Calorie restriction potentiates epigallocatechin-3-gallate-mediated Nrf2 activation in hepatocytes of aged rats
2023-11-13RajeswariRavindranMalathiManuelThangarajeswariMohanRavindranJaganathanKalaiselviPeriandavan
Rajeswari Ravindran, Malathi Manuel, Thangarajeswari Mohan, Ravindran Jaganathan, Kalaiselvi Periandavan✉
1Preclinical Department,Faculty of Medicine,Universiti Kuala Lumpur,Royal College of Medicine Perak,30450,Ipoh,Malaysia
2Department of Medical Biochemistry,Dr A.L Mudaliar Institute of Basic Medical Sciences,University of Madras,Taramani,Chennai 600113,India
ABSTRACT Objective: To explore the combinatorial effect of epigallocatechin-3-gallate (EGCG) and calorie restriction on activation of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor involved in the antioxidant defense system of aged rats.Methods: Aged male Wistar rats were calorie-restricted and treated with EGCG orally for 45 days.The initial body weight of aged rats was recorded, and the final body weight was measured at the end of the experimental period.Serum lipid and lipoprotein status,oxidative stress markers such as free radicals and malondialdehyde levels, and reduced glutathione were assessed.In addition, RT-PCR and Western blotting analyses were performed.Results: Calorie restriction potentiated the effect of EGCG on enhancing antioxidant status, improving the levels of serum lipid and lipoproteins, upregulating Nrf2 and Bcl2, and downregulating Keap1, cullin3, Bax and cytochrome c in aged rats.Conclusions: Calorie restriction can promote EGCG-mediated Nrf2 activation in aged rats.This preliminary finding paves the way for a combinatory approach to replenishing the antioxidant status during aging, thereby reducing the risk for age-associated degenerative diseases.
KEYWORDS: Aging; Reactive oxygen species; Calorie restriction;EGCG; Nrf2; Antioxidant
1.Introduction
Aging is a natural process that leads to a decline in cellular function,resulting in an elevated risk of mortality and serving as the primary contributor to a wide range of age-related illnesses[1].The generation of free radicals, telomere shortening, increased DNA damage,accumulation of mutations, defective DNA repair, and dysregulation of gene expression ultimately lead to symptoms of aging[2].Oxidative stress is a situation whereby cellular levels of reactive oxygen species (ROS) or reactive nitrogen species overwhelm the cellular antioxidant capacities[3].Free radical and mitochondrial theories of aging state that elevated levels of intracellular free radicals have an impact on mitochondria, resulting in functional alterations and a decline in cellular regenerative capacity.The impact of ROS further increases with aging as the mitochondrial respiratory capacity, particularly cytochrome c oxidase, declines with aging,which further leads to increased generation of superoxide anion radicals or hydrogen peroxide[4].Hence, enhancing the antioxidant defense system will not only result in a decreased production of free radicals but also improve the functional decline of mitochondria[5].The mammalian liver serves various essential physiologicalfunctions, including carbohydrate metabolism, synthesis of plasma proteins, and lipid metabolism[6].It plays a vital role in drug clearance and detoxification processes.One prevalent observation in the aging liver is the heightened occurrence of apoptosis, which could potentially contribute to the increased incidence of liver diseases, such as non-alcoholic fatty liver disease[7], adverse drug reactions, and alterations in drug clearance associated with aging.Calorie restriction has an action against the aging process and agerelated diseases.It attenuates many detrimental effects of aging and consequently promotes health resulting in longevity of organisms[8].Calorie restriction is thought to decrease ROS generation, enhance the plasma membrane redox system, reduce inflammation, and enhance insulin signaling pathways, all of which are believed to be correlated with improved mitochondrial function.Calorie restriction has been demonstrated to enhance mitochondrial performance significantly, consequently reducing the production of ROS within the mitochondrial electron transport chain complexes.As a result,this enhanced mitochondrial efficiency under calorie restriction can sustain cellular metabolism while reducing the accumulation of oxidative stress damage, ultimately leading to a reduced rate of aging at both the cellular and organismal levels[9].Earlier literature evidence suggests that Nrf2, the master regulator of antioxidant defense systems, has been shown to restore the life span of aging mice by modulating ROS levels notably during calorie restriction[10].The Nrf2 pathway is one of the pathways that respond to ROS.Nrf2 is a Cap‘n’collar-basic region leucine zipper (CNC-bZIP)transcription factor that regulates the antioxidant response elementregulated antioxidant and cytoprotective gene expression.It is highly expressed in tissues like the intestine, lung, and kidney,where detoxification processes are frequently carried out[11].In typical cellular circumstances, cytosolic Nrf2 is consistently kept at minimal levels due to its continuous degradation by the proteasome.However, when Nrf2 specifically binds to its cytosolic inhibitor KEAP1[12], it promotes the sequestration of Nrf2 within the cytoplasm and its subsequent ubiquitination by the cullin-3 E3 ubiquitin ligase complex.This, in turn, results in the degradation of Nrf2viathe proteasome.Upon exposure to various stresses or inducers, Nrf2 is released from KEAP1 and translocates into the nucleus[13].The nuclear import and export of KEAP1 and/or Nrf2 lead to the cytoplasmic nuclear shuttling of Nrf2.Nrf2 is a central player in antioxidant protection.An age-dependent decline in the antioxidant enzyme response is caused by declining efficiency of the Nrf2/EpRE signaling.Many phytochemical antioxidants are shown to upregulate Nrf2 levels[14].
