From Discovery to Mass Production:A Perspective on Bio-Manufacturing Exemplified by the Development of Statins
2023-11-14XiaoLingTangJiaWeiYuYuHengGengJiaRuiWangRenChaoZhengYuGuoZheng
Xiao-Ling Tang, Jia-Wei Yu, Yu-Heng Geng, Jia-Rui Wang, Ren-Chao Zheng, Yu-Guo Zheng,*
a Key Laboratory of Bioorganic Synthesis of Zhejiang Province, College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310014, China
b Engineering Research Center of Bioconversion and Biopurification of the Ministry of Education, Zhejiang University of Technology, Hangzhou 310014, China
Keywords:Bio-manufacturing Statins Microorganisms Bioconversion Chemoenzymatic synthesis
ARTICLEINFO The increasingly complex molecular structures and high requirements of advanced industries are triggering a transformation in chemical production modes.Bio-manufacturing provides efficient strategies and brings the advantages of high atomic economy, few side reactions, and strong adaptability to processes,as well as environmental friendliness, which can contribute toward global efforts against greenhouse effect and environmental pollution.The significance of bio-manufacturing can be specifically illustrated by examining the bio-manufacturing process from the scientific discovery of a key compound to its technological integration and engineering innovation.The development of statins—important drugs for hypercholesterolemia treatment—is a good example of the progress and application of biomanufacturing.The production of the first-generation statins from microorganisms, the secondgeneration statins using bioconversion, and the third-generation statins through an evolution from total chemical synthesis to chemoenzymatic synthesis demonstrates the technological and engineering revolution of bio-manufacturing,which is of great importance for energy conservation,cost saving,and waste emission reduction.With advances in cutting-edge biotechnologies,as well as the integration of multiple disciplines, bio-manufacturing is expected to promote the advancement of more intelligent processes to realize sustainable and green industrial development.
1.Introduction
With the high environmental and industrial standards of advanced industries, issues with traditional manufacturing processes for chemicals have become increasingly evident, particularly regarding production cost, manufacturing steps, and waste emissions[1].In general,the molecular structures of many modern medicines tend to be complex, with multiple chiral active centers and functional groups, and their synthesis requires long reaction steps with continuous protection/deprotection and resolution.In addition,the optical purity of chiral chemicals for use must usually be very high,requiring repeated enrichment steps,which results in a low yield and the generation of serious byproducts and pollutants.Therefore, manufacturing processes often fail to meet the requirements of industrialization.
Bio-manufacturing is a manufacturing mode that utilizes biological systems, including enzymes, tissues, and living cells, to produce commercially important molecules with renewable resources as raw materials[2].Compared with traditional chemical routes,bio-manufacturing has the advantages of high atomic economy; strict regional, stereo-, and chemical selectivity; few side reactions; and strong adaptability to process requirements.The application of bio-manufacturing has extended to the chemical,pharmaceutical, agricultural, food, and environmental industries.Thus, a driving and permeable system of bio-manufacturing is being established,promoting sustainable development of the economy and society.In fact, bio-manufacturing has been listed as a strategic focus of scientific and technological development by numerous countries around the world [3].
The significance of bio-manufacturing can be specifically illustrated by examining the bio-manufacturing process from the scientific discovery of a key compound to its technological invention and engineering innovation.The emergence of cuttingedge technologies, the integration of engineering processes, and the needs of the market have promoted the development and application of bio-manufacturing.For example, after penicillin was coincidentally discovered, its antibacterial activity was identified, its purification process was established, and its culture conditions were regulated with advances in microbiology technology.Once these technologies were dynamically embedded into an engineering system in an orderly way, large-scale production of penicillin was achieved, and penicillin was developed into an essential antibiotic used to treat diseases and save lives [4].Similarly, based on developments in manufacturing, important compounds such as avermectins [5] and validamycin [6,7]transitioned from newly discovered compounds to key pesticides for dealing with parasitic disease or rice sheath blight [8].In the present article,the significance of bio-manufacturing is elaborated using the development of statin drugs.From the discovery of valuable compounds to their mass production, bio-manufacturing has realized the transformation of the bioindustry and promoted the economic development of related fields.
2.The development of statins
With the changes of human lifestyle that have occurred in modern society, the morbidity and mortality of cardiovascular and cerebrovascular diseases are increasing, and these diseases have become leading causes of human disability and death.According to related reports, the number of patients with cardiovascular and cerebrovascular diseases in China has reached 330 million,ranking first in the world,and the prevalence is still rising.Hyperlipidemia is a risk factor for cardiovascular and cerebrovascular diseases.It is mainly manifested by a high level of low-density lipoprotein (LDL)—a kind of cholesterol transporter, which accumulates on blood vessel walls and causes arteriosclerosis,resulting in various cardiovascular-related diseases.It has been clinically proven that the risk of ischemic cardiovascular disease can be effectively controlled by reducing the LDL level in bodies.
Statins are the most pharmaceutically important cholesterollowering drugs for the treatment of hyperlipidemia-related diseases, and these drugs hold a large market share.During cholesterol synthesis in the human body, the enzyme hydroxymethylglutaryl (HMG)-coenzyme A (CoA) reductase(HMGCR) is a rate-limiting enzyme that catalyzes the reduction of HMG-CoA into mevalonate, a crucial cholesterol intermediate[9].Statins have structures similar to that of HMG-CoA, but with a higher affinity to HMGCR.They block the intracellular mevalonate biosynthesis pathway to prevent the buildup of plaque inside the arteries and reduce cholesterol synthesis by competitively inhibiting endogenous HMG-CoA.Meanwhile, statins promote the activity of LDL receptors, accelerate the catabolism of LDL, and play a role in eliminating serum cholesterol [9,10].Since the discovery of the first cholesterol synthesis inhibitor, mevastatin, in the 1970s, statin families have been developed rapidly,with the successive development of natural statins, semisynthetic statins, and fully synthetic statins, in what has become a milestone in the history of drugs for the treatment of cardiovascular and cerebrovascular diseases [10] (Fig.1).From a thermodynamics perspective, the first-generation statins were not enthalpically optimized, and the third-generation statins exhibit more favorable binding enthalpies,indicating that they show more thermodynamic stability than the previous statin family [11,12].
