Sericin alleviates pentylenetetrazole kindling epilepsy and associated comorbidities via modulation of GABA-T enzyme and mitochondrial activity
2023-11-13SaniaGroverRajKumarNarangShamsherSingh
Sania Grover, Raj Kumar Narang, Shamsher Singh
1I.K.Gujral Punjab Technical University,Jalandhar,Punjab,India
2Department of Pharmaceutics,ISF College of Pharmacy (An Autonomous College), Moga-142001,Punjab,India
3Neuroscience Division,Department of Pharmacology,ISF College of Pharmacy (An Autonomous College), Moga-142001,Punjab,India
ABSTRACT Objective: To assess the effect of sericin against pentylenetetrazole(PTZ) kindling epilepsy and its associated comorbidities.Methods: Epilepsy was induced with PTZ at the dose of 30 mg/kg i.p. on alternative days for 25 days in rats.Sericin was administered orally at the doses of 250, 500, and 1 000 mg/kg for 35 days.The behavioral activities were performed using an elevated plus maze,forced swim test, and Morris water maze test.A PTZ challenge test was conducted on day 32.On day 35, rats were sacrificed to perform oxidative stress, mitochondrial dysfunction, neuroinflammation,neurotransmitters, GABA-T activity, and histopathological analyses.Results: Sericin at 500 and 1 000 mg/kg significantly reduced behavioral changes and neuroinflammatory cytokines, as well as improved oxidative stress, mitochondrial enzyme complex activity,neurotransmitter level, and GABA-T enzymatic activity (P<0.05).Moreover, sericin improved the neuronal survival altered by PTZ kindling in rat hippocampus.Conclusions: Sericin mitigates epilepsy-associated secondary complications possibly by the modulation of mitochondrial enzyme complexes and GABA-T enzymatic activity.
KEYWORDS: Pentylenetetrazole; Sericin; GABA-T; Epilepsy;Anxiety; Cognitive impairment
1.Introduction
Epilepsy is a prevalent central nervous system (CNS) disorder,indicated as unprovoked seizures owing to excessive discharge of neurons in the brain.It is associated with long-lasting comorbidities, for instance, anxiety, depressive behavior, and memory impairment[1,2].Patients having epileptic seizures are more susceptible to death and it is a challenge to treat comorbid conditions associated with epilepsy[3].Epileptogenesis is the process of developing epileptic seizures and various mechanisms like oxidative stress[4], neuroinflammation[5], mitochondrial dysfunction[6],neurotransmitters alteration [γ-aminobutyric acid (GABA),glutamate, and serotonin (5-HT)][7], altered Nrf2/HO-1 pathway[8],anomalous second messenger (cAMP, phosphoinositides, calcium ions) activity[9], and increased γ-aminobutyric acid-transaminase(GABA-T) activity[10] are involved in the process of epileptogenesis.Clinically, it is found that mitochondrial dysfunction and oxidative stress are responsible for neuronal damage in epileptic patients by oxidizing proteins, lipids, and nucleotides which further leads to diminished cellular activity, cell injury and cell death[11].Prolonged oxidative stress increases neuronal inflammation by activating proinflammatory cytokines which in turn results in the stimulation of transcription factors like nuclear factor kappa B(NF-κB), mitogen-activated protein kinases (MAPKs), motivating the development of neuronal excitability and ictogenic activity[5].However, mitochondrial dysfunction increases oxidative stress and inflammatory insult, causing alterations in neuronal homeostasis which directly or indirectly affect neurotransmitter levels.Available clinical reports revealed that patients having chronic epileptic seizures exhibit elevated glutamate levels and reduced GABA and 5-HT levels[1].Furthermore, excessive neuronal stress and reduced mitochondrial activity degrade GABA and promote glutamate synthesis responsible for refractory seizures[11].The metabolism of GABA to succinic semialdehyde is caused by a mitochondrial enzyme, GABA-T.Increased GABA-T activity in neuropsychiatric disorders like epilepsy affects the neuronal signaling in the hippocampus, striatum, and cortical areas of the brain[10].Therefore,there is a need to establish an animal model that can reflect the unprovoked seizures in animals as likely found in humans.Following the International League against Epilepsy, 2017, epilepsy is categorized as generalized and focal (partial).Temporal lobe epilepsy (TLE) is a focal (partial) form of epilepsy, originating from the temporal area of the brain, which affects adults and manifests as impulsive reoccurring seizures along with cognitive and affective comorbidities like depression and anxiety[12].Kindling is a validated animal model that mimics human temporal lobe epilepsy[13].For the induction of kindling in animals, the sub-convulsive dose of pentylenetetrazole (PTZ) is persistently administered, leading to seizure development, and associated with cognitive deficit.It is well documented that the repeated exposure of rat brains to PTZ causes neuronal damage specifically in hippocampal CA1 and CA3 regions,further resulting in cognitive impairment.Additionally, some preclinical reports also concluded that PTZ kindling is blameworthy for bringing out variations in neurotransmitter (GABA, glutamate,5-HT, and their metabolites) levels in the brains of animals[1].
In the current scenario, complete seizure suppression is a matter of concern as the available drug therapy for epilepsy is effective symptomatically, not improving the underlying cause of epileptogenesis.Even though, the newer anti-epileptic drugs like topiramate, lamotrigine,etc., clinically endorsed by the Food and Drug Administration for the cure of epilepsy, are now considered as second-line therapy for refractory seizures.Moreover, the available anti-epileptic drugs are known to cause cognitive side effects[14],pharmacoresistance, economic burden, withdrawal symptoms,deleterious interactions with other drugs, chronic toxicity, and recurrent seizures[15].Therefore, researchers are increasingly turning their attention to natural drugs which can suppress epileptogenesis and have cognition-improving properties.A well-known natural fiber, silk, which is obtained fromBombyx mori(silk moth), contains two major proteins: fibroin and sericin[16].Having a proteinaceous nature, sericin consists of 18 amino acids (both essential and nonessential) and exhibits 32% of serine (non-essential amino acid).About 20%-30% of sericin is found in the total weight of the cocoon, which is wrapped over the fibroin to maintain its shape and hardness[17].Sericin is resistant to protease owing to which it is not digested in the gastrointestinal tract by proteolysis[18].During the degumming process of silk manufacturing, sericin is procured as waste material, that is 50 000 tons of sericin is acquired from 400 000 tons of dry cocoons.Therefore, by recycling it from waste, it can be utilized for economic and social benefits as a valuable product.During pharmacological therapy, sericin showed activities such as antibacterial, antioxidant, wound healing, anti-tumor, anti-Alzheimer,anti-anxiety, anti-depressant, anti-Parkinson, and neurological lesions in diabetes.In addition, it is used in cosmetic industries like sunscreen formulations, shaving gels, cosmetic powders in sericincoated forms, and nail varnishes[19].
