an alternative energy storage technology to LABs and LIBs in various application fields due to their low material cost, promising electrochemical performance, and high level of safety.However, daunting challenges remain that need to be addressed for SIBs to reach market-readiness.The performance of SIBs mainly depends on the designed battery chemistry; therefore, many different SIB prototypes for different purposes can be assembled.In particular, the development of high-performance electrode materials with high energy density and stable cycling is of great importance.Cathode materials play a decisive role in controlling the overall cost and energy density of a cell.SIB cathode materials that have prospects for industrialization can be categorized into three types: layered metal oxides [50,51],polyanion-type materials [52–57], and Prussian Blue analogs[58].These cathode materials stand out among the various available SIB material chemistries, due to their high gravimetric and volumetric energy density,and have already been used in commercial battery products with various application fields in the current SIB market.Anodes are electrode materials with low electrochemical potential in a cell.Considering the total energy density of SIBs,it is desirable to develop an anode material with a theoretically low potential and high specific capacity [59–61].
3.1.Faradion, Ltd.
Faradion,the first non-aqueous-SIB company in the world,was founded in 2011 in the UK by Jerry Barker, Chris Wright, and Ashwin Kumaraswamy, in order to identify and exploit the potential of rechargeable SIBs.Since its founding, Faradion has focused on developing non-aqueous sodium-ion technology and bringing it to the market.With its headquarter established in Sheffield,UK, Faradion has gathered a powerful technical team of experts in the field of industrial batteries with an impressive array of skills and experience.Faradion has developed a wide-reaching intellectual property (IP) portfolio comprising 21 patent families (including eight that have been granted) and focusing on three key areas of SIB technology: cell materials, cell infrastructure, and safety and transportation.With more patents in this area than competing SIB companies, Faradion has cemented its position as the world’s market leader of non-aqueous SIBs.
Faradion runs and commercializes non-aqueous SIBs based on a layered nickelate oxides cathode with an HC anode and a nonaqueous liquid electrolyte.Strenuous efforts had been made to screen active material combinations through extensive testing that enables the active materials to provide the optimal overall electrochemical performance in terms of specific capacity, cycling stability, rate capability, and safety.
At first, Faradion’s investigation into cathode materials focused on polyanion-type materials, which exhibit a robust host framework with high voltage and high structural stability [62,63].Faradion filed its first two patents in September 2011 and February 2012 on condensed mixed phosphates [64] and sulfate [65]polyanion materials, respectively.Despite the high discharge voltage and good cycling performance shown by polyanionic cathode materials,the low reversible capacities(<100 mA·h·g-1)delivered in the SIB full cell could not fulfill the demands of high-power applications.Faradion therefore quickly shifted its research attention to layered-oxide cathode materials with high theoretical specific capacities.Since 2012,Faradion has been delving into various types of sodium-containing layered oxides with rich and complex structures.The first-generation Faradion battery product scaled up to the multi-kilogram level had a stoichiometry of Na0.950Ni0.317Mn0.317Mg0.158Ti0.208O2and exhibited a typical O3 phase.Based on the reversible Ni2+↔Ni4+redox couple with irreversible O2–oxidation,Faradion’s first-generation cathode material delivered a capacity of 157 mA·h·g-1cycled at a ±C/10 rate under the voltage window of 1.0–4.3 V, showing high energy and good cyclability.This material was used for Faradion’s first-generation battery pack demonstrations, including an e-bike and an escooter [49].Subsequently, Faradion shifted its focus to the second-generation cathode material, using a mixed O3–P2 phase with different O3/P2 ratios.The stoichiometry of the P2 phase was Na0.677Ni0.300Mn0.600Mg0.033Ti0.067O2.In the half-cells, closetheoretical capacities of 156,147,and 121 mA·h·g-1were achieved at C/5 in different voltage windows of 4.35–2.00, 4.25–2.00, and 4.40–2.00 V, respectively (Fig.7(a)).After being paired with an HC anode, a sodium-ion full cell demonstrated stable cycling in excess of 3000 cycles with a 20% capacity loss rate at 4.00–1.00 V.Faradion’s SIB design not only provides a high energy density, but also displays excellent rate capability under relatively high rates.In a mixed O3–P2 phase cathode||HC 0.1 A·h pouch cell,high capacity retentions of 91% and 84% were realized at 4C and 10C, respectively.Remarkably, Faradion’s SIBs also demonstrate a fast charging capability for crucial end-user applications including EVs and portable electronic devices.They exhibit a safe charge acceptance as high as 4C(15 min for total charge)with no capacity drop at C/5 during the first charge/discharge stage (Fig.7(b)).