Bio-manufacturing has played a key part in the synthesis of statins, from the first to the third generations.Core technologies of bio-manufacturing, such as strain breeding, microbial fermentation, enzyme screening and modification, and biocatalytic conversion, have provided strong technical support for the development of statins (Fig.2) [11,12].
3.Production of first-generation statins
3.1.First-generation statins
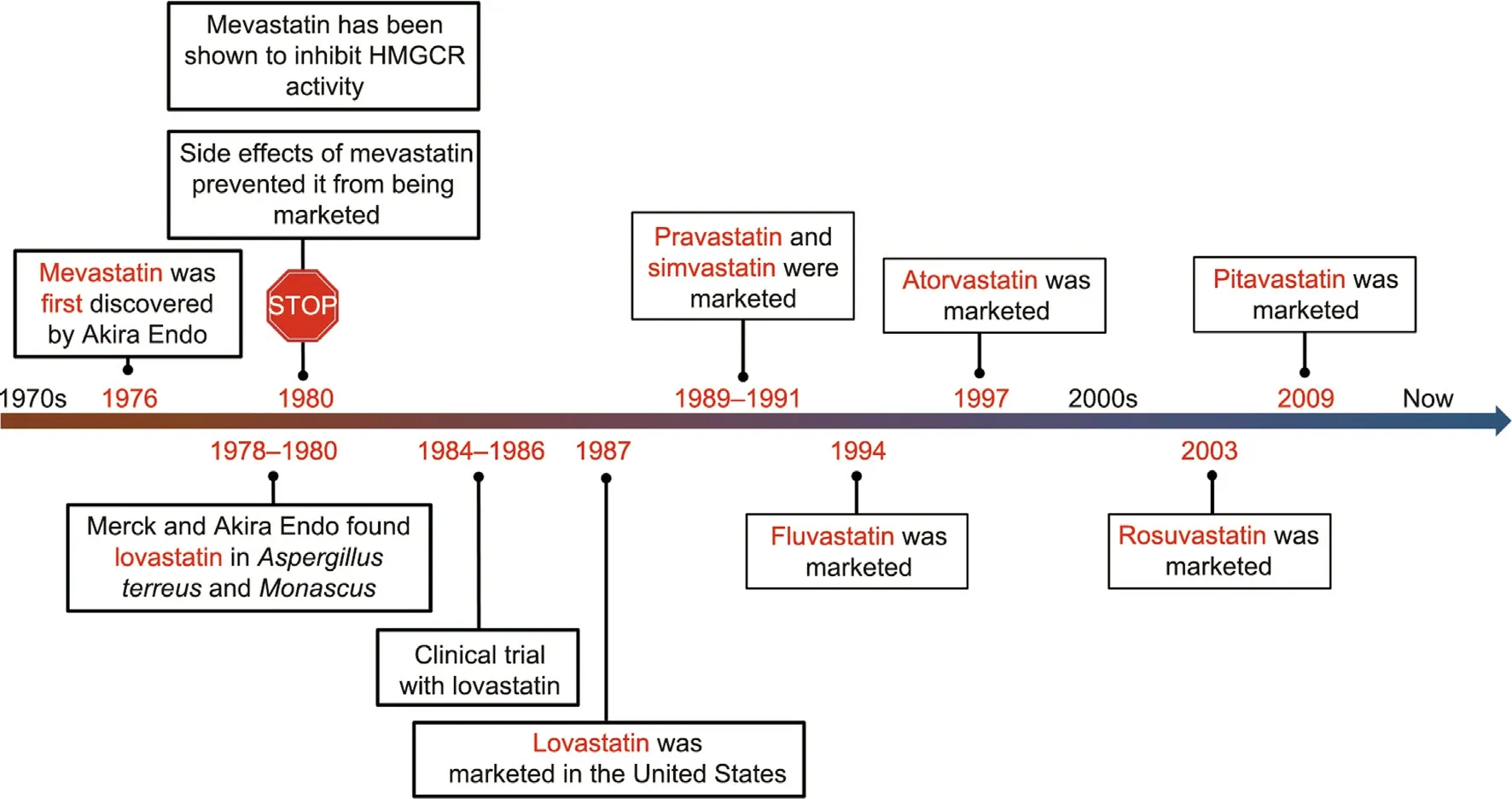
Fig.1.The history of the development of statins.

Fig.2.(a)Overview of production routes for the first-,second-,and third-generation statins.(b)Thermodynamic parameters of the ΔH and ΔS of statin molecules binding to the immobilized artificial membrane at pH 7.0 and 25 °C.(c)Thermodynamic parameters of the ΔG,ΔH,and-TΔS of statin molecules binding to HMGCR at pH 8.0 and 25 °C.ΔH represents the enthalpy of binding;ΔG represents the Gibbs free energy;ΔS represents entropy change;ΔG=ΔH-TΔS.1 cal=4.19 J.Reproduced from Refs.[11,12]with permission.
Microorganisms are a vast treasure trove of drug production.Many drugs—including antibiotics such as penicillin and streptomycin,antitumor drugs such as actinomycin D,and enzyme inhibitors such as lovastatin and acarbose—have first been found in microorganisms.In 1976, mevastatin was discovered for the first time in the fermentation broth of Penicillium citrinum (P.citrinum)[13],and it showed potential effect to control hypocholesterolemia by inhibiting HMGCR activity and reducing cholesterol in vivo[14,15].The application of mevastatin to patients with heterozygous familial hypercholesterolemia and combined hyperlipidemia was found to significantly reduce serum cholesterol by about 30%, shedding light on this lethal disease [16].Thus, mevastatin is regarded as the first active compound of a statin drug to be identified and initially used clinically in the 1980s.However, prolonged experimental treatment with a high dose of mevastatin on a dog was found to increase the proportion of malignant lymphomas, so it was withdrawn from the market in the early 1990s[10].Later, an active substance that strongly inhibits cholesterol synthesis was found in the metabolites of Monascus and was named monacolin K(MK).This substance is able to reduce cholesterol and preferentially reduce low-density lipoprotein-cholesterol(LDL-C) [17].Another compound, mevinolin, which has a similar structure to mevastatin, was discovered using Aspergillus terreus(A.terreus) [18].It was further named as lovastatin [10].In 1987,lovastatin was approved by the US Food and Drug Administration(FDA) for marketing.With sales of more than 260 million dollars in the first year, lovastatin ranked as the drug with the highest annual sales [19].The first-generation statin drugs of mevastatin and lovastatin were identified from microorganisms, and their development was promoted by key biological technologies such as the screening and identification of microorganisms, efficient breeding, metabolic engineering,and intelligent fermentation regulation, leading to a breakthrough in the history of treatment for hyperlipidemia-related diseases.