Therefore, the current study aimed to evaluate the anti-epileptic and protective effect of sericin against PTZ kindling epilepsy and its secondary complications (anxiety, depression, and cognitive impairment) by performing various behavioral, biochemical,mitochondrial enzyme complex, and neurotransmitter analyses.
2.Materials and methods
2.1.Experimental animals
Adult Wistar rats of either sex having body weight 220-250 g were acquired from Central Animal House, ISF College of Pharmacy,Moga Punjab, India, and housed in a group of three animals in propylene cages containing husk bedding.In this study, we followed standard laboratory methods and maintained environmental conditions, specifically, animals were maintained at a 12-hour light and 12-hour dark cycle, (25 ± 2) ℃, and the relative humidity of (60± 10)%.Additionally, the animals were provided with free access to food and water.These measures were typically taken to minimize the influence of environmental factors on the experimental results and ensure that the study conditions were consistent and reproducible.The experimental protocol was executed between 09:00 to 15:00.
2.2.Drugs and chemicals
PTZ and sodium valproate were procured from Sigma-Aldrich(St Louis, MO, USA) and sericin from Seidecosa Skincare India Pvt.Ltd.(a division of Indian Silk Fiber Co, Bengaluru, Karnataka,India).PTZ (30 mg/kg) and sodium valproate (200 mg/kg) were solubilized in 0.9% saline and sericin (250, 500, and 1 000 mg/kg) in distilled water.Based on the previously published data, the doses of PTZ and sodium valproate were selected, whereas the dose of sericin was purely selected in conformity with OECD guidelines.PTZ and sodium valproate were administered by the intraperitoneal route and sericin was by using oral gavage[20-22].All these drug solutions were freshly made before administration.In contrast, test kits for interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α) were procured from Krishgen Bio.Sys, Ashley ct Whittier, CA, USA.In this study, analytical-grade chemicals were used.
2.3.Induction of kindled seizures
The sub-convulsive dose of PTZ (30 mg/kg) was delivered intraperitoneally (i.p.) to the rats on alternate days for 25 d (13 injections)[20].The animals were placed in a transparent acrylic chamber (30 cm × 24 cm × 22 cm) with in-between partitioning,where the CNS excitation phenomena were observed for 20 min following the administration of each injection.The extent of seizure induction was analyzed by using Racine’s scale[11]: Stage 0: no response; Stage 1: Mouth and facial movements; Stage 2:Head nodding; Stage 3: Forelimb clonus; Stage 4: Rearing, falling,hindlimb clonus and forelimb tonus; Stage 5: Tonic extension of the hind limb, status epilepticus, and/or death.
Animals showing convulsions on the first day after PTZ injection were rejected from the study.The rats indicating stage 4 seizures three times were assumed as fully kindled.
2.4.Experimental design
In this protocol, Wistar rats were arbitrarily divided into six groups.Sericin administration was initiated on day 1 along with PTZ (till day 25) and continued up to day 35.Several behavioral tests were conducted on day 26i.e., 24 h after the final PTZ injection.On day 26, rats were directed to an elevated plus maze (EPM), and its retention was observed on day 27.On day 28, the rodents were exposed to training for the forced swim test (FST) and its retention was recorded on day 29.Similarly, the rodents were trained in the Morris water maze (MWM) on day 30 and its retention was noted on day 31.On day 32, the PTZ challenge test was carried out and retention was noticed.This PTZ challenge test was carried out to ascertain the onset of generalized clonic seizures.On day 35 (10 d after the final PTZ injection), the animals were euthanized by cervical dislocation, and their brains were taken out and analyzed for biochemical, neuroinflammatory, neurotransmitters, and mitochondrial enzyme complex estimations (Supplementary Figure 1).

Figure 1.Effect of sericin treatment on pentylenetetrazole (PTZ)-induced (A) seizure severity and (B) depressive-like behavior in rats.Data are expressed as mean ± SD.aP<0.05 vs. the normal control, bP<0.05 vs. the PTZ group, cP<0.05 vs. PTZ (30 mg/kg) + Sericin (250 mg/kg), dP<0.05 vs. PTZ (30 mg/kg) +Sericin (500 mg/kg), eP<0.05 vs. PTZ (30 mg/kg) + Sericin (1 000 mg/kg).Statistical analysis is performed by two-way ANOVA followed by Bonferroni’s multiple comparison test.
2.5.Experimental grouping
In this study, 48 rats were employed, and they were allocated into six groups as follows: Group Ⅰ: Normal control (treated with 0.9%saline); Group Ⅱ: PTZ (30 mg/kg;i.p.); Group Ⅲ: PTZ (30 mg/kg;i.p.) + Sodium valproate (200 mg/kg;i.p.); Group Ⅳ: PTZ (30 mg/kg;i.p.) + Sericin (250 mg/kg;p.o.); Group Ⅴ: PTZ (30 mg/kg;i.p.) + Sericin (500 mg/kg;p.o.); Group Ⅵ: PTZ (30 mg/kg;i.p.) +Sericin (1 000 mg/kg;p.o.).