Polyphenols represent secondary plant metabolites and play a pivotal role in shaping the sensory and nutritional characteristics of fruits, vegetables, and various other plant-based foods[15].Epigallocatechin-3-gallate (EGCG) exhibits a range of biological and pharmacological properties, including its ability to scavenge free radicals, act as an antioxidant, chelate iron, and mitigate lipid peroxidation induced by various types of radicals[16].EGCG, the main and the most important polyphenol in green tea is a common phytochemical that is claimed to have many potential health benefits[17].The potential for EGCG to enhance longevity in stressful conditions could be linked to its direct scavenging of ROS and its indirect role in scavenging free radicals by increasing the expression of stress-resistance-related genes like superoxide dismutase-3,heat shock protein (HSP)-16.2, and skinhead-1[18].These results emphasize the potential of EGCG to extend the human average life expectancy by protecting against oxidative stress.Therefore, the present study aims to test the synergism between calorie restriction and EGCG supplementation in attenuating senescence-associated deterioration in aged rat liver.
2.Materials and methods
2.1.Chemicals
EGCG and bovine serum albumin were purchased from Sigma-Aldrich, USA.Other chemicals used were obtained as analyticalgrade materials from suppliers including Merck Chemical Supplies(Darmstadt, Germany), Sisco Research (SRL, India), and S.D.Fine Chemicals (Mumbai, India).
2.2.Animals
Healthy male Wistar-aged rats were utilized for the study.Aged(24 months old) rats were used throughout the study.Rats that are twenty-four months old are considered aged because animals are deemed aged when they surpass two-thirds of their average life expectancy.The animals were bought from Central Animal House Facility, Dr.ALM PGIBMS, University of Madras, Taramani Campus, Chennai, India, and maintained under controlled conditions,including a temperature of (25 ± 2) ℃ and a 12/12-hour light/dark cycle with free access to food and water.The experimental animals consisted of four groups of six animals each: Group Ⅰ-aged control,Group Ⅱ-aged rats treated with EGCG, Group Ⅲ-caloricallyrestricted aged rats, and Group Ⅳ-calorically-restricted aged rats treated with EGCG.Calorie-restricted animals were given 60% of the average feed consumed byad libitum-fed age-matched controls.EGCG (100 mg/kg of body weight per day)[19] was suspended in a physiological saline solution (0.89%) and deliveredviaoral gavage for 45 d.Calorie restriction was based on a 40% reduction in daily consumption.Rats were kept on anad libitumdiet initially for two weeks, the average food consumption was measured during that period.From that average value, the volume of food was reduced by 40%.
2.3.Collection of tissues and serum
After the experimental period, rats were sacrificed.Before sacrifice,they were anesthetized with ketamine (22 mg/kg,i.m.), and the blood samples were collected in respective test tubes for the separation of plasma and serum samples.Liver tissues were excised immediately,immersed in ice-cold physiological saline, and weighed.A portion of the liver tissue was homogenized immediately in 0.01 M tris HCL buffer (pH 7.4) to give a 10% homogenate.The remaining tissue was kept at -80 ℃ for gene expression analyses and other studies.