3.2.Production of first-generation statins from microorganisms
The goal of bio-manufacturing has always been to discover new biologically active secondary metabolites and increase their yields.The first-generation statins were originally found in fungi, which produce metabolites with complex chemical structures and are used to produce many valuable products.The metabolites of A.terreus,Monascus, Penicillium, Mortierella maculate,Streptomyces,Paecilomyces, Trichoderma, and Pleurotus ostreatus contain the active ingredients of statins [20–26].Among them, Monascus and A.terreus are the major host strains.
3.2.1.Strains for production
The fermentation products of Monascus have been used as food and health products in China for more than 1000 years, and their use later expanded to other countries such as the United States,Japan, Republic of Korea, Thailand, and Indonesia [26].The secondary metabolites of Monascus contain a variety of active substances, endowing Monascus with multiple functions including lowering blood lipids and blood pressure,regulating blood ammonia, and antitumor activity.The important components of the metabolites were found to be citrinin, monascus pigments, dihydromonacolins, γ-aminobutyric acid, and dimerumic acid [27–29].The composition of statins in Monascus is complex.In addition to the well-known MK, there are cholesterol-lowering analogs of lovastatin, including monacolin J (MJ), monacolin L, monacolin X,dihydromonacolin K, and dihydromonacolin L [10].Researchers applied the strategy of exogenous induction to the production of active products generated by Monascus and, using the screening method of high-performance liquid chromatography and citrininrelated gene analysis,were able to obtain a strain with a relatively high yield of MK [30].When this strain was cultivated with Discorea,the yield of MK was slightly increased[31].The fermentation process was also optimized.However, the lovastatin production level of Monascus remained low, restricting its industrialization.
In addition to Monascus,as described above,A.terreus has been shown to produce active ingredients of statins with a higher yield[32].Originally discovered as a potent producer of lovastatin, the production process of A.terreus has been continuously improved,making it the best lovastatin-producing strain; it was commonly used in the industrial production of the first-generation statins.To improve the productivity of A.terreus, mutagenesis breeding,metabolic engineering, and fermentation regulation were performed,in order to meet the requirements of industrial production.
3.2.2.Strategies to improve the production of first-generation statins
(1)Mutagenesis breeding.Since the wild-type A.terreus that is isolated from the natural environment has a low capacity for statin production, random mutagenesis was carried out to enhance its productive potential.Physical mutagenesis mainly involves ultraviolet (UV) irradiation and high-radiation heavy ion beams, while chemical mutagenesis commonly involves ethyl methyl sulfone(EMS), N-methyl-N′-nitro-N-nitrosoguanidine, and nitrous acid[32–35].In a recent study, with several rounds of UV irradiation mutagenesis breeding,the lovastatin yield of A.terreus was tripled,and the tolerance of the strain to lovastatin was significantly strengthened [34,35].Mukhtar et al.[36] obtained a strain with a high yield of lovastatin by means of co-application of UV radiation and nitrous acid.Compared with that of the wild type,the strain’s yield of lovastatin was 3.5 times higher.Vilches Ferrón et al.[33]obtained a new strain after EMS mutagenesis,with a yield of lovastatin four times higher than that of the wild type.In addition,there are methods that combine physical mutagenesis with chemical mutagenesis.Sreedevi et al.[37] used UV irradiation and EMS mutagenesis to obtain mutant strains; their highest yielding mutant strain had a yield of 663 mg·L-1,which was 1.8 times that of the original strain.
(2) Metabolic engineering.Based on an understanding of the molecular mechanisms of the metabolic pathways of statin biosynthesis,metabolic regulation has emerged as an effective strategy to improve the productivity of the microorganisms [20].Hasan et al.[38,39] increased lovastatin production by more than 40% using a promoter replacement method to maintain the expression levels of the key enzymes in the most suitable state.Meanwhile, the key enzyme acetyl-CoA carboxylase was overexpressed and competition pathways such as geodin biosynthesis were blocked to reduce the dispersion of metabolic flow.As a result,the production of lovastatin was further increased by 80%.Askenazi et al.[40]correlated global gene expression patterns with the production of specific metabolites through a comprehensive assessment of gene expression.With this method, high-yielding strains were successfully engineered,and the yield of lovastatin was increased by nine times.
(3) Fermentation regulation.The fermentation process is an extremely important link in the microbial production of target products, as it is directly related to the production efficiency and product quality.Highly efficient regulation of the fermentation process is of great significance in improving the productivity of microorganisms.Studies indicate that the productivity of lovastatin by A.terreus is influenced by nutrients such as carbon and nitrogen.Numerous other environmental factors also affect lovastatin production,such as agitation,temperature, pH,and moisture content.As such, to further improve the production of lovastatin,fermentation regulation strategies have been established [41,42].Researchers have considered the synergy between the growth and metabolism of lovastatin-producing strains in order to regulate global fermentation [36,43,44].Mukhtar et al.[36] optimized various culture conditions for a high-yielding strain and expanded it to a laboratory-scale fermenter, resulting in an eight-fold increase in lovastatin yield.Ansari et al.[43] studied the effects of hydrodynamic behaviors such as bubble size on fungal growth and lovastatin production by A.terreus in a bubble column bioreactor.The highest yield of lovastatin was obtained when the bubble diameter was 0.18 cm,which resulted in a yield of 443 mg·L-1.The yield of lovastatin under these conditions was 1.7 and 3.5 times higher than that of a bubble culture with a diameter of 0.36 and 0.09 cm, respectively.Raina et al.[44] were the first to present a correlation between the increase in lovastatin production and changes in the transcription levels of its biosynthetic genes lovB and lovF through elicitation with butyrolactone.The addition of butyrolactone can stimulate the metabolic production of A.terreus and significantly increase the yield of lovastatin.In their work,the exogenous addition of 100 nmol·L-1of butyrolactone I to submerged cultures resulted in 2.5-fold increase in lovastatin production, as compared with control cultures.All of the metabolic regulation described above not only significantly improved the productivity of microorganisms in producing the first-generation statins, but also promoted the industrialization process of statins.