2.6.Behavioral assessments
The rodents were exposed to a series of behavioral tests 24 h after receiving the final PTZ injection as mentioned in Supplementary Figure 1.These behavioral tests were performed chronologically in a manner from least aversive to the most aversivei.e.EPM for anxiety,FST for depression followed by MWM for cognitive impairment.The animals were subjected to these behavioral tests if the seizures were not detected for at least 30 min before the tests.If the seizures persist during testing, the test is discontinued, and the animal is placed in its respective cage.The test was re-initiated 1 h after the seizure cessation.
2.6.1.EPM test
For the determination of anxiety-like behavior in rats, the EPM test was conducted on the 26th, 27th, and 32nd days.EPM test was performed as per the method mentioned by Sharma and Kulkarni[23].which is based on the scary behavior of animals towards height and open spaces and preference towards the closed arena.The EPM contains two open and two closed arms having the dimensions of 50 cm × 10 cm and 50 cm × 10 cm × 40 cm respectively, elevated 50 cm from the floor.The rodents were placed one by one in the center area facing away from the experimenter.When all four paws were kept into any arm, it was considered as one entry into that arm.This test was performed for 5 min and the two parametersi.e.the number of entries into the open arm and the number of entries into the closed arm were determined for each rat.A decrease in the number of entries into the open arm indicated the anxious behavior of the animal[23].
2.6.2.FST
A FST was accomplished to confirm depressive-like behavior in epileptic rats.For this test, the individual rat was allocated to a glass tank filled 2/3rd with water [maintained at (24 ± 1) ℃], for 6 min.Immobility time or floating time (not including swimming or climbing time) was observed as an indicator of behavioral despair.For the first 2 min, all the animals were trying to escape from the water therefore the immobility time of the last 4 min was considered.All the animals were examined meticulously for 6 min and immediately brought out from the water if they were recognized to be at imminent risk of getting drowned or unable to maintain their head above the water level.After a 6-minute test session, animals were taken out, tended to be dried, and returned to their home cage where they were anticipated to get active and started grooming to get rid of excessive water.If any animal failed to do so, then it was dried by placing a heating pad (37 ℃) beneath the cage or by keeping a clean paper towel in the animal cage for 10-15 min[24].This behavioral test was performed on the 28th, 29th, and 32nd days.
2.6.3.MWM test
The MWM test was performed to determine spatial learning and memory in epileptic animals.MWM was accomplished as per the procedure described by Morris[25].It consists of a water basin of circular shape having a diameter of 180 cm and height of 60 cm,which is pooled with water [(25 ± 1) ℃] up to 40 cm height.Nonfat fresh milk was introduced to create the opaqueness of tank water.The tank was marked equally into 4 quadrants and an escape platform was kept in any one of the 4 quadrants, sunken 2 cm underneath the water level, whose position remained constant during the whole experiment.Rodents were given 120 s of unrestricted swimming time in the tank to allocate the obscured platform during the training phase.If the rat was unable to locate the platform within 120 s, then it was gently pushed towards the platform and retained for 30 s on it.The escape latency (in s) was the measure of spatial learning and memory in rodents.The amount of time taken by the rat to locate the hidden platform is considered the “escape latency”.In the present study, the acquisition trials were carried out on the 30th,31st, and 32nd days.After the culmination of the acquisition phase,a probe trial was carried out on the 32nd day by withdrawing the hidden platform to determine the animal’s learning and memory.For this, time spent in the target quadrant from where the platform was removed, is considered as a measure of spatial memory[25].
2.7.Dissection and homogenization
On day 35, the animals were euthanized by cervical dislocation.The brains were taken out and drenched with ice-cold saline and then kept on dry ice.For the preparation of 10% (w/v) brain tissue homogenates,0.1 M phosphate buffer (pH 7.4) was utilized.Then the homogenates were centrifuged at 10 000 ×gfor 15 min at 4 ℃.The aliquoted supernatants were then taken and used for oxidative stress parameters,neuroinflammatory, neurotransmitter, and GABA-T analyses.
2.8.Estimation of oxidative stress parameters
2.8.1.Estimation of lipid peroxidation
The method described by Kauret al.[26] was used to quantify the malondialdehyde (MDA), which assesses the degree of lipid peroxidation in tissue homogenates.This test is based on the reaction between the thiobarbituric acid (TBA) and MDA, resulting in the formation of a pink-colored complex MDA-TBA, which is further analyzed by spectrophotometric method at 530-535 nm and expressed as nmol/mg protein[26].
2.8.2.Estimation of reduced glutathione(GSH)
The procedure mentioned by Kauret al.[26] was utilized for the determination of GSH in tissue homogenates.This was accomplished by adding 1 mL of 4% sulfosalicylic acid to precipitate 1 mL of supernatant, which was then frozen at 4 ℃ for 1 h.Subsequently, the material was centrifuged at 1 200 ×gfor 15 min.One mL supernatant was then added to 2.7 mL of phosphate buffer (0.1 M, pH 8) and 0.2 mL of 5,5’-dithiobis(2-nitrobenzoic acid), resulting in the development of yellow color, which was then analyzed at 412 nm using spectrophotometry and expressed as µmol/mg protein[26].
2.8.3.Estimation of nitrite
According to the procedure reported by Kauret al.[26], the levels of nitrite in brain tissue supernatant were determined using the Griess reagent [0.1%N-(1-naphthyl) ethylenediamine dihydrochloride, 1%sulphanilamide, and 2.5% phosphoric acid].The concentration of nitrite was determined by using the sodium nitrite standard curve and expressed as µg/mL[26].
2.8.4.Estimation of superoxide dismutase(SOD)
The Dhamiet al.[27] method was employed to assess SOD levels.With this method, the SOD’s propensity to scavenge superoxide anion radical (O2˙-) increases, slowing down the reaction chains and the rate of pyrogallol autoxidation, which is expressed as U/mg protein[27].
2.8.5.Estimation of protein
The method of Paudelet al.[28] was followed to determine the protein concentration in tissue supernatant.