The protein concentration of the tissue homogenate was assessed using the standard procedure, employing bovine serum albumin as a reference standard.
2.4.Body weight gain
Body weight gain was determined as follows:
Body weight gain (g) = Final body weight (g) – initial body weight(g).
2.5.Lipid and lipoprotein profile
Total cholesterol (TC), triglycerides (TG), high-density lipoproteins(HDL), serum levels of low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL) were assessed by commercial kits obtained from Spinreact and analyzed using a semi-automated analyzer (Rx Monza, Randox, U.K).
2.6.Oxidative stress markers
2.6.1.Estimation of lipid peroxidation(LPO)
The assay mixture (2.0 mL) was comprised of 1.6 mL of 0.15 M Tris-HCl buffer (pH 7.4), 0.2 mL of 10 mM KH2PO4, and 0.2 mL of liver homogenate.This mixture was agitated for 20 min at 37 ℃.Subsequently, about 1.0 mL of tricarboxylic acid (10%) was introduced for protein precipitation.Following this step, 1.5 mL of 1% thiobarbituric acid was added, and the mixture was heated for 15 min.After cooling, the test tubes were subjected to centrifugation at 3 000 rpm for 10 min.The color intensity was then measured at 532 nm using a spectrophotometer with a reagent blank.The levels of lipid peroxides are quantified as µmol of MDA per mg of protein.
2.6.2.Estimation of superoxide radical
For the analysis, 0.6 mL of homogenate was combined with 0.2 mL of a 4 mM nitroblue tetrazolium (NBT) solution, and 150 mM ascorbic acid.This mixture was then incubated at 37 ℃ for 10 min.Following the incubation, 2.0 mL of a solution containing 0.1 M sodium hydroxide and 24 mM sodium bicarbonate was added.After centrifugation, the resulting precipitate was dissolved in 5.0 mL of 1,4-dioxane, and its absorbance was measured spectrophotometrically at 520 nm, with 1,4-dioxane alone serving as the blank.The changes between these two measurements are directly related to the reduction of NBT, specifically by superoxide radicals.The levels of superoxide radicals in the liver tissue are quantified as μmol of NBT reduced per min per mg of protein.
2.6.3.Estimation of hydroxyl radical
The reaction mixture consisting of 1.0 mL suitably diluted liver homogenate, 0.2 mL 1 M phosphate buffer (pH 7.4), 0.1 mL MgCl2, 0.1 mL sodium azide, 0.1 mL dimethyl sulfoxide, and 0.1 mL NADPH was kept at 37 ℃ for 10 min.Next, 0.5 mL of a 1% chromotrophic acid solution was added, and the mixture was subjected to a 30-minute incubation in a boiling water bath.The hydroxyl radical (•OH) content was quantified by measuring the absorbance at 570 nm using a spectrophotometer.The findings are presented as µmol per min per mg of protein.
2.6.4.Estimation of hydrogen peroxide radical
H2O2generation was assessed using a fluorometric method based on the rate ofp-hydroxyphenylacetic acid oxidation (20 µg/mL)catalyzed by horseradish peroxidase (50 µg/mL) in the presence of H2O2.In brief, the reaction mixture consisted of 1.0 mL of appropriately diluted liver homogenate, 154 mM KCl, 5 mM potassium phosphate, 3 mM MgCl2, 0.1 mM EGTA (pH 7.4), parahydroxyphenyl acetate (166 µg/mL), and horseradish peroxidase(83 units).This mixture was then added to 20 µg of protein.The rate of H2O2release was monitored by following the increase in fluorescence at an excitation wavelength of 317 nm and emission wavelength of 400 nm.The known concentrations of H2O2were used as standards.The results are represented as µmol/min/mg protein.
2.6.5.Estimation of glutathione(GSH)
Briefly, 1 mL of 10% TCA was used to precipitate 1 mL of homogenate, and the resulting precipitate was separatedviacentrifugation.To 0.5 mL of the supernatant, 2 mL of a 0.6 mM 5,5-dithiobis(2-nitrobenzoic acid) solution was introduced, and the total volume was adjusted to 3 mL using 0.2 M phosphate buffer at pH 8.0.The absorbance was then measured at 412 nm.The concentration of glutathione was quantified and expressed as µg per gram of protein.