For anode materials, Faradion has carried out fundamental studies on HC materials based on an overall consideration of the significance of performance and commercialization.In terms of industrial application,advantages such as cost,scalability,tap density, surface area, and attainable purity are as important as a high energy density when employing HC for SIB commercialization[66,67].Faradion’s proprietary HC anode material demonstrates a specific capacity that exceeds 330 mA·h·g-1at C/20 with a high initial Columbic efficiency of over 91% when applying a carbonateester solvent electrolyte (Fig.7(c)) [68].Moreover, Faradion has explored a NaPF6salt in ethylene carbonate (EC):DEC:PC = 1:2:1(w:w:w) mixed solvents with a certain additive as its firstgeneration sodium-ion electrolyte to boost the overall performance of the Faradion battery systems [69].
Based on the aforementioned advanced design philosophies,Faradion’s SIBs can deliver an energy density as high as 140–160 kW·h·kg-1in a 32 A·h pouch cell at 4.2–1.0 V, with a good cycling lifetime of 1000 or 3000 cycles over 4.0–1.0 V.Such a sodium-ion energy performance can be projected to be at an intermediate level between commercial LIBs based on LiFePO4and those based on LiCoO2cathode materials.Faradion’s SIBs can be an excellent alternative to LABs as low-cost batteries for electric transport, such as e-scooters, e-rickshaws, and e-bikes.Fig.7(d) illustrates a 3 A·h Faradion pouch cell and its culmination in a 400 W·h battery pack with the specific energy density of ~80 W·h·kg-1.Thanks to their enhanced energy density in comparison with LABs and their improved cyclability in comparison with LIBs in a wider temperature range,Faradion’SIBs exhibit potential for use as a 12 V battery for starter-lighting-ignition or as a mild hybrid EV 48 V battery.
3.2.HiNa Battery Technology Co., Ltd.
HiNa Battery Technology Co., Ltd., (hereinafter referred to as HiNa) China’s leading supplier of high-power, long-cycle-life,low-cost, and safe SIB products, is located in Liyang, Jiangsu Province.As a spin-off from the Institute of Physics,Chinese Academy of Sciences, in 2017, HiNa became the first high-tech company to focus on the research,development,and commercialization of SIBs in China.Led by Liquan Chen and Yong-sheng Hu, the company possesses a core technical team that is mastering the state-ofthe-art SIBs technology.
Since 2011,the researchers in Hu’s group have been committed to the exploration and development of SIB technology based on their more than 30 years of research and accumulated experience in LIBs.At the time it was founded,HiNa had a clear idea of seeking to investigate and develop a new-generation energy storage system based on low-cost, high-performance, environmentally friendly,and safe SIBs.Now,HiNa is working with IP and manufacturing patents to bring more exciting battery products to market.It has applied for more than 30 core SIB patents on new electrode materials, key components, manufacturing, and applications.Among them, 12 have already been authorized, including one US patent and one Japanese patent.