4.Production of second-generation statins
Second-generation statins, which are derivatives of firstgeneration statins, include pravastatin and simvastatin.Pravastatin is a hydroxylated derivative of mevastatin that was initially separated from the urine of a dog fed with compactin ML-236B(the closely related compound as mevastatin, which was first isolated from P.citrinum),indicating that pravastatin can be produced from mevastatin.Pravastatin selectively inhibits cholesterol synthesis in the liver and small intestine, while cholesterol synthesis in peripheral cells is largely unaffected[45].Therefore,pravastatin is suitable for the primary and secondary prevention of hypercholesterolemia.Pravastatin was synthesized by the Japanese company Daiichi Sankyo and underwent clinical trials.In 1991,it was demonstrated that pravastatin significantly reduces the incidence of heart disease, and the statin was soon approved for marketing in Europe and the United States.
Simvastatin is a methylated derivative of lovastatin.The difference between the chemical structures of simvastatin and lovastatin is that the α-carbon atom of the butyrate sidechain at the C8 position of simvastatin has one more methyl group than that of lovastatin [46].Compared with lovastatin, simvastatin exhibits stronger activity in lowering LDL-C, with fewer side effects.The results show that the clinical activity of two doses of lovastatin is just comparable to that of one dose of simvastatin [47].With the application of biotechnologies and innovation in the production process, the cost of simvastatin has been significantly reduced,while its medicinal effect has increased.In 2006, simvastatin became the second-best-selling statin in the world.
Since the second-generation statins show high structure similarity to the first generation,researchers have focused on their efficient production from mevastatin or lovastatin.Through a combination of microbial fermentation with efficient bioconversion,a transformation in the traditional production process of statins was realized,resulting in a decrease of production cost,as well as significant energy conservation and emissions reduction.
4.1.Two-step fermentation process for pravastatin production
Pravastatin has a hydroxyl group (–OH) at the 6β position,which is a structural modification of the mevastatin skeleton.Since it is not easy to perform the hydroxylation reaction for pravastatin using conventional synthetic methods, only a few chemical methods have been reported for pravastatin production[48].Therefore,microbial hydroxylation was adopted to produce pravastatin.This was realized through a two-step process involving microbial fermentation and bioconversion.First, with the development of fermentation engineering technologies, the microbial strain producing mevastatin was optimized to perform high-density fermentation.The mevastatin could then be converted to pravastatin through hydroxylation (Fig.3).
Thus far, various microorganisms—including Actinomadura,
Micropolyspora, Streptomyces, and Pseudonocardia [49–51]—have been explored to determine their capability for hydroxylating mevastatin at the 6β position to form pravastatin.Based on an extensive screening program, Mucor was found early on to be effective for the hydroxylation of mevastatin, with a conversion ratio between 30%and 90%.However,Mucor is very sensitive to mevastatin,and cannot tolerate a substrate concentration of 0.05%of the lactone form of mevastatin.In order to overcome this limitation,high-yielding strains were bred.Researchers conducted multiple rounds of UV mutagenesis on Streptomyces flavus and, using high-throughput screening strategies, obtained the mevastatinresistant strain S33-1.In addition, online monitoring methods were set up to maintain the concentration of mevastatin at the optimal level.As a result,the biotransformation rate of mevastatin to pravastatin reached 91% when the mevastatin concentration was maintained at a constant level[52].As an alternative solution,an intermittent addition of compactin in the reaction mixture was attempted with Actinomadura sp.as the biocatalyst in order to increase pravastatin production.The results showed that the yield reached 0.8 mg·L-1.By further regulating the fermentation process of Actinomadura sp., both the cell biomass and conversion rate increased [53].
4.2.Key enzymes in pravastatin biosynthesis
Although various microorganisms exhibit activity in converting mevastatin to pravastatin, the key enzymes responsible for the conversion are different.Thus far, two major enzymes—namely,cytochrome P450 (CYP450) monooxygenase and a new hydrolase system presented in Actinomadura—have been demonstrated to be effective for the production of pravastatin.CYP450 is an enzyme containing heme that belongs to the class of monooxygenases widely distributed in nature.CYP450 can catalyze various types of reactions, such as hydroxylation,epoxidation, alcohol and aldehyde oxidation, O-dealkylation, N-dealkylation, oxidative dehalogenation, and oxidative C–C bond cleavage [54].Therefore, it participates in many important metabolic pathways in eukaryotes and prokaryotes.A new CYP450 was identified and cloned from Amycolatopsis orientalis.When it was used for the conversion of mevastatin,in addition to hydroxylation at the correct C6 position of the substrate, the stereoisomer 6-epi-pravastatin was obtained,indicating the unsatisfactory stereoselectivity of the enzyme.Therefore, molecular modification was performed on it.Based on a structure–function relationship analysis, the electron density in the enzyme’s active site and the conformational landscape were determined to affect the stereochemistry during the enzymecatalyzed process.Using an error-prone polymerase chain reaction strategy, the P450 mutant was screened, increasing the ratio of pravastatin to 6-epi-pravastatin from 3:97 to 96:4.Furthermore,researchers reprogrammed the antibiotic-producing fungus Penicillium chrysogenum (P.chrysogenum) by the insertion of the P450 mutant, in order to construct a single-step fermentative route for pravastatin production[55].By deleting the penicillin biosynthetic genes,strengthening the expression of the compactin gene cluster,and reducing the esterase activity in P.chrysogenum, the yield of the precursor statin compactin was significantly increased.Based on this,with the P450 mutant fused to Rhf reductase and randomly integrated into the engineered strain,the pravastatin yield reached 6 g·L-1in 10 L fed-batch fermentations [55,56].
Another hydroxylase for the conversion of compactin to pravastatin,which was found in Actinomadura sp., requires nicotinamide adenine dinucleotide (NADH) or reduced nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor.Compared with the normal CYP450 monooxygenase, this hydroxylase does not depend on compactin induction and is not inactivated by carbon monoxide(CO),and thus shows industrial potential for pravastatin production.
4.3.Production of simvastatin from lovastatin
The traditional production of simvastatin mainly involves sidechain synthesis and direct methylation.The lovastatin undergoes multiple reaction steps including hydrolysis, lactonization, the trimethylsilylation protection of hydroxyl groups at the C11 and C13 positions,acylation of α-dimethylbutyryl chloride,and deprotection.Next, a methyl group is added to the α-carbon of the C8 butyrate sidechain to obtain simvastatin, as shown in Fig.4 [57–60].This chemical synthesis has the disadvantages of low comprehensive yield (50%–80%); the requirement for excessive quantities of toxic reagents such as toluene, lithium metal, dimethyl amide,and methyl bromide; and the generation of a large amount of liquid waste [61–64].