2.7.Reverse transcription-polymerase chain reaction (RTPCR)
Total RNA was extracted from the liver tissue using the TRI(Total RNA Isolation) reagent, following the established protocols.Subsequently, cDNA was synthesized from the mRNA template using dNTPs and reverse transcriptase (Superscript-Ⅲ Reverse Transcriptase from Bio-Rad, USA).Once the cDNA was created from the initial single-stranded mRNA, a standard PCR was initiated using PCR Ready Mix DNA polymerase obtained from Ampliqon in Denmark.Synthesized oligonucleotide primer sequences for the chosen genes in RT-PCR were obtained from Sigma-Aldrich (St.Louis, MO, USA) and shown in Table 1.The resultant amplified products were electrophoresed on a 2% agarose gel and detected using ethidium bromide staining.The specificity of amplification was verified by comparing the size of the amplified products to a reference 100 bp DNA ladder (BioVision, USA).Subsequently, the intensity of the bands was quantified using Quantity One Software from Bio-Rad (USA).
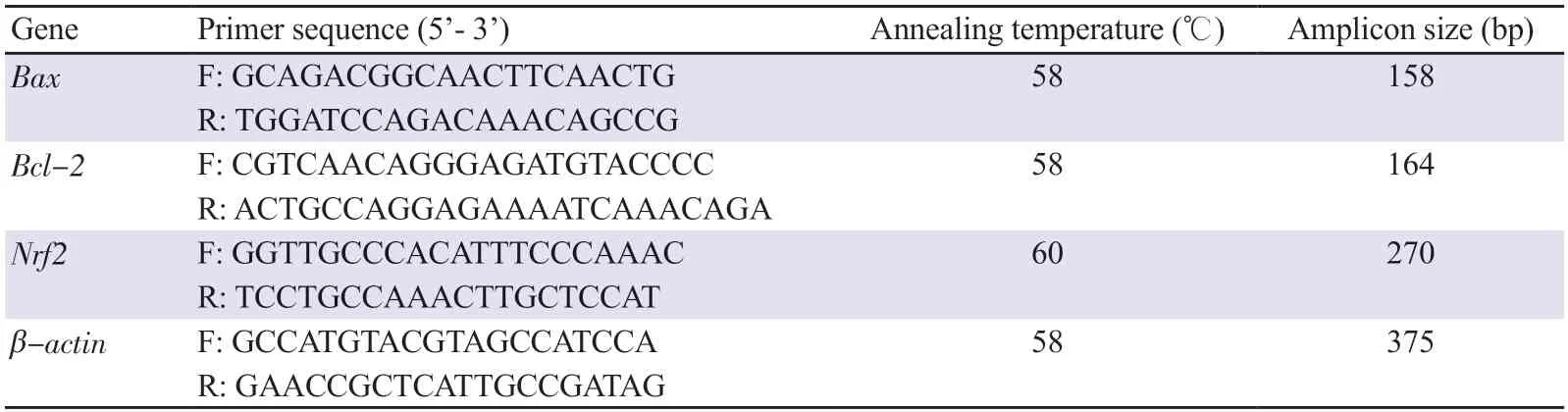
Table 1.Primer sequences of genes in RT-PCR analysis.
2.8.Western blotting
Protein expression analysis was conducted following the established procedures.The samples were dissolved in a reducing loading buffer containing 62.5 mM Tris (pH 6.8), 6 M urea, 10%glycerol, 2% sodium dodecyl sulfate (SDS), 0.003% bromophenol blue, and 5% 2-mercaptoethanol.Subsequently, they were subjected to electrophoresis on 10% SDS polyacrylamide gels and subsequently transferred onto PVDF membranes.These membranes were then subjected to incubation with specific primary antibodies targeting Nrf2, Keap1, Cullin3, Bax, Bcl-2 and cytochrome c(provided by Cell Signaling Technology), following the standard protocol.Antibodies used were as follows: anti-Nrf2 (Cell signaling Technology, 20733, 1:1 000), anti-Keap1 (Cell signaling Technology,4678, 1:1 000), anti-cullin 3 (Cell signaling technology, 10450,1:1 000), anti-Bax (Cell signaling Technology, 5023, 1:1 000),anti-Bcl-2 (Cell signaling Technology, 15071, 1:1 000) and anticytochrome c (Cell signaling technology, 11940, 1:1 000).To ensure the consistency of protein distribution and transfer effectiveness among the test samples, we conducted additional probing of the membranes using beta-actin (β-actin) antibodies from Cell Signaling Technology (3700, 1:1 000).Immunoreactive bands were developed with Immobilon Western-Chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA, USA) and the band intensities were visualized by using an enhanced chemiluminescence system (Chemi-Doc, BioRad, USA) and presented in comparison to β-actin expression.