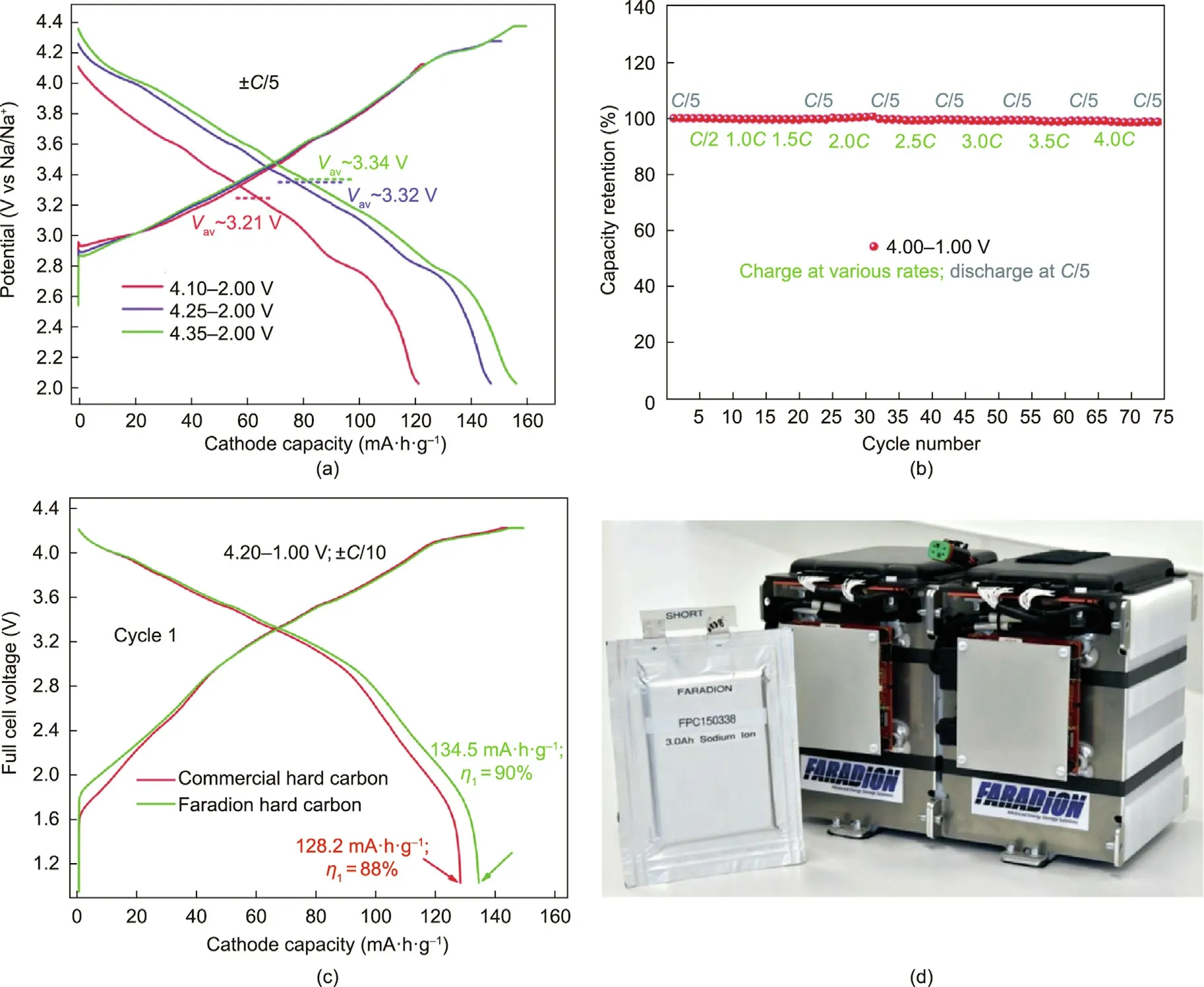
Fig.7.Demonstration of Faradion’s SIBs: (a) charge/discharge profiles of Faradion’s second-generation cathode material cycled in half-cells at C/5 within different voltage windows; (b) the fast-charge performance of Faradion’s second-generation cathode material||HC 0.1 A·h full cell, charging at 4C (15 min total charge) with no obvious capacity drop; (c) the higher initial Columbic efficiency (≥90%) of the HC anode in a 0.1 A·h full cell when paired with the second-generation cathode material; and (d) a Faradion 3.0 A·h Na-ion pouch cell with the 400 W·h battery pack system.Vav: average voltage; η1: initial Coulombic efficiency.(a–c) Reproduced from Ref.[69] with permission.(d) Credit: Faradion Ltd., with permission.
HiNa’s SIB products are based on a unique copper (Cu)-based oxides chemistry and anthracite-derived soft carbon as the cathode and anode materials, respectively.In 2014, the research team at HiNa first discovered the electrochemical activity of the Cu3+/Cu2+redox couple in a P2-phase Na0.68Cu0.34Mn0.66O2material[70].This was not only an innovative and fundamental study, but also a significant breakthrough in using element copper to build novel layered-oxide cathode materials that are low in cost and environmentally friendly.Similar to nickel (Ni) and cobalt (Co), which are widely used for LIB cathode materials,the introduction of copper enhances the electronic conductivity and the overall Na+-storage performance.It is worth noting that the price of CuO is only half of that of NiO.On the basis of the Cu3+/Cu2+redox reaction,the P2-Na0.68Cu0.34Mn0.66O2material delivers ~70 mA·h·g-1at a current density of C/10(referring to 0.34 Na extraction per formula unit in 10 h)within 2.50–4.20 V and displays a high average voltage plateau of ~3.7 V versus Na+/Na.With the aim of increasing the specific capacity of SIB cathodes, HiNa selected the low-cost element iron(Fe)to be introduced into the oxides’lattice.Under the same voltage window,the P2-Na7/9Cu2/9Fe1/9Mn2/3O2material reaches a capacity of ~90 mA·h·g-1at 10 mA·g-1[71].Remarkably,it shows excellent air/water stability, as its crystal structure is well maintained after being soaked in water.Through precise control of the atomic ratio of Cu,Fe,and manganese(Mn),O3-type Na0.90Cu0.22Fe0.30Mn0.48O2with an improved reversible capacity(~100 mA·h·g-1at 10 mA·g-1at 2.50–4.05 V)was designed and successfully synthesized in 2015[72].To maximize the specific capacity and minimize cost, HiNa sought to take advantage of a combination of Cu–Fe–Mn-based oxides with Li, which enabled a specific capacity as high as~130 mA·h·g-1at 10 mA·g-1at 2.50–4.00 V.The charge/discharge profiles for the abovementioned cathode materials are displayed in Fig.8(a)[73].