2.9.Estimation of mitochondrial complex enzymes
2.9.1.Isolation of rat brain mitochondria
For the determination of mitochondrial enzyme complex activities,mitochondria were isolated from the rat brain by employing the method of Thakuret al[29].The brains were homogenized in the isolated buffer containing ethylene glycol tetraacetic acid (EGTA)and the pellets obtained were then centrifuged at 13 000 ×gfor an additional 5 min at 4 ℃.The resultant supernatants were put in fresh tubes, washed off with an isolation buffer containing EGTA,and centrifuged one more time at 13 000 ×gfor 10 min at 4 ℃.Pure mitochondria-containing pellets were resuspended in an isolation buffer that was devoid of EGTA[29].
2.9.2.Succinate dehydrogenase(complex Ⅱ)activity
Succinate dehydrogenase activity was quantified spectrophotometrically as per the method described by Thakuret al[29].This method is principally based on the tendency of potassium ferricyanide (an artificial electron acceptor) to oxidize succinate.The reaction mixture included 0.6 M succinic acid, 0.03 M potassium ferricyanide, 1% bovine serum albumin, and 0.2 M phosphate buffer(pH 7.8).The mitochondrial sample was introduced to commence the reaction, which was then followed by a 2-minute change in absorbance at 420 nm[29].
2.9.3.Cytochrome oxidase(complex Ⅳ)activity
Using the technique of Paudel and his colleagues[28], cytochrome oxidase activity was assessed in brain mitochondria.The assay solution carried 75 mM of phosphate buffer and 0.3 mM of reduced cytochrome c.The solubilized mitochondrial sample was added to initiate the process, and for 2 min, the variation was determined in absorbance at 550 nm[28].
2.10.Estimation of neuroinflammatory markers
Proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α were examined using the rat immunoassay kit (KRISHGEN BioSystem,Whittier, CA).This kit makes use of a microtiter plate reader that reads at 450 nm in conjunction with a solid-phase sandwich enzymelinked immunosorbent assay (ELISA).The TNF-α, IL-1β, and IL-6 values were determined using the depicted standard curves and indicated as pg/mg protein.
2.11.Estimation of neurotransmitters
2.11.1.Estimation of brain catecholamine and its metabolite(5-HT and 5-HIAA)
High-performance liquid chromatography (HPLC) was employed to assess the levels of brain catecholamine (serotonin or 5-HT) and its metabolite (5-hydroxy indole acetic acid or 5-HIAA) using an electrochemical detector.According to the procedure mentioned by Thakur and her co-workers, the quantitative estimation of 5-HT and 5-HIAA was carried out[29].
2.11.2.Estimation of GABA and glutamate
O-phthalaldehyde/β-mercaptoethanol was used to derivatize homogenate solution to quantify amino acids (GABA and glutamate), following a previously described procedure by Kaur and her coworkers[26], GABA and glutamate in the brain homogenate were estimated[26].
2.12.Estimation of GABA-T activity
The colorimetric assay method described by Kandedaet al.[30] was used to assess the activity of GABA-T.For this assay, graduated 10 mL tubes were used which were loaded with 0.1 mL of homogenate supernatant, 0.1 mL of 5% methanol, 15 mmol of I-oxoglutarate, 15 mmol of GABA, and 10 mg of pyridoxal phosphate.With the help of Tris-HCl buffer (50 mM, pH 7.4), the mixture’s final volume was raised to 3 mL.After that, these tubes were incubated for 30 min at 37 ℃.After the incorporation of 0.5 mL of glacial TCA (20%,Sigma-Aldrich), the reaction was accomplished.Then, the resultant products of the reactioni.e.semialdehyde succinic acid complex and 3-methyl-2- benzothiazole-2-hydrazone were stained with 12%FeCl3followed by spectrophotometric estimation to quantify how much semialdehyde succinic acid was formed during the mixture’s incubation, and the absorbance at 610 nm was observed after 30 and 90 s in comparison to a blank.The extent of these products get stained is the measure of GABA-T activity in the tissue homogenate and is indicated as pg/min/mg of tissue[30].
2.13.Histological analysis
Following the decapitation process, the animal brains were taken out and kept in 5% formaldehyde.Then, the rat brains were fixed in liquid paraffin wax, thereafter, slicing (5-μm) and staining with hematoxylin and eosin (H & E) were carried out[31].The examination of these stained slices was performed by using a fluorescence microscope (Model: 102M, Motic Microscopes, China)with a magnification power of 40×.
2.14.Statistical analysis
The result outcomes are shown as mean ± SD.Seizure severity and behavioral data (EPM, FST, and MWM) were analyzed by employing a two-way ANOVA followed by a Bonferroni’spost hoctest for multiple comparisons, whereas data on biochemical,mitochondrial complexes, neuroinflammatory cytokines,neurotransmitter, and GABA-T activity were analyzed using a one-way ANOVA and Tukey’s test.P<0.05 was established as the significant level in each test.
2.15.Ethical statement
This study was conducted after the approval of the Institutional Animal Ethics Committee (IAEC) during the internal meeting as per the meeting and protocol no.ISFCP/IAEC/CPCSEA/Meeting No: 27/2020/Protocol No.447.Further, the study was conducted as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals required in our country(India) and all the experimental procedures were carried out by research scholars in coordination with research scientists.
3.Results
3.1.Effect of sericin treatment on seizure severity in PTZinduced kindling in rats
The seizure severity was markedly worsened by chronic PTZ(30 mg/kgi.p.) administration given every other day for 25 d (13 injections).On the 25th day, the maximum score was achieved,and most of the rats of the PTZ control group experienced stage 4 seizures, although a few rats experienced stage 5 seizures.In comparison to the PTZ-treated rats, sericin (500 and 1 000 mg/kgp.o.) significantly (P<0.001) lowered the seizure severity.A PTZ challenge test was conducted on day 32 and the PTZ-kindled rats did not experience any variation in seizure severity, whereas the sericintreated group (500 and 1 000 mg/kgp.o.) further demonstrated a significant (P<0.001) reduction in seizure severity in the challenge test.However, the rats treated with sericin (250 mg/kgp.o.) did not show any significant decrease in seizure severity as compared to PTZ-treated rats.In addition to this, the rats treated with sericin (500 and 1 000 mg/kgp.o.) exhibited a reduction in seizure intensity in a dose-dependent manner in contrast to rats treated with sericin (250 mg/kgp.o.) (Figure 1A).