2.9.Statistical analysis
The findings are presented as mean ± standard deviation (SD).To assess variations between groups, a one-way analysis of variance(ANOVA) was conducted using the SPSS software package for Windows (Version: SPSS 20.0).Subsequently, apost hoctest was carried out for comparisons between different groups using the least significant difference (LSD) test.Statistical significance was set atPvalues less than 0.05.
2.10.Ethical statement
All animal experiments, sanctioned by the Institutional Animal Ethical Committee (IAEC No.01/14/12), were conducted in strict accordance with applicable laws and institutional protocols.
3.Results
3.1.Effect of EGCG and calorie restriction on the body weight of aged rats
The impact of EGCG and calorie restriction on the body weight of experimental rats is represented in Supplementary Table 1.All the treatment groups, especially the calorie-restricted groups, exhibited a substantial decrease in body weight at the end of the experimental period with the calorie-restricted and EGCG-treated aged group showing a maximum decrease of 15.12% compared to the aged rats.EGCG supplementation in calorie-restricted aged rats caused a considerable decline of 8.42% in body weight when compared with aged rats with EGCG administration alone.
3.2.Effect of calorie restriction and EGCG supplementation on the lipid and lipoprotein profile of aged rats
Figure 1A and 1B shows the effect of calorie restriction and EGCG supplementation either in separation or in combination on the lipid profile of rats.A considerable reduction (P<0.05) in the levels of serum TC, TG, LDL, and VLDL, along with a concomitant increase in the HDL, was found in all the treatment groups when comparedto the control group with the maximum difference noticed in the calorie-restricted aged group treated with EGCG [TC (46.27%),TG (34.80%), LDL (45.53%), VLDL (61.17%), HDL (96.70%)].Although the rats in the calorie-restriction group showed lower lipid levels compared to the aged control rats, a considerable decline was noticed in calorie-restricted and EGCG-treated rats.

Figure 1.Effect of EGCG and calorie restriction on serum lipid profile of aged rats.Values are represented as mean ± SD (n=6).aP<0.05 compared with the aged control group; bP<0.05 compared with the EGCG-treated group.TC: total cholesterol, TG: triglycerides, HDL: high-density lipoproteins, LDL: lowdensity lipoproteins, VLDL: very low-density lipoproteins, EGCG: epigallocatechin-3-gallate, CR: calorie restriction.
3.3.Effect of calorie restriction and EGCG supplementation on malondialdehyde and GSH level of aged rats
Figure 2A and 2B shows the effect of calorie restriction and EGCG supplementation on the levels of MDA and GSH in aged rats.The level of MDA was significantly reduced in all treatment groups in comparison to the aged control group.Calorie restriction potentiated the reducing effect of EGCG supplementation on MDA level.In addition, GSH level was markedly increased in aged rats given calorie restriction and EGCG supplementation alone or in combination, showing a more significant effect.
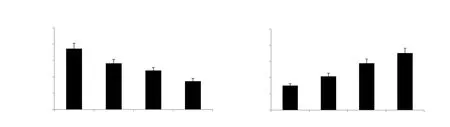
Figure 2.Effect of EGCG and calorie restriction on malondialdehyde and non-enzymatic antioxidant levels in hepatic tissues of aged rats.Values are represented as mean ± SD (n=6).aP<0.05 compared with the aged control group; bP<0.05 compared with the EGCG-treated group.MDA: malondialdehyde,GSH: glutathione.