HiNa has carried out other investigations into the use of amorphous carbon materials as the anodes for SIBs on the basis of a deep understanding of the Na+-storage mechanism.Representative data from sodium-ion half-cells featuring HiNa’s proprietary soft carbon materials are shown in Fig.8(b).In 2016, HiNa reported a pyrolyzed anthracite (PA) anode material with superior low cost and a high degree of safety.The PA anodes demonstrate a high Na+-storage capacity of 222 mA·h·g-1at 0–2.0 V in half-cells.After the PA anode is assembled with Na0.90Cu0.22Fe0.30Mn0.48O2in a prototype pouch cell,an energy density of ~100 W·h·kg-1and a good cycling stability can be realized.The anode’s easy synthesis method, low cost, and high carbon yield of over 90% are very promising for practical applications [74].Furthermore, HiNa has proposed a pitch (carbon yield of 67%) precursor for the largescale production of SIB anode materials.After undergoing an easy pre-oxidation treatment in air,the pitch-derived soft carbon delivers 300.6 mA·h·g-1with a high first-cycle Coulombic efficiency of 88.6% [75].HiNa is taking the lead in integrating next-generation proprietary carbonaceous material derived from formaldehyde resin (PF) with ethanol as the pore-making agent.The obtained optimal carbon anodes deliver a Na+-storage capacity of~410 mA·h·g-1, which exceeds that of graphite for Li+storage in LIBs.Coupled with NaNi1/3Fe1/3Mn1/3O2, a prototype pouch cell exhibits an energy density of ~300 W·h·kg-1with an initial Coulombic efficiency of 83% [76].
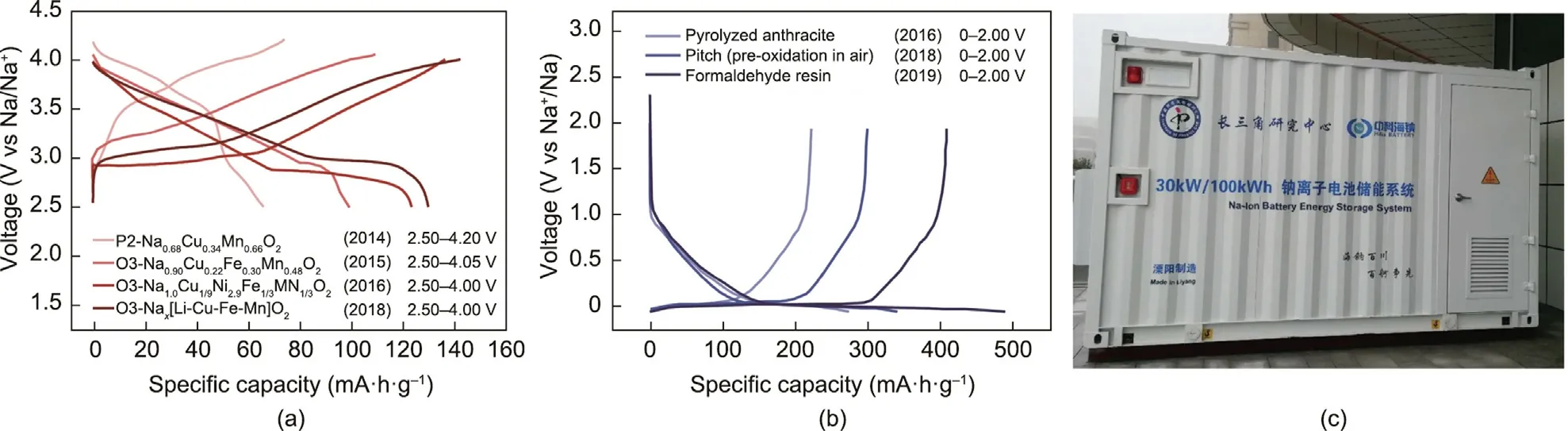
Fig.8.HiNa’s SIB chemistry and technology: typical initial charge/discharge profiles of (a) Cu-based oxides cathode materials and (b) amorphous carbon anode materials.Reproduction from Ref.[73] with permission.(c) HiNa’s 30 kW/100 kW·h SIB energy storage system.Reproduction from Ref.[77] with permission.