3.2.Effect of sericin treatment on PTZ kindling-induced depressive-like behavior in FST
On the 28th, 29th, and 32nd (after the challenge test) days, chronic administration of PTZ was found to exacerbate depressive-like behavior in rats.Treatment with sericin (500 and 1 000 mg/kgp.o.)daily alleviated the depressive-like behavior in animals, as evidenced by significantly reduced immobility time (P<0.001), in contrast to the PTZ-kindled group, which showed a significant increase in the immobility time (P<0.001).In terms of immobility time, there was no discernible difference between the PTZ group and the sericin(250 mg/kgp.o.)-administered group (Figure 1B).
3.3.Effect of sericin treatment on PTZ kindling-induced anxiety-like behavior of rats in EPM test
Rats exhibited anxiety-like behavior after receiving PTZ at the subconvulsive dose of 30 mg/kg on alternate days until the 25th day.On days 26 and 27, the number of entries in the closed arm was significantly increased and the number of entries in the open arm decreased significantly in the PTZ-kindled group (P<0.001), whereas sericin (500 and 1 000 mg/kgp.o.) markedly decreased the number of entries in the closed arm and increased the number of entries in the open arm (P<0.001), compared to the PTZ-kindled group.Rats were evaluated again for retention on the plus maze on day 32(30 min following the challenge test), and the number of entries in both arms was counted.Treatment with sericin (500 and 1 000 mg/kgp.o.) displayed a significant improvement in the number of entries in both arms.Additionally, in contrast to the PTZ-treated group,the rats administered with sericin (250 mg/kgp.o.) did not exhibit any appreciable improvement in the number of entries in either arm(Figure 2).
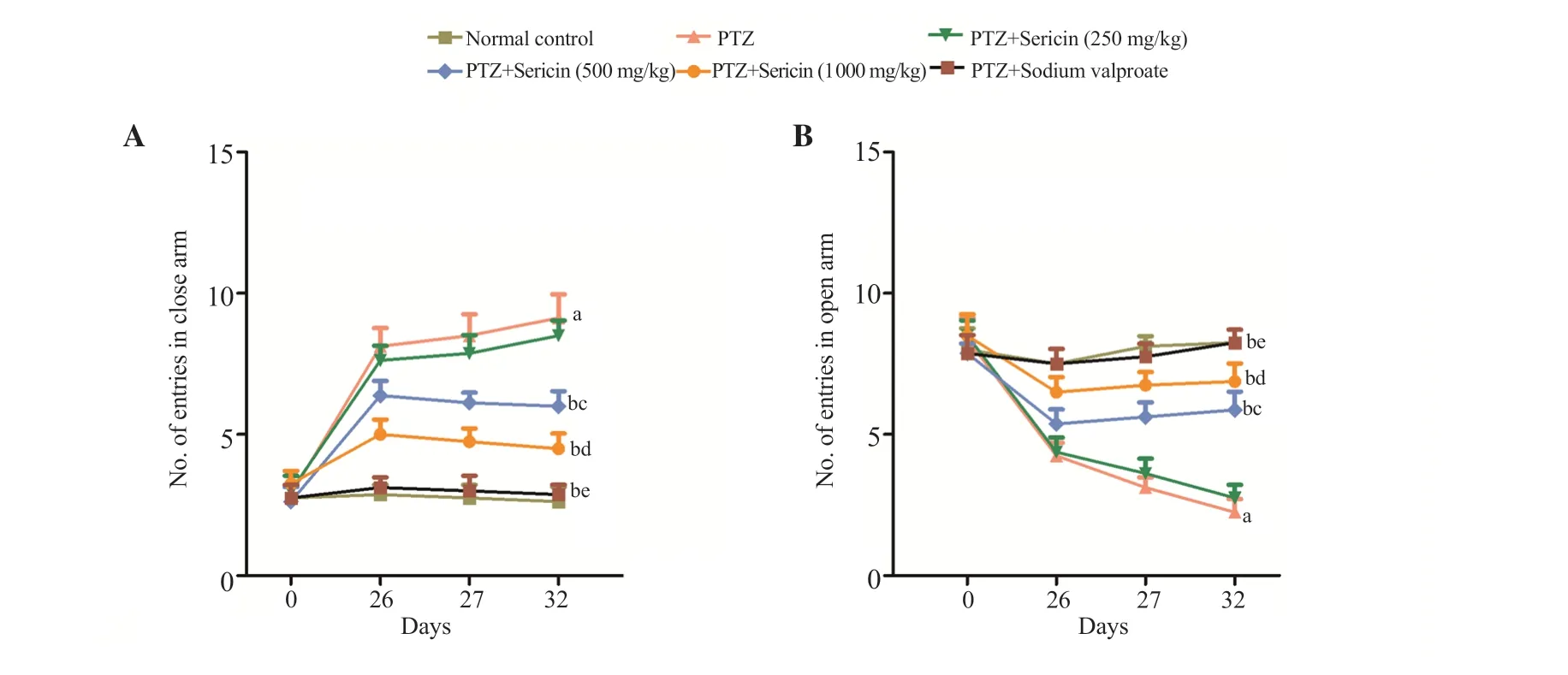
Figure 2.Effect of sericin treatment on PTZ kindling-induced anxiety-like behavior in elevated plus maze test.Data are expressed as mean ± SD.aP<0.001 vs. the normal control, bP<0.001 vs. the PTZ group, cP<0.001 vs. PTZ (30 mg/kg) + Sericin (250 mg/kg), dP<0.001 vs. PTZ (30 mg/kg) + Sericin (500 mg/kg),eP<0.001 vs. PTZ (30 mg/kg) + Sericin (1 000 mg/kg).Statistical analysis is performed by two-way ANOVA followed by Bonferroni’s multiple comparison test.