Fig.3.Two-step fermentation process for pravastatin production.Mevastatin is first produced via microbial fermentation and is then bioconverted into pravastatin.CYP450:cytochrome P450.

Fig.4.Synthetic pathways of simvastatin, including the chemical route and the biosynthetic route.TBS: tert-butyldimethylsilane; Mel: iodomethane.
With the development of bio-manufacturing, biocatalytic conversion has been introduced into the industry as a means of producing simvastatin.Theoretically, lovastatin can be converted to simvastatin via methyltransferase in one step since, in terms of its chemical structure, simvastatin only has one more methyl group at the C8 position of the butyrate sidechain compared with lovastatin [61].Methyltransferase was developed and has been applied to catalyze erythromycin for clarithromycin with high catalytic efficiency, providing important guidance for constructing a new route for simvastatin biosynthesis.However,no methyltransferase with ideal catalytic activity toward lovastatin has been found thus far, and the one-step biosynthesis of simvastatin remains under exploration.
During the biosynthetic pathway of lovastatin in A.terreus, the two- and three-carbon units of acetic acid and malonic acid are condensed to MJ with the enzymes polyketide synthase encoded by lovB/lovC and P450 oxygenase encoded by lovA.Meanwhile,diketone synthase encoded by lovF catalyzes the condensation of acetic acid and malonic acid into a methylbutyric acid sidechain,which is transferred to the hydroxyl group at the C8 position of MJ by acyltransferase encoded by lovD, thereby generating lovastatin.On the other hand, MJ can be directly converted to simvastatin by means of acyltransferase with α-dimethylbutyryl-Nacetylcysteine thioester as the acyl donor (Fig.4).As such, MJ is the precursor of both lovastatin and simvastatin.Interruption of the lovastatin biosynthetic pathway or the hydrolysis of lovastatin provides an important substrate for simvastatin production.
Blocking the activity of diketone synthase or acyltransferase has been found to result in an accumulation of MJ.However, the fermentation level of the existing strains is still low, making them unable to meet the requirements of the industrial production of simvastatin.In recent decades, simvastatin has mainly been biosynthesized from lovastatin with chemical hydrolysis and enzymatic catalysis.After lovastatin is produced by microbial fermentation, its hydrolysis is carried out to form MJ, which is further acylated to simvastatin under the action of an acyltransferase[65–67].Researchers have obtained the acyltransferase LovD from A.terreus, which has a wide range of specificity for acyl substrates and acyl receptors.Modification of the enzyme resulted in improved catalytic properties.After expression of the enzyme in Escherichia coli(E.coli),a whole-cell catalyzed biosynthesis system of simvastatin from MJ was established [68].Meanwhile, an activated and membrane-permeable substrate, α-dimethylbutyryl-Smethyl-mercaptopropionate, was found to be an effective acyl donor for acyltransferase.As a result, the substrate conversion reached 99% without depending on chemical protection, and the extraction yield of the final product reached 90% with a purity of 98% [64].
Taking the synthetic pathway of simvastatin described above as inspiration, researchers are also focusing on the exploration of a bio-step for lovastatin hydrolysis to generate MJ, so as to replace the chemical hydrolysis process and thus realize higher atomic economy and greater environmental friendliness [69].In recent years, a new esterase PcEST was identified from P.chrysogenum;compared with previous reported hydrolases, it displayed a 232-fold higher catalytic efficiency for the in vitro hydrolysis of lovastatin to generate MJ.When PcEST is overexpressed in A.terreus with the capability for the industrial production of lovastatin,MJ is efficiently produced through the single-step fermentation and conversion of lovastatin, with a yield of about 95%.With the further co-use of acyltransferase, the synthetic steps used to produce simvastatin are all expected to be realized through biological methods, solving the bottlenecks caused by the chemical steps(Fig.4).
4.4.Key enzymes involved in simvastatin production
4.4.1.Lovastatin hydrolase—PcEST
The discovery of lovastatin hydrolases with the potential for industrial applications is very limited.As early as 1997, Merck had isolated and purified a specific lovastatin esterase.In 2004,Morgan et al.[70] reported another specific lovastatin esterase,EcBla4.However,the high Kmvalues(Kmis the Michaelis constant)of the reported enzymes toward lovastatin limit their application for MJ production.When the lovastatin hydrolase PcEST from P.chrysogenum was identified and characterized, its high activity toward lovastatin inspired researchers to aim for the one-step biosynthesis of MJ.Directed evolution was performed on PcEST and compared with wild-type PcEST,the solubility of the beneficial mutant Q140L increased by 2.2 times, the whole-cell activity showed an 18-fold improvement, and the T5100value increased by 3 °C(T5100is defined as the temperature where 50%of initial enzyme activity is lost following 10 min heat treatment)[71].The catalytic mechanism and structure–function relationship of PcEST and its mutant were revealed:The catalytic triplet,the hydrogen bonding network around the active site, and the specific substrate binding the channel together determine the catalytic efficiency of PcEST for the hydrolysis of lovastatin.On this basis, PcEST was further rationally designed, and the mutant D106A, which has improved soluble expression and thermal stability, was obtained [72].With A.terreus harboring the PcEST mutant as a host strain, the generated lovastatin was completely converted to MJ, realizing the single-step bioproduction of MJ in a more effective way.