Based on the aforementioned electrode materials, HiNa has developed NaCP08/80/138 and other specifications of soft-pack SIBs,as well as cylindrical NaCR26650 and NaCR32138 SIBs,which demonstrate an internationally advanced sodium-storage performance level.The obtained technical progress in HiNa’s SIBs is as follows: ①a working voltage of 3.2 V; ②a working temperature from -40 to 80 °C, with a capacity retention >99% at 55 °C and >88% at -20 °C; ③a cyclelife ≥4500 cycles at 83% (2C/2C);④a gravimetric specific energy density ≥145 W·h·kg-1; ⑤a rate capability of 90%capacity retention at 5C-rate to 1C-rate;⑥a storage of 100%SOC at RT for 28 d,with a capacity retention ≥94%and capacity recovery ≥99%; and ⑦in regard to safety, meets the national standard GB/T31845–2015.
HiNa provides advanced battery technologies that can integrate into a wide variety of critical power and industrial applications ranging from electric transport, household energy storage, and industrial energy storage.In 2018, HiNa demonstrated a lowspeed EV powered by SIBs; in 2019, HiNa successfully installed the world’s first 30 kW/100 kW·h SIB energy storage system(Fig.8(c)) [77].
3.3.Aquion Energy, Inc.
Aquion Energy,Inc.,which is located in Pittsburgh,PA,USA,was founded in 2008.The company develops aqueous SIBs (salt-water batteries) as an alternative to LIBs and other energy storage systems for grid storage.Aquion Energy’s batteries use a Mn-based oxide cathode and a titanium (Ti)-based phosphate anode with aqueous electrolyte (<5 mol·L-1Na2SO4) and a synthetic cotton separator.The aqueous electrolyte is easier to work with than non-aqueous electrolytes, simplifies the manufacturing process,and greatly decreases the material cost.In 2011, the company achieved 1.5 kW·h in an individual battery stack and 180 kW·h in a shipping-container-sized unit.In 2014, Aquion Energy launched second-generation aqueous hybrid ion (AHI) batteries with a 40%increase in energy density,reaching 2.4 kW·h in a single stack and 25.5 kW·h hold in a multi-stack model.
Aquion’s proprietary AHI batteries with an environmentally friendly electrochemical design were the first SIBs to be Cradle to Cradle Certified as qualifying under the methodology’s comprehensive criteria.Compared with the flammable organic solvent used in LIBs and the caustic sulfuric acid used in LABs, Aquion’s Aspen batteries are claimed to be non-flammable and nonexplosive and to be made from abundant and nontoxic materials,resulting in the cleanest and safest batteries on the market, while meeting the strict performance requirements of residential,commercial, or industrial energy storage applications.Aspen batteries, which are nominal 2.2 kW·h systems at 48 V, can be connected in series or parallel for various configuration designs.Fig.9 provides a clear illustration of different Aspen battery stacks with a flexible structural design.
In order to promote the aqueous SIB development in the field,Aquion Energy is driven to improve its SIB chemistry and battery quality.The corporation’s continued efforts include improvements in energy/power density, cycling lifetime, and a corresponding capacity decay rate for the Aquion SIB products.A new battery chemistry that is environmentally sustainable, safe, and costeffective will soon be perfected, making Aquion Energy batteries a promising choice for energy storage applications.
3.4.Novasis Energies, Inc.