3.4.Effect of sericin treatment on PTZ kindling-induced cognitive impairment in MWM test
On days 30, 31, and 32 (after the challenge test), escape latency time was recorded, and on day 32, time spent in target quadrant was determined.PTZ-exposed rats demonstrated a substantial rise(P<0.001) in escape latency time and a reduction in time spent(P<0.001) in the target quadrant, showing altered spatial memory up to the 32nd day of the learning process.In comparison to the PTZ group, sericin treatment (500 and 1 000 mg/kgp.o.) mitigated the impaired memory and significantly (P<0.001) lessened the escape latency time and raised the time spent in the target quadrant.However, sericin (250 mg/kgp.o.) did not induce any significant changes in PTZ rats (Figure 3A & 3B).

Figure 3.Effect of sericin treatment on PTZ kindling-induced cognitive impairment (A: escape latency; B: time spent in target quadrant) in Morris water maze test.Data are expressed as mean ± SD.aP<0.001 vs. the normal control, bP<0.001 vs. the PTZ group, cP<0.001 vs. PTZ (30 mg/kg) + Sericin (250 mg/kg), dP<0.001 vs. PTZ (30 mg/kg) + Sericin (500 mg/kg), eP<0.001 vs. PTZ (30 mg/kg) + Sericin (1 000 mg/kg).Statistical analysis is performed by two-way ANOVA followed by Bonferroni’s multiple comparison test.
3.5.Effect of sericin treatment on oxidative stress parameters in PTZ-kindled rats
After chronic delivery of PTZ (30 mg/kg) on every alternate day for 25 d, kindled rats demonstrated a significant (P<0.05) rise in the levels of MDA and nitrite, and decreased activity of GSH, SOD, and total protein content in contrast to the normal group.The increased levels of MDA and nitrite along with lowered levels of total protein,GSH and SOD in rat brains were significantly (P<0.05) mitigated by pre-exposure to sericin (500 and 1 000 mg/kgp.o.) in comparison to PTZ-treated rats.However, sericin (250 mg/kg) did not reveal any remarkable difference in the levels of these oxidative parameters(Table 1).
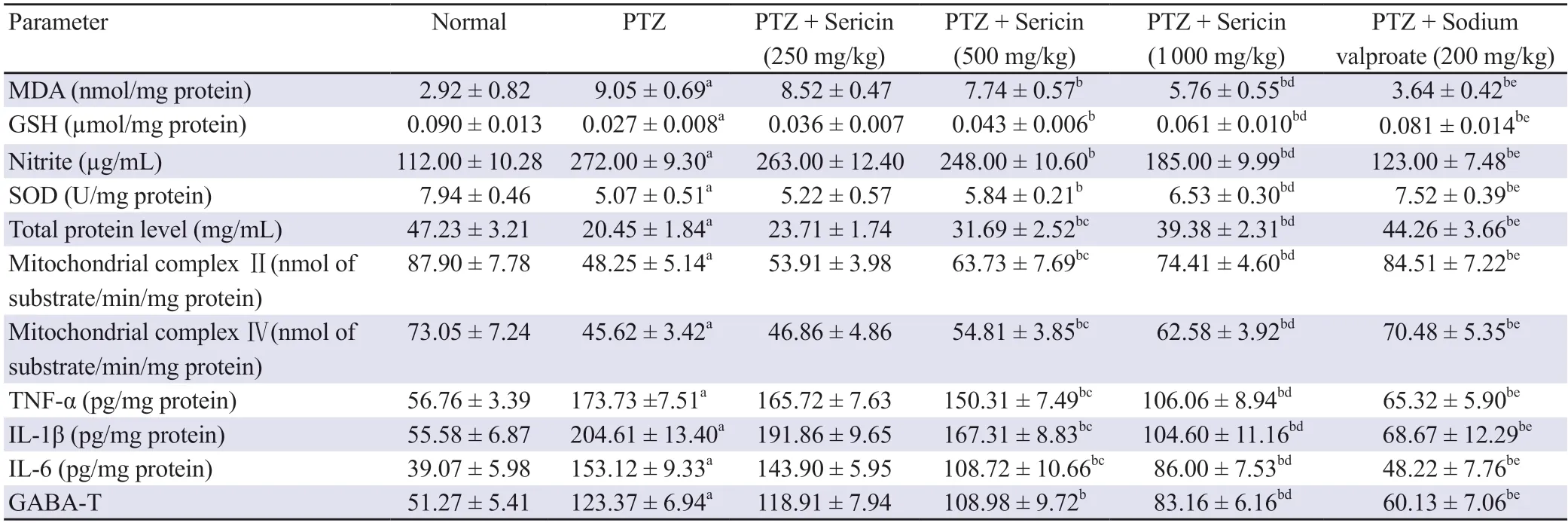
Table 1.Effect of sericin treatment on oxidative stress parameters, neuroinflammatory markers, as well as mitochondrial complex enzyme and GABA-T activities in PTZ-kindled rats.
3.6.Effect of sericin treatment on mitochondrial complex enzyme activity (complex Ⅱ and Ⅳ) in PTZ-kindled rats
Chronic PTZ (30 mg/kg) exposure significantly decreased mitochondrial complex enzyme (Ⅱand Ⅳ) activity in contrast to the normal rats.Sericin treatment (500 and 1 000 mg/kgp.o.)significantly (P<0.05) elevated mitochondrial complex enzyme(Ⅱand Ⅳ) activity.No significant change was observed in rats treated with 250 mg/kg sericin (Table 1).
3.7.Effect of sericin treatment on neuroinflammatory markers(TNF-α,IL-1β,and IL-6) in PTZ-kindled rats
Cytokine (TNF-α, IL-1β, and IL-6) production is one of the important processes implicated in neuroinflammation in epilepsy and other neurological disorders.The levels of TNF-α, IL-1β, and IL-6 in the brain were significantly (P<0.05) raised after intraperitoneal injection of PTZ in contrast to the normal group.The rats treated with sericin(500 and 1 000 mg/kgp.o.) manifested a significant (P<0.05) reduction in the levels of TNF-α, IL-1β, and IL-6, whereas the rats administered with the low dose of sericin (250 mg/kg) did not reveal any significant changes in contrast to PTZ-treated rats (Table 1).