4.4.2.Acyltransferase—LovD
The acyltransferase is responsible for the key step of converting MJ to lovastatin or simvastatin,so its development and application is of great significance in constructing a biosynthesis route for the second-generation statins.In 2006, researchers cloned the lovD gene encoding an acyltransferase from Aspergillus and overexpressed it in E.coli.In this way,they were able to characterize this acyltransferase,which selectively transfers α-methylbutyryl to the C8 hydroxyl group of MJ,thereby producing lovastatin.It was then discovered that LovD can catalyze the direct acylation of MJ via acyl-CoA thioesters, which was a promising biocatalyst to synthesize simvastatin with α-dimethylbutyryl-S-methyl thioglycolate as the substrate.Researchers replaced the two cysteine residues in LovD with alanine residues and improved the catalytic performance of the enzyme.The mutants were subjected to high cell density fermentation to convert 45 mmol·L-1of MJ sodium salt into simvastatin sodium salt within 18 h [61,68,73].In addition,the natural enzyme LovD was modified so that the enzyme can accept the free unnatural acyl donor α-dimethylbutyryl-Smethylmercaptopropionate, and the acyl carrier protein is no longer needed.The resulting mutant is 1000 times more efficient than the wild-type LovD in the synthesis of simvastatin [74].Finally,a whole-cell biocatalysis platform for the synthesis of simvastatin was established.In bio-manufacturing,the use of LovD for the catalytic production of simvastatin has many advantages,such as reducing the use of toxic and hazardous substances including tert-butyldimethylsilane chloride, methyl iodide, and n-butyl lithium.Since the reaction is carried out at ambient temperature and near atmospheric pressure, the energy efficiency is improved.The only byproduct 3-mercapto methyl propionate can be recovered,and the main waste stream produced is biodegraded in a biological treatment facility.
5.Production of third-generation statins by introducing key biocatalysis steps in a chemoenzymatic system
The third-generation statins include rosuvastatin, atorvastatin,fluvastatin, and pitavastatin.Compared with the first- and second-generation statins, the third-generation statins exhibit a better lipid-lowing effect with longer half-lives and reduced liver and kidney toxicity, making them the first-selected drugs for the prevention and treatment of hyperlipidemia at present and granting them an important position in the international market, with fast-growing annual sales.Atorvastatin calcium was first launched by Pfizer Pharmaceuticals Ltd.in the United States and was approved by the US FDA under the trade name Lipitor.It has been the sales champion of the world’s pharmaceutical market for nine consecutive years and was the only single compound to exceed 100 billion USD in the sale history of human medicine.Rosuvastatin and pitavastatin have better liver selectivity and muscle safety,and their small-dose use can effectively reduce LDL-C.In addition,they are less metabolized by the liver, resulting in lower liver toxicity.
The third-generation statins all contain a universal sidechain with a chiral diol structure as their main pharmacophore [75],and the synthesis of this sidechain is of great importance for the production of these statins.However, since the chiral diol sidechains contain two chiral hydroxyl groups,generating four epimers,independent stereoselective construction of the chiral centers is extremely difficult, and the synthesis cost accounts for half of the total cost of statin materials.For a long time, the synthesis of third-generation statins was extremely challenging.In the last decades, however, scientists have successively developed a variety of chemical methods for the synthesis of chiral diol sidechains,including monocarbonyl reduction methods,dicarbonyl reduction methods, aldehyde–ketone reduction coupling formation methods, and epoxide asymmetric ring-opening methods [76–78].Nevertheless,these methods generally present the problems of low enantiomeric and diastereomeric excess values,high cost of precious metal catalysts, and harsh reaction conditions.For example, during the process of atorvastatin synthesis, the cheap and readily available ethyl 4-chloroacetoacetate (A3) is used as a starting material.It is catalyzed for the synthesis of (S)-4-chloro-3-hydroxybutyric acid ethyl ester (A4) and further catalyzed to produce (R)-4-cyano-3-hydroxybutyric acid ethyl ester (A5), which is the first Rconfiguration chiral center at the 5′-position.The chemical dehalogenation and cyanation of A3 to A5 requires three steps:trimethylsilyl protection, sodium cyanide cyanidation, and deprotection.The product yield is only 56.7% [76].In the process from A5 to A7 (in the construction of the second chiral center at the 3′-position), the reagent borane is used under cryogenic conditions.The destruction of enantiomers (de) value of the target product,which represents the percentage of one enantiomer that is converted to its opposite, is always lower than 98% and, in order to increase the optical purity,multiple purification steps are required,resulting in low productivity[77,78].
With the development of enzyme engineering,the key steps for atorvastatin synthesis have been redesigned to meet industrial requirement, and enzymes—including carbonyl reductase (CR)and halohydrin dehalogenase (HHDH)—have been applied to construct an entire chemoenzymatic system for third-generation statins.CR is an important enzyme in the construction of the chiral hydroxyl groups of the chiral diol sidechain, as it catalyzes the asymmetric transfer of hydrogen on the nicotinamide ring to an oxidized substrate[79].HHDH can selectively catalyze the conversion from epoxides to O-haloalcohols through an intramolecular nucleophilic substitution mechanism [80].With the coapplication of CR and HHDH, the two chiral centers of the chiral diol sidechain are successfully built via simple reaction steps,under mild reaction conditions, and with high biosynthesis efficiency.
During atorvastatin synthesis,CR is applied for the bioreduction of A3 to A4 [81], which then further undergoes biological dehalogenation by HHDH to produce A5,generating the first chiral center of the chiral diol unit[82].Next,A5 is converted into 6-cyano-(5R)-hydroxy-3-carbonylhexanoic acid tert-butyl ester (A6) through a Claisen ester condensation reaction and is then reduced to 6-cyano-(3R,5R)-tert-butyl dihydroxyhexanoate (A7) (Fig.5)[83,84], which is reacted with 2,2-dimethoxypropane (DMP) to obtain (4R,6R)-6-cyanomethyl-2,2-dimethyl-1,3-dioxane-4-tertbutylacetate(A8).At this point,all chiral centers have already been constructed by the three-step biocatalytic synthesis described above.Compared with the traditional chemical steps, the biocatalytic route for A8 eliminates the use of borane,potassium borohydride,methanol,tetrahydrofuran,and liquid nitrogen.Through the additional steps of hydrogenation, condensation with the parent nucleus, hydrolysis, and recrystallization, the chiral diol sidechain is successfully applied for the synthesis of atorvastatin calcium.In this way,an industrial production line for atorvastatin calcium has been established [83].
Recently, another statin synthesis route with an enzymatic aldol reaction involving 2-deoxyribose 5-phosphate aldolase(DERA) has been proposed [85,86] (Fig.6).Combined with the application of HHDH,optically pure super statins such as rosuvastatin and atorvastatin are expected to be obtained.A few DERAs have been reported and characterized from different microorganisms, including Paenibacillus sp., Hyperthermus butylicus, Yersinia sp.,Pyrobaculum aerophilum,Thermus thermophiles,and Streptococcus mutans [87–91].However, most wild-type enzymes have low affinity toward acetaldehyde, limiting their application [92].Therefore,further research is needed to realize the practical application of the pathway.