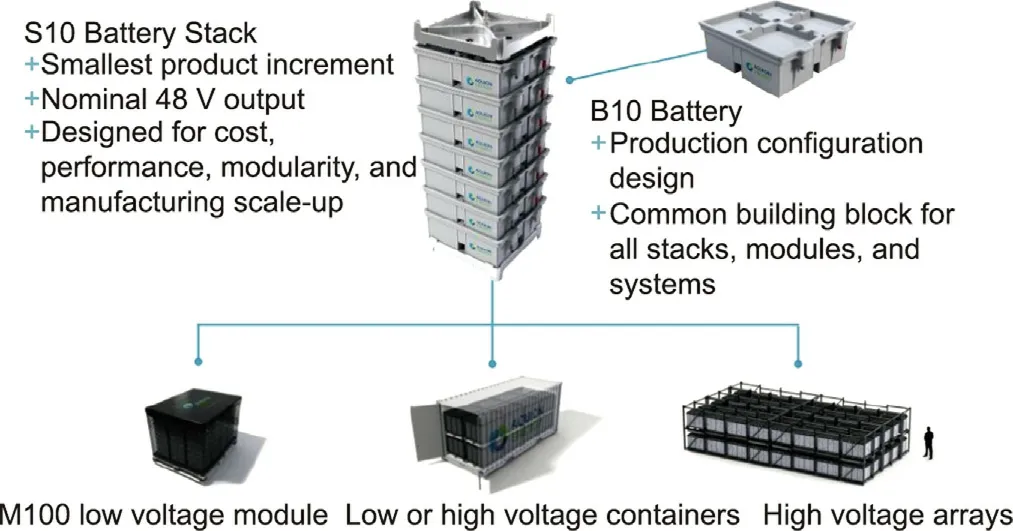
Fig.9.Aquion Energy’s battery supply for energy storage.Credit: Aquion Energy,Inc., with permission.
Novasis Energies,Inc.,grew from a start-up that was founded by academic scientists in John B.Goodenough’s group in 2010 and was then further developed by former researchers from Sharp Laboratories of America with funding support from the Advanced Research Projects Agency-Energy (ARPA-E) during 2012–2016.Novasis Energies specializes in the design,development,and manufacturing of innovative SIB solutions for mobile and stationary energy storage applications.Novasis’SIB chemistry comprises proprietary technology based on Prussian blue analog(PBA)cathodes,HC anodes,and a non-aqueous sodium-ion electrolyte.PBA,a classic intercalation host material for Na+,has been demonstrated to be a nontoxic pigment for widespread application.The general chemical composition is represented as AMFe(CN)6, where A represents an alkali metal ion and M represents a transition metal cation[78,79].Novasis’s commercialized PBA cathode material,NaxMnFe(CN)6, enables a high capacity of around 160 mA·h·g-1,which is close to its theoretical capacity value,and an ~83%capacity retention of 10C-rate to 0.1C-rate.Benefitting from the relatively low sodiation potential of HC anodes and the single flat sodiation plateau at around 3.5 V of PBA-based cathodes, Novasis SIB full cells display an average output voltage of 3.2 V.In terms of a full-cell evaluation in a pouch cell format, normal energy in the range of 100–130 W·h·kg-1or 150–210 W·h·L-1can be realized according to the cell’s size and capacity.Moreover, the synthetic method for PBA active materials is fairly straightforward and is easy to scale up for production.The fabrication of the PBA materials can be easily realized through a co-precipitation reaction process in which the Mn2+ions react with ferrocyanide ions to form rhombohedral NaxMnFe(CN)6crystals.The whole process is carried out under aqueous solution at a relatively low temperature, and a high-temperature calcination step is not required.Furthermore,all the raw chemicals involved in the process are readily available,and rare elements and hazardous substances are not necessary.Overall,the PBA materials offer promising scale-up synthesis and the fabrication of active materials, which enable great reduction in both energy consumption and manufacturing cost relative to the production of other LIB and SIB cathode materials.
3.5.Natron Energy
Natron Energy, a battery company based in Santa Clara, CA,USA,is developing SIB technology for various energy storage applications, including critical backup power systems, transportation,material handling, renewable smoothing, microgrids, and regulatory services.Natron Energy(formerly Alveo Energy)was founded as a spin-off of Stanford University in 2012.Natron develops and provides disruptive new battery products based on PBA electrode SIB chemistry and targeting utility applications with higher power density, lower cost, faster recharge, longer cycle life, and greater safety.Natron’s PBA electrodes charge and discharge through a single-phase reaction mechanism within the stable electrochemical window of the sodium-ion electrolyte,which effectively eliminates irreversible phase transformation via conversion reactions and suppresses the electrolyte decomposition that limits the lifetime of LIBs and LABs,indicating improved battery safety.Recently,Natron Energy achieved an Underwriters Laboratories (UL) listing for its BlueTray 4000,demonstrating its safety for stationary applications.In addition,Natron’s PBA-based SIBs realize a quick charge of power; they are capable of discharging in 2 min or less and charging in 8 min.Remarkably,Natron’s batteries provide a service life of 5 years or more and perform 35 000 cycles without capacity loss.Combining the low cost and facile scale-up synthesis of its products, Natron Energy aims its SIB chemistry and technology at applications including data centers, electric forklifts, and small electrical grids.Supported by 35 million USD in Series D funding in 2019 and 20 million USD funding from ARPA-E in 2020, Natron Energy plans to conduct continuous efforts to increase their batteries’ energy density and scale up battery production in the near future.