3.4.Effect of EGCG and calorie restriction on scavenging free radicals in hepatocytes of aged rats
The effect of EGCG and calorie restriction on scavenging free radicals such as (H2O2, OH˙-and O2˙-) in the liver tissue of aged rats is depicted in Figure 3A and 3B.In accordance with the fact that aging accelerates ROS production and associated damaged macromolecules, the control rats exhibited the highest levels of free radicals such as H2O2, OH˙-and O2˙-.Although the calorierestricted group/EGCG alone supplemented group showed reduced ROS production, the maximum decrease was observed in the combinatorial group.
Figure 3.Effect of EGCG and calorie restriction on the levels of free radicals in hepatic tissue of aged rats.Values are represented as mean ± SD (n=6).aP<0.05 compared with the aged control group; bP<0.05 compared with the EGCG-treated group.
3.5.Impact of EGCG and calorie restriction on mRNA and protein expression of Nrf2 in hepatic tissues of experimental rats
Figure 4A and 5F signifies the impact of EGCG and calorie restriction on mRNA and protein expression of Nrf2 in hepatic tissues of aged rats.Both mRNA and protein expression of Nrf2 were found to be markedly decreased in the aged control rats.Although the EGCG-supplemented rats showed a significant upregulation in the mRNA (1.62 fold) and protein expression (52.14%) of Nrf2 compared to the aged controls, the percentage of increase was further elevated in the combinatorial group with calorie restriction and EGCG intake (mRNA 1.75 fold and protein expression 97.5%).In addition, EGCG and calorie-restricted animals showed 29.81% increase in protein expression and 1.07 fold increase in mRNA expression respectively compared with aged rats supplemented with EGCG alone.

Figure 4.Effect of EGCG and calorie restriction on mRNA expressions of (A) Nrf2, (B) Bax and (C) Bcl-2 in hepatic tissues of aged rats.Values are represented as mean ± SD of three independent experiments.aP<0.05 compared with the aged control group; bP<0.05 compared with the EGCG-treated group.M:DNA marker.
3.6.Effect of EGCG and calorie restriction on protein expression of Keap1 and cullin3 in hepatic tissues of aged rats
Figure 5A and 5B shows the effect of EGCG and calorie restriction on protein expression of Keap1 and cullin3 in hepatic tissues of aged rats.The levels of Keap1 and cullin3, the important regulators of Nrf-2 function, were found to be decreased in all treatment groups with the maximum effect observed in the calorie-restricted and EGCG-treated group showing a striking decline in Keap1 and cullin3 expression compared to the aged rats.

Figure 5.Effect of EGCG and calorie restriction on protein expression of (A) Keap1, (B) cullin3, (C) Bax, (D) Bcl-2, (E) cytochrome c and (F) Nrf2 in hepatic tissues of aged rats.Values are represented as mean ± SD of three independent experiments.aP<0.05 compared with the aged control group; bP<0.05 compared with the EGCG-treated group.
3.7.Effect of EGCG and calorie restriction on the mRNA and protein expression levels of Bax in aged rats
Figure 4B and 5C depicts the effect of EGCG and calorie restriction on the mRNA and protein expression levels of apoptotic protein Bax in aged rats.There was a noteworthy increase in the mRNA and protein levels of Bax in aged rats.In contrast, EGCG and calorie restriction alone or in combination markedly decreased the mRNA and protein levels of Bax.The combined treatment showed a more significant effect on downregulating the mRNA and protein levels of Bax.
3.8.Impact of EGCG and calorie restriction on mRNA and protein expression of Bcl-2 in aged rats
Figure 4C and 5D portrays the impact of EGCG and calorie restriction on the mRNA and protein expression of the anti-apoptotic protein Bcl-2.Bcl-2 expression was upregulated in all treatment groups.There was a marked increase (P<0.05) in mRNA (1.6 fold)and protein levels (48.54%) of Bcl-2 in the EGCG-treated and calorie-restricted rats compared to the aged rats fed with EGCG alone.
3.9.Effect of EGCG and calorie restriction in protein expression of cytochrome c in hepatic tissues of aged rats
The cytosolic cytochrome c levels were assessed in hepatocytes of aged rats and depicted in Figure 5E.The protein expression of the cytosolic fraction of cytochrome c was significantly low in the groups given EGCG treatment alone or calorie restriction, and the calorie restriction plus EGCG treatment group compared to the aged controls.