5.1.Key enzymes involved in third-generation statins production
5.1.1.Engineering HHDH for third-generation statins production
To introduce key biocatalysis steps into the chemoenzymatic system of third-generation statins, researchers have focused on identifying and screening novel HHDHs with catalytic potential.A high-throughput screening technique for HHDH activity based on the color reaction between azide ion and Fe3+was established,and the HHDH from Parvibaculum lavamentivorans DS-1 was screened and mutated [93,94].After quickly screening from 2500 colonies, two mutants were obtained, F176M and A187R, which respectively possessed 2.1- and 1.8-fold higher relative activities than the wild type.With the synergistic mutation of two amino acid residues, the double mutant F176M/A187R exhibited a 2.8-fold improvement in catalytic activity [93].Bioinformatics technologies were used to analyze the amino acid composition and the structure–function relationship of HHDH.The catalytic pocket and ion channels for the product were determined to play a key role in determining the enantioselectivity of the enzyme.The hot spots determining both the enzyme activity and the stability were identified.This coupling modification greatly improved the industrial application properties of HHDH[95].For HHDH from Agrobacterium radiobacter (HheC), site mutagenesis was performed on the key amino acid residues.Based on the protein modeling and molecular docking results, six residues were selected for cooperative mutagenesis;after screening,the activity of the obtained variants increased by 15 times [96].
5.1.2.Engineering of CR for third-generation statins production

Fig.5.Synthetic route for atorvastatin calcium.The carbonyl reductase(CR)-and halohydrin dehalogenase(HHDH)-catalyzed steps were introduced for the construction of the A5 and A7 chiral centers.AKR: aldehyde-ketone reductase; DMP: 2,2-dimethoxypropane.

Fig.6.Synthesis route of atorvastatin and rosuvastatin with an enzymatic aldol reaction.
The gene-mining strategy shows good practical application prospects for the discovery of new enzymes based on protein databases.A series of CRs were screened for the effective production of third-generation statin intermediates [97].Since the reagent 2,4-dinitrophenylhydrazine and A6 involved in the synthesis route of atorvastatin form a red-brown hydrazone derivative whose color is positively correlated with the concentration of A6, a high-throughput screening method with a color reaction between 2,4-dinitrophenylhydrazine and A6 was created to improve the activity of CR in the reaction step from A6 to A7 [98].Rational design was performed on key amino acid residues near the coenzyme binding region and the substrate catalytic pocket.With enzyme engineering, a new CR was created with high affinity to the coenzyme, as well as excellent catalytic activity and stereoselectivity, resulting in good industrial performance in constructing the chiral center of atorvastatin calcium [98].
In addition, aldehyde-ketone reductase (AKR) can perform the same function as CR in the reduction of A6 to A7 [99].Luo et al.[100]rationally designed the wild-type aldehyde-ketone reductase from Kluyveromyces lactis (KlAKR) based on homology modeling and molecular docking.The highest catalytic efficiency of the mutant KlAKR-Y295W-W296L(M1)reached 12.37 s-1·(mmol·L)-1,which was 11.25 times higher than that of the wild-type KlAKR.To further improve its catalytic performance, semi-rational engineering of M1 was performed, and the mutant KlAKR-Y295W-W296LI125V-S30P-Q212R-I63W (M8) was developed.The catalytic efficiency value of M8 was 36.31 s-1·(mmol·L)-1, which was 1.9-fold higher than that of the parent M1 [101].In order to achieve the regeneration of in situ cofactors, the researchers constructed a strong co-expression system for M8 and glucose dehydrogenase(GDH) from Exiguobacterium sibiricum (EsGDH), developed a biocatalytic method with an increased substrate load,and reduced cell culture burden[83].Under these optimized conditions,a load of up to 80 g·L-1of A6 was completely converted in 1.5 h by M8 along with EsGDH for cofactor regeneration, producing A7 with space–time yield of 660 g·L-1·d-1[101].
5.2.Regulation of the production process
Through a fed-batch fermentation mode with constant dissolved oxygen feedback regulation, the high-density fermentation of recombinant strains harboring CR and HHDH was established[102].With Zn2+supplemented in a 50 L batch bioreactor, the cell biomass was increased, and the maximum HHDH activity was increased by 9.80%[103].Liu et al.[104]carried out the fermentation of recombinant E.coli harboring CR in 500 and 5000 L fermenters, and the biomass and specific activity reached about 9.7 g dry cell weight (DCW) per liter and 15 750 U·g-1DCW, and 11.0 g DCW per liter and 19 210 U·g-1DCW, respectively.The obtained cells were successfully applied for the efficient production of A7.Scale-up synthesis of A7 was performed in a 5000 L bioreactor with 400 g·L-1substrate at 30 °C, resulting in a space–time yield of 13.7 mmol·L-1·h-1·g-1DCW.
Zhang et al.[105] used recombinant E.coli cells containing CR and GDH to efficiently produce A7.After 12 h of reaction,the substrate conversion rate reached 98.8%, the yield was 95.6%, and an enantiomeric excess (ee) value >99.0% at substrate concentration of 350 g·L-1.Furthermore, a fed-batch strategy was adopted to increase the substrate concentration to 400 g·L-1.After 12 h of reaction, the product yield was increased to 98.5% and the space–time yield was 1182.3 g·L-1·d-1, which was the highest value for the CR–GDH coupling system in the literature.
In addition, Xue et al.[106] and Wan et al.[107] studied the thermodynamics of the unit reactions including biological reduction of A3, biological dehalogenation of A4 and cyanidation(Fig.7).Through the coupling of above three-enzymatic catalyzed steps, the conversion from A3 to A5 was significantly improved.When the input amount of A3 was 280 kg·m-3, the substrate conversion reached 100%, with an ee value for A5 of >99%.Compared with the most advanced Pfizer–Codexis technology reported in the previous literature, the substrate dosage and space–time yield of the multi-enzyme ‘‘one-pot” catalytic process were doubled[108–110].
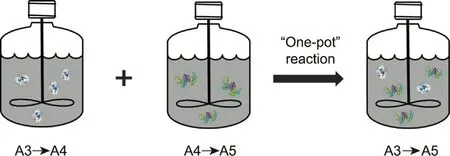
Fig.7.The ‘‘one-pot” synthesis of A5 via a combination of the A3 biological reduction, A4 biological dehalogenation, and cyanidation.