3.8.Effect of sericin treatment on brain catecholamine and its metabolite (5-HT and 5-HIAA) in PTZ-kindled rats
A significant reduction in brain catecholamine and its metabolite(5-HT and 5-HIAA) levels was observed after intraperitoneal injection of PTZ.Treatment with sericin (500 and 1 000 mg/kgp.o.)pronouncedly (P<0.05) raised the 5-HT and 5-HIAA levels, whereas a low dose of sericin (250 mg/kg) did not induce any substantial changes in 5-HT and 5-HIAA levels (Table 2).
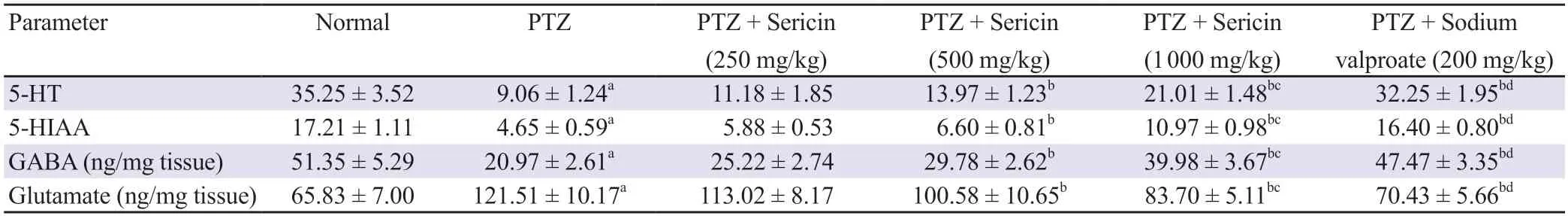
Table 2.Effect of sericin treatment on brain catecholamine and its metabolite (5-HT and 5-HIAA) as well as GABA and glutamate in PTZ-kindled rats.
3.9.Effect of sericin treatment on GABA and glutamate in the brain of PTZ-kindled rats
The kindled-group animals manifested a significant decline in GABA levels and a rise in glutamate levels in contrast to normal animals.However, treatment with sericin (500 and 1 000 mg/kgp.o.)dose-dependently elevated the level of GABA and diminished the level of glutamate.Sericin at 250 mg/kg had no significant effect on GABA and glutamate levels (Table 2).
3.10.Effect of sericin treatment on GABA-T activity in the brain of PTZ-kindled rats
GABA-T, as the main GABA degradative enzyme, is responsible for regulating the levels of GABA.In the current study, the intraperitoneal administration of PTZ exhibited a significant elevation in GABA-T activity in comparison to normal rats.In contrast, sericin (500 and 1 000 mg/kgp.o.) dose-dependently reduced the GABA-T activity,but the low dose of sericin (250 mg/kg) did not reveal any significant difference, in contrast to PTZ-treated rats (Table 1).
3.11.Effect of sericin treatment on histopathological damages in the brain of PTZ-kindled rats
For the assessment of the extent of neuronal damage in the hippocampal region of the brain, H & E staining was employed.In the normal control group, the neurons of the hippocampal region were found with intact nuclear membranes and cell membrane integrity.The hippocampus of PTZ-kindled rats showed neuronal apoptosis, leading to a significant reduction in the number of neurons as compared to non-epileptic rats.Treatment with sericin (250, 500 and 1 000 mg/kg) dose dependently manifested less hippocampal degeneration (Figure 4).

Figure 4.Histopathological analysis of hippocampal neurons using hematoxylin and eosin staining (magnification: 40×, scale bar: 20 μm).A: The hippocampus of normal control rats shows normal neurons.B: PTZ-kindled rats show apoptotic (black arrow) nuclei in rat’s hippocampus.C, D & E: Sericin treatment (250, 500 and 1 000 mg/kg) significantly improves neuronal cells and declines apoptotic cells.F: Rats administered with sodium valproate (200 mg/kg)show significant regeneration of hippocampal neuronal cells.
4.Discussion
The current research confirmed the therapeutic prospective of sericin against PTZ-kindling epilepsy, its associated comorbidities,and the neurochemical changes in rats.Kindling is the well-approved method to determine the seizures, the pattern of seizure development,and the epilepsy-associated comorbidities.It is well known that the seizures developed by kindling mimic human temporal lobe epilepsy[32].Various reports revealed that the hippocampus(specifically CA1, CA3, and dentate gyrus), entorhinal cortex,and amygdala are more susceptible to neurodegeneration by PTZ administration[33].The hippocampus plays a key role in regulating normal brain functioning such as mood, learning, and memory[34],and kindling with PTZ leads to anxiety-like and depression-like behavior, as well as memory impairment[2], which resembles the clinical epileptic manifestations.In this study, the chronic exposure of PTZ on every alternative day for 25 d significantly developed seizures and associated complications (anxiety, depression, and cognitive impairment) in rats.Pre-administration of sericin (500 and 1 000 mg/kgp.o.) significantly alleviated the seizure severity and ameliorated the anxiety-like and depression-like behavior, as well as learning process in rats.The kindling of animals with PTZ leads to the progression of oxidative stress in the brain because of the generation of free radicals and reduction in the antioxidant enzymes,resulting in the excessive firing of excitatory neurons and seizure spawning[1].Similarly, in the current study, kindled animals showed elevated levels of oxidative stress (MDA and nitrite) and diminished levels of antioxidants (GSH and SOD), whereas treatment with sericin (500 and 1 000 mg/kgp.o.) dose-dependently reversed these oxidative stress parameters.Furthermore, mitochondria play a vital role in performing various cellular functions that further regulate neuronal excitability along with adenosine triphosphate formation,oxidation of fatty acids, excitotoxicity, regulation of apoptosis,neurotransmitter synthesis, and maintaining cytosolic Ca2+levels.Free radical generation mainly takes place in mitochondria which is why mitochondria are highly susceptible to oxidative damage,having a key involvement in regulating neuronal excitability.It is believed that the occurrence of episodic attacks for prolonged duration leads to superoxide production which overwhelms the mitochondrial antioxidant defensive system with a series of events such as increased neuronal firing, the exaggerated release of glutamate,N-methyl-D-aspartate receptor stimulation, cytosolic and mitochondrial calcium influx, and raised adenosine triphosphate consumption.Various reports revealed that mitochondrial dysfunction associated with oxidative damage has a key involvement in neurodegenerative diseases[35].Moreover, many studies affirmed epileptogenesis is associated with abnormalities in mitochondrial functions[11].Likewise, in our study, the repetitive administration of PTZ significantly impeded the mitochondrial enzyme complex activities (Ⅱand Ⅳ), which evidenced the correlation of mitochondrial dysfunction with epilepsy.However, sericin treatment(500 