4.Discussion
Calorie restriction can exert a more significant influence on oxidative stress by decreasing obesity and consequently, aging.However, even though calorie restriction has the potential to lower the ROS generation, it may not efficiently detoxify the generated ROS during the aging process.This is due to the reported decline in the mechanisms that regulate ROS accumulation, specifically antioxidants, including both enzymatic and non-enzymatic components, as individuals age[20].This vicious cycle may not be completely stopped, but it can be slowed down using antioxidant supplementation.Green tea is a source of catechins, with the most potent and plentiful ingredient EGCG.EGCG has demonstrated a wide range of biological and pharmacological characteristics, such as scavenging free radicals, functioning as an antioxidant, binding to iron, and mitigating lipid peroxidation triggered by various forms of radicals[21,22].
Excess body weight and adiposity trigger insulin resistance,inflammation, and numerous factors that promote atherosclerosis,steatosis, neurodegeneration, and aging[23].Hence, the present study aims to address the hypothesis that the combinational efficacy of calorie restriction and EGCG supplementation might retard the process of aging.
Aged rats have shown an incremental body weight gain, while the EGCG-treated rats did not show any incremental weight gain.Calorie restriction individually or along with EGCG had a decremented impact on body weight.More interestingly, cotreatment with calorie restriction and EGCG showed a more significant decline in body weight.Studies have confirmed the beneficial role of leanness in promoting health and life span[24].There are qualms about the soundness ofad libitumfeeding since it often results in energy overconsumption and weight gain.It has been reported that calorie restriction, which involves reducing calorie intake without harming overall health, is a highly promising dietary method for extending lifespan[25].On the other hand, a study reported that EGCG supplementation resulted in moderate loss of their original body weight in mice[26].
Moreover, calorie restriction and EGCG supplementation resulted in a drastic reduction in TC.A similar trend was observed for TG,LDL, and VLDL.The HDL-boosting ability of EGCG was found to be higher when combined with calorie restriction.In a previous study, rats fed with a high-cholesterol diet and supplemented with EGCG showed reduced TC and LDL and increased HDL levels[27].Studies have shown that green tea catechins reduce cholesterol absorption from the intestine by forming micelles in animal models.It is concordant with the results obtained from the previous studies in our laboratory as well as other laboratories[28].Calorie restriction decreased the TG, and LDL and increased the HDL-C[29].Aging which is accompanied by an altered lipid profile leads to hepatic steatosis-associated liver damage and inflammation which modulates other risk factors of life-threatening diseases like atherosclerosis[30].
As an organism age, there is a rise in ROS production, leading to the buildup of damaged macromolecules associated with aging.This, in turn, amplifies ROS production even further.In the present study, an increased level of free radicals such as H2O2, and OH˙-and O2˙-was noticed in aged control rats.Studies have demonstrated that calorie restriction can lead to highly efficient mitochondrial function,achieving adenosine triphosphate production while diminishing the generation of ROS within the mitochondrial electron transport chain complexes[31].Consequently, this enhanced mitochondrial efficiency resulting from calorie restriction could potentially play a role in sustaining cellular metabolism with reduced accumulation of molecules damaged by oxidative stress.A further reduction in free radical levels was seen in the EGCG-treated and calorie-restricted group compared to calorie-restricted aged rats, which might be due to the ability of polyphenolic catechins of EGCG to scavenge ROS[32].
Lipid peroxidation is considered one of the fundamental processes responsible for cellular harm induced by free radicals.It results in the oxidative degradation of polyunsaturated lipids.This process begins with the removal of hydrogen atoms from the side chains of polyunsaturated fatty acids within membrane lipids.Lipid peroxidation in biological membranes can induce changes in fluidity,a decrease in membrane potential, heightened permeability to hydrogen ions and other ions, and ultimately, membrane rupture,leading to the release of cellular and organelle contents.Hence, lipid peroxidation can be crucial in inflammation, cancer, and cardiac diseases[33].We observed increased levels of MDA, a secondary product of lipid peroxidation which is an indicator of tissue damage in the liver tissue of aged control rats.EGCG supplementation brought down the levels of lipid peroxidation and boosted the antioxidant status in aged rats under calorie restriction.This could be due to EGCG’s metal chelating activity, especially iron and copper, which in turn inhibits the generation of hydroxyl radicals and the formation of lipid peroxides, leading to reactive aldehyde formation[34].