4.Conclusion and perspective
As we face a social transition into greener energy and a greener economy, increasing the penetration of renewable energy stands out as a vital factor in realizing this ultimate object.To curb the intrinsic intermittency of renewable energy sources and integrate them into the current grid or the future smart grid, cost-efficient EES is both significant and necessary.RT SIBs, as a relatively nascent energy storage technology, have received considerable attention due to abundant sodium reserves and to SIBs’electrochemical behavior being similar to that of commercial LIBs.In terms of practical application, the cost per kilowatt-hour and the cost per cycle life become the most important parameters.Hence, SIBs are a promising option for EES in large-scale station or grid applications,where size is not the first consideration.
The key challenge for SIBs is to achieve both high energy density and a long cycle life.The excellent electrochemical performance of a battery depends on the material science involved in its creation.Many essential problems in state-of-the-art of SIB technology remain to be resolved, from the cell level to commercial products for practical applications.These have been summarized as critical challenges in SIB chemistry and technology toward the development of the next generation of SIBs.
4.1.Anodes
As graphite-based materials for LIBs cannot be directly used for SIBs, alternative carbonaceous materials such as hard/soft carbon with a low sodiation/desodiation voltage have been proposed.Thus far, carbon-based anode materials for SIBs delivering about 300 mA·h·g-1can be realized,which approaches the limits of their theoretical specific capacity value.Moreover, such materials are commonly obtained from various biomass materials and chemical industrial byproducts; thus, they bring cost and environmental benefits to applications for stationary and grid-scale energy storage.However, the most intrinsic challenge that remains for carbon-based anode materials is their low initial Columbic efficiency, which results from solid electrolyte interface (SEI) formation with partial Na+consumption.Improvements in the mitigation of the initial capacity loss are urgently needed, based on the exploitation of Na+-storage and structure–degradation mechanisms.Manipulating the interface phases between the electrolyte and electrode by the addition of sacrificial salts offers a possible solution for maximizing the initial Columbic efficiency.
4.2.Cathodes
Polyanion-type materials usually deliver a higher redox potential but lower specific capacity than oxide cathodes, due to the inductive effect of the polyanionic units and the relatively high molar mass.Benefitting from a robust and open framework structure,polyanions show excellent cycling stability,thermal stability,and high safety.The drawbacks of polyanions are of their intrinsically low electronic conductivities.In addition, the use of electrochemically active elements such as vanadium results in increased environmental concerns.PBAs have already demonstrated their vast potential in the development of aqueous SIBs, thanks to their high energy density and strong structural stability.Nevertheless,PBAs are inconvenient for use in non-aqueous batteries because of the inevitable existence of a certain amount of coordinated water in the lattice,which could react with the organic electrolyte in the system.Another disadvantage of PBA materials lies in their synthesizing process; it is difficult for scale-up production and manufacturing due to the potential safety hazard involved, which includes the leakage of liberated CN–ions and the generation of toxic waste.
In regard to layered metal oxide cathode materials, cost and performance are the most sensitive parameters for practical SIB applications.The selection of expensive metal elements for the material chemistry design,such as cobalt and nickel,is truly favorable for increasing the energy density and overall electrochemical performance, yet inadvertently diminishes the cost merits of SIBs.The exploration of layered oxides that are free of cobalt and nickel but possess equal or greater sodium-storage performance is highly encouraged.Here, low-cost Mn-based layered oxides with high specific capacity and adequate operating voltage stand out as possible contenders.A small amount of doping or substitution with accurate regulation of the selected elements and atomic ratios to optimize the stoichiometries can be used to significantly enhance the performance of this family of Mn-based materials.Detailed studies on anionic redox behavior,a reversible O2–to On–transfer,should be carried out in order to further explain the origins of the anomalous excess capacity and to elaborate the reasons behind the mechanism.