and 1 000 mg/kgp.o.) improved the mitochondrial complexⅡand Ⅳ activities in a dose-dependent manner in contrast to PTZtreated animals, whereas the low dose of sericin (250 mg/kg) failed to improve these mitochondrial complex activities.Besides this,neuroinflammation serves a key role in the emergence of epilepsy[36].Pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) trigger NFκB and result in the excessive formation of nitric oxide, leading to the distortion of proteins and DNA.These pro-inflammatory markers further impelled the inflammatory and apoptotic cascade,followed by neurodegeneration.TNF-α binds with the TNFR1-type receptor on neurons resulting in the initiation of an apoptotic cascade[37].In this study, PTZ kindling caused a remarkable rise in the levels of TNF-α, IL-1β, and IL-6, whereas treatment with sericin(500 and 1 000 mg/kgp.o.) significantly diminished the levels of these cytokines.However, sericin at the dose of 250 mg/kg could not significantly reverse these cytokine levels.In numerous animal models, it is well known that monoaminergic neurotransmitters affect seizure susceptibility.Various reports evidenced that in kindled animals, the levels of monoaminergic neurotransmitters were decreased in several brain areasi.e.the hippocampus and cortex[7].The exposure of PTZ to animals causes exaggerated glutamatergic transmission followed by declined GABA-ergic and monoaminergic transmission, resulting in depressive-like behavior in animals.The depressive-like behavior of rats, confirmed by the FST, is assumed to be due to abnormal 5-HT transmission and decreased 5-HT release.It can be concluded that alteration in the 5-HT levels might be responsible for depression in epileptic animals[1].Likely, in our study,PTZ exposure exhibited a decline in 5-HT levels followed by depletion in its metabolite (5-HIAA) levels.However, treatment with sericin (500 and 1 000 mg/kgp.o.) significantly reversed the alterations in the levels of 5-HT and 5-HIAA, whereas sericin at its low dose (250 mg/kg) did not reveal any significant variation in 5-HT and 5-HIAA.It is wellapproved that the hyperexcitability of neurons occurs because of an imbalance between excitatory (glutamate) and inhibitory (GABA)neurotransmitters.In the human epileptic brain, glutamatergic neurotransmission gets exaggerated, whereas GABAergic neurotransmission gets understated[1,38].These reports are in favor of our findings that PTZ-kindled rats manifested elevated levels of glutamate and diminished levels of GABA in the brain.However, the rats treated with sericin (500 and 1 000 mg/kgp.o.) dose-dependently restored the levels of glutamate and GABA in rat brain, but sericin at 250 mg/kg dose caused no significant alteration in the levels of these excitatory and inhibitory neurotransmitters.In correlation with the GABA levels in the epileptic brain, it is well-stated that the decline in GABA levels in the brain during epileptic episodes is due to the intensified GABA-T enzymatic activity (the GABA degradative enzyme) and consequently, the inhibitory tone of GABA gets abolished, in due course the excitatory cascade gets switchedon, leading to Ca2+influx and results in burst-firing of neurons[10].These findings support our study results that the GABA-T activity gets reinforced with PTZ kindling which might be responsible for the reduction in GABA levels.However, the animals treated with sericin at medium and high doses hindered the GABA-T enzymatic activity and restored the GABA levels, whereas sericin (250 mg/kg)did not show any potential to alter the GABA-T activity.
It can be concluded that sericin manifested a potential neuroprotective effect against PTZ-induced epilepsy and its associated secondary complications which may be attributed to the inhibition of mitochondrial dysfunction-mediated excitotoxicity and GABA-T activity.Therefore, by targeting the GABA-T enzyme and altered mitochondrial functions, the therapeutic perspective in the treatment of epilepsy and its associated comorbidities is further suggested in the upcoming research.In the present study,we mainly focused on a few parameters such as behavioral assessments, oxidative damage, neuroinflammation, neurotransmitter alterations, mitochondrial dysfunction, altered GABA-T activity,and histopathological changes induced by PTZ kindling in rats, but in the future, researchers could emphasize on the molecular level or pathway-specific research using Western blotting technique or immunohistochemistry.Furthermore, the incorporation of sericinper segroup may add more clarity, which is missing in this study.Therefore, before drawing any definitive conclusions, more research is necessary.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are highly thankful to Mr.Parveen Garg, Chairman,and Prof.(Dr.) G.D.Gupta, Director-cum-Principal, ISF College of Pharmacy, Moga, and I.K.Gujral Punjab Technical University,Jalandhar, Punjab, India for providing an excellent research platform.The authors extend their special thanks to Prof.(Dr.) Y.K.Gupta,President, AIIMS Bhopal, and Jammu for spending their valuable time in reading and editing the manuscript.
Funding
The authors received no extramural funding for the study.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
All authors contributed to the study conception and design.Study design, experimental work, data collection, data analysis and writing were performed by SG.RKN contributed to literature analysis,critical revision and manuscript drafting.Manuscript drafting, final analysis and approval were performed by SS.All authors read and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Kaempferol and its derivatives: Biological activities and therapeutic potential
- Calorie restriction potentiates epigallocatechin-3-gallate-mediated Nrf2 activation in hepatocytes of aged rats
- Naringenin suppresses NLRP3 inflammasome activation via the mRNA-208a signaling pathway in isoproterenol-induced myocardial infarction