Immobilization is always used as an important technology to improve the stability and reusability of enzymes or recombinant cells.Wang et al.[111]were the first to develop Celite–polyethyleneimine–glutaraldehyde (GA) immobilized cells expressing CR to catalyze the synthesis of chiral A7.In their work, the stability of the immobilized cells was improved.In particular, in regard to thermostability, the half-life of the immobilized cells was prolonged to 120 min at 50 °C—a two-fold improvement over that of free cells.Application of the immobilized recombinant cells realized the production of A8 with an annual output of 200 t.We calculated that the unit consumption of raw materials in the process was reduced by 88.6%, the energy consumption was reduced by more than 70%, and the total production costs were reduced by approximately 54% compared with the total chemical synthesis[77,78,83,111].To increase the stability and recyclability of the biocatalyst, Qiu et al.[112] and Liu et al.[113] exploited a combinatorial immobilization technique of activated carbon adsorption,metal–organic framework zeolitic imidazolate framework (ZIF)-8 coating, and GA cross-linking.They applied this immobilization method to the previously constructed GDH and CR co-expressing strain of E.coli BL21(DE3)/pcDFDUET-gdh-cr.Under the optimized conditions, the active recovery of the immobilized cells reached 82.6%.The immobilized biocatalyst could be used for nine batches,with an overall yield of 23.75 g product per gram of immobilized cells in a diastereomeric excess of >99.5%.Moreover, a selfsufficient biocatalyst based on CR and a NADP+immobilization strategy was developed, and the cost of the catalyst continued to decrease [114,115].
5.3.Crystallization technology and quality control
Preliminarily prepared third-generation statins always contain numerous impurities, so the statins must undergo a series of separation and purification processes.Crystallization is an important step in product refining.However, the common occurrence of the phenomenon of homogeneous polycrystals makes it difficult to prepare a single crystal of the product.To address the problem of the product purity, researchers have established a synthesis process for the atorvastatin calcium precursor, which is generated from the condensation of (4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tert-butylacetate (A9) and the parent nucleus.After optimizing the hydrolysis and salting process of the atorvastatin calcium precursor—which involves defluorination, epoxidation, methoxidation, and the degradation of impurities—the preparation of a high-purity atorvastatin calcium crystal was achieved [116–118], providing technical support for the establishment of an atorvastatin calcium quality-assurance system.
In third-generation statins production, it can be seen that the enzyme-catalyzed reaction plays a primary role in the biomanufacturing process[119].This reaction meets the requirement of green chemistry, since the reaction is carried out under mild reaction conditions and uses water as reaction medium under ambient temperature.In this way, it avoids steps such as functional activation, protection, and deprotection, which are commonly required in traditional organic synthesis [120].Based on the core technologies of enzyme design and modification, a high degree of chemical–enzymatic coupling, and good matching of the reactions and separations, chemoenzymatic synthesis processes for the efficient production of target products can be established, thereby enabling successful industrialization by employing modern bio-manufacturing technologies.
6.Conclusion and outlook
The integration of bio-manufacturing throughout the development of statins can clearly be seen, in the development of microbial fermentation to produce the first-generation statins,biotransformation to produce the second-generation statins, and the chemoenzymatic synthesis to produce third-generation statins.With the increasing complexity of drug molecules and high industrial standards, traditional synthetic processes fail to meet the requirements of industrialization.In addition,economic and social development is facing severe challenges such as declining resources and increasing environmental pollution.As such, it is urgent for green transformation to occur in the supply of raw materials, processing modes, and product upgradation, in order to achieve coordinated and sustainable development.Biomanufacturing is the core integration of technologies and engineering in the bioindustries.Its application scope covers the value chain from resources and technologies to industries, including modern biotechnologies in the fields of medicine, agriculture,energy, materials, and environmental protection.
Thorough bio-manufacturing, green production has been achieved for numerous batches of chemicals thus far,including statins,providing good examples of the process of modern industrialization.Looking to the future, with advances in the cutting-edge biotechnologies of bioinformatics, synthetic biology, and protein engineering, as well as the integration of multiple disciplines including system engineering, process engineering, information technology, and management science, bio-manufacturing is expected to move toward more intelligent processes, in order to realize in-depth machine learning design, multi-parameter online monitoring, and intelligent regulation of industrial processes.For example, the development of genetic editing technologies such as clustered regularly interspaced short palindromic repeats(CRISPR)interference screening provides a powerful tool to map complex prokaryotic genetic networks in a precise and high-throughput manner [121,122].Its application greatly promotes the engineering and regulation of microbial cell factories for efficient chemical production.With the emergence of technologies such as the microbial microdroplet culture system and gel microdropletbased high-throughput screening, integrated platforms for microbial screening, cultivation, and adaptive evolution can be constructed, promoting the screening and cultivation efficiency of high-yielding microorganisms [123,124].Advances in multiomics analysis enable the redesigning of more powerful methods to decipher the code of life, which will directly eliminate the screening step and provide greater convenience for biomanufacturing [125].Similarly, with the development of bioinformatics, enzymes can be easily redesigned with excellent catalytic performance, in order to broaden their application in biological manufacturing [126].Furthermore, the intelligent control of biological processes and the development and application of new bioreactors will be an inevitable trend, which will together promote the high-quality development and upgrading of the bio-manufacturing industries.
Acknowledgment
This work was supported by the National Key Research and Development Project of China (2018YFA0901400).
Compliance with ethics guidelines
Xiao-Ling Tang, Jia-Wei Yu, Yu-Heng Geng, Jia-Rui Wang,Ren-Chao Zheng, and Yu-Guo Zheng declare that they have no conflict of interest or financial conflicts to disclose.
杂志排行
Engineering的其它文章
- Dynamic Deformation Measurement of an Intact Single Cell via Microfluidic Chip with Integrated Liquid Exchange
- The Rational Design and Development of Microalgae-Based Biohybrid Materials for Biomedical Applications
- DART Mission Shows Potential for Planetary Defense by Smashing Asteroid into New Orbit
- Electric Racers Hit the Track, but Still Catching Up
- Micro-Cantilever Electric Field Sensor Driven by Electrostatic Force
- Theory and Practice of Hydrodynamic Reconstruction in Plain River Networks