As hepatic lipid accumulation is linked with increased oxidative stress, the enzymatic and non-enzymatic antioxidants and lipid peroxidation were assessed in the hepatic tissue.Calorie-restricted rats treated with EGCG displayed a notable increase in the level of GSH compared to rats treated with EGCG alone.Earlier studies also reported a notable decline in the level of GSH during the aging process[35].EGCG administration also resulted in a significant increase in level of GSH in aged rats.The anti-oxidative properties of calorie restriction are well-established and are in accordance with the aging theory that emphasizes oxidative stress[36].
The expression levels of antioxidant enzymes, both under normal conditions and in response to stressors, are controlled, to some extent, by the activation of the Nrf2/EpRE signaling pathway.It is widely recognized that Nrf2 serves as the primary transcription factor responsible for regulating the expression of numerous antioxidants and detoxifying enzymes, both in their basal state and when induced by various stimuli[37].Age-related declines of the antioxidant defense are closely involved in the expression of Nrf2[38].The levels of Nrf2 were slightly elevated in the EGCG alone treated groups,while calorie restriction can further enhance the effect of EGCG on Nrf2 level.Increasing evidence suggests that the age-related decline in the antioxidant enzyme response can be attributed to a decrease in the effectiveness of the Nrf2/EpRE signaling[39].However, the combined treatment of EGCG and calorie restriction could elevate Nrf2 levels.This observation suggests that calorie restriction might potentiate EGCG’s ability to enhance Nrf2 activity, potentially protecting against the detrimental biological consequences of ROS.
Nrf2 activity is, in part, controlled by the actions of the associated Keap1 protein.Initially, it was proposed that Keap1 functions by sequestering the transcription factor in the cytoplasm.Keap1 can suppress Nrf2 activity by directing it toward a cytoplasmic Cul3-based E3 ligase[40].Keap 1 also enters the nucleus and escorts Nrf2 out to the cytoplasm for degradation under stressed conditions[41].In aged rats treated with EGCG or under calorie restriction, Keap1 and cullin3 levels were reduced.The combined treatment further diminished their levels.
Apoptotic cell death in hepatocytes often occurs in both hepatic steatosis and the aging process, consistently associated with liver damage[42].In the current study, Bcl-2 level was upregulated and Bax level downregulated in calorie-restricted and EGCG-treated rats.Our findings are consistent with the previous study in which EGCG decreases Bax, rather than increasing Bcl-2, in aged animals to safeguard them from hepatocyte apoptosis[43].
Although the study implies that calorie restriction and EGCG supplementation might positively influence aging-related factors, a comprehensive exploration of the precise mechanisms underpinning these effects is necessary.A more thorough scientific investigation into these mechanisms is vital for gaining a deeper molecular-level understanding of how these interventions function.To attain a more extensive scientific assessment of the impacts of calorie restriction and EGCG, it is essential to consider a wider range of markers and physiological parameters.This would enable a more comprehensive exploration of the combined effectiveness of EGCG and calorie restriction in aging-related processes.
In conclusion, while EGCG has been demonstrated to increase antioxidant levels in rats with unrestricted diets, its positive impact can be further amplified when combined with calorie restriction.This highlights the importance of incorporating polyphenols or antioxidants into the rigorous dietary plan of calorie restriction.However, long-term supplementation might be required for validation of this combinatorial efficacy to be deemed as an antiaging strategy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
The authors wish to acknowledge the financial support offered by UGC SAP Programme, University of Madras, Chennai, India for conducting this study.
Funding
The article was financially supported by UGC SAP Programme,University of Madras, Chennai, India.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
Material preparation, data collection and analysis were performed by MM, RR and KP.Study design and conception were done by MM, RR, RJ and KP.The first draft of the manuscript was written by MM and RR.Previous version and revised version of the manuscript were commented by RR, RJ, TM and KP.Final manuscript was read and approved by MM, RR, RJ, TM and KP.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Kaempferol and its derivatives: Biological activities and therapeutic potential
- Sericin alleviates pentylenetetrazole kindling epilepsy and associated comorbidities via modulation of GABA-T enzyme and mitochondrial activity
- Naringenin suppresses NLRP3 inflammasome activation via the mRNA-208a signaling pathway in isoproterenol-induced myocardial infarction