Nevertheless, layered oxides still present challenges such as moisture sensitivity and sodium deficiency, which irreparably inhibit their practical application.Although air stability can be realized through dry treatments during the production process,an increase in manufacturing cost is inevitable.Innovative strategies including protective layer coating,lattice doping,and/or ethanol washing have been demonstrated to mitigate this issue of layered oxides.In terms of reducing the sodium deficiencies(there is often 25%–40%Na deficiency in the stoichiometry of layered oxides)and mitigating the contiguous side reaction,the use of sacrificial sodium salts has been shown to be promising.However, a fundamental understanding of the selected salt chemistries and the corresponding compensating mechanism is still required.
4.3.Electrolytes
Investigations into a new electrolyte system with specific formulations, an optimized solvent, sodium salts, and additives are needed.As large-scale EES applications have higher requirements for SIBs to perform well under more restricted climatic and intermittent conditions, the organic liquid electrolytes applied in SIBs should have a wider temperature range tolerance while enabling safe and stable cycling.Furthermore, there is still much room for improvement in alternative electrolyte systems that have the potential to prolong the cycling lifetime and improve safe operation.For example, the modification of ionic liquids and solidstate electrolytes has exhibited effective results.In particular, indepth studies and analyses on electrolyte/electrode interface issues are very necessary, as a stable interface between the electrolyte and active particles is vital for sodium-storage performance.Electrolyte degradation and continuous SEI growth lead to increased interface resistance and low Columbic efficiency,which dramatically affect the overall performance of SIBs.Designing and optimizing a stable electrolyte/electrode interface is thus highly required, in combination with the exploration of interface reaction mechanisms by using advanced characterization technologies.
4.4.Manufacturing process
Manufacturing-related factors are expected to play an important role as SIBs enter the battery market and are used in largescale EES applications, considering their overall advantages in material costs and energy density.First,aluminum foil can be used as the current collector for both the cathode and anode in SIBs, as Na+ions do not alloy with aluminum at the anode side.The use of an aluminum current collector in SIBs with the same capacity saves two-thirds of the cost of the aluminum and copper collectors used in LIBs;it not only lowers the SIB costs,but also reduces the transportation risks,as SIBs can be loaded for transportation completely discharged,at 0 V.Subsequently,it is anticipated that the development of SIB technologies will be able to refer to the mature manufacturing technologies of LIBs.According to the characteristics of SIBs, more suitable manufacturing technologies are expected to be further optimized to meet the needs of large-scale production in future.Finally, it is appropriate to mention the necessity for the development of manufacturing technologies for commercialscale SIBs at the pack level; this will involve developing cell-topack(CTP)technology,which is of great significance for improving the energy density of the battery pack.
4.5.Industrial developments
The following issues remain to be addressed for the industrial development of SIBs: ① Cost, performance, and safety issues remain as key parameters for SIB development and commercialization for energy storage applications.② Although the firstgeneration commercial SIB products have already entered the energy storage market, aiming at light mobility, SIBs are only in a preliminary stage.To realize the stationary and/or grid storage applications of SIBs, research efforts must shift from the academic level toward the cell/pack level, supported by industrial investments and inputs,along with policy orientations.③Further reducing the cost per energy density will make SIBs much more compatible with LIBs.Next-generation SIBs should aim for the following targets:reaching an energy density of 220 W·h·kg-1applied with a 200 mA·h·g-1cathode,a 500 mA·h·g-1carbon-based anode,and an average output potential of 3.3 V.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (52101267) and China Postdoctoral Science Foundation Project (2021M690117).
Compliance with ethics guidelines
Lina Zhao, Teng Zhang, Wei Li, Tao Li, Long Zhang, Xiaoguang Zhang, and Zhiyi Wang declare that they have no conflict of interest or financial conflicts to disclose.
杂志排行
Engineering的其它文章
- Dynamic Deformation Measurement of an Intact Single Cell via Microfluidic Chip with Integrated Liquid Exchange
- The Rational Design and Development of Microalgae-Based Biohybrid Materials for Biomedical Applications
- DART Mission Shows Potential for Planetary Defense by Smashing Asteroid into New Orbit
- Electric Racers Hit the Track, but Still Catching Up
- Micro-Cantilever Electric Field Sensor Driven by Electrostatic Force
- Theory and Practice of Hydrodynamic Reconstruction in Plain River Networks