Peanut proteins: Extraction,modifications,and applications: A comprehensive review
2023-11-09SongCuiDaviJulianMClementsXingfengXuBoJiaoLiyangZhouHualuZhouLiuXiongQiangWangQingjieSunLeiDai
Song Cui ,Davi Julian MClements ,Xingfeng Xu ,Bo Jiao ,Liyang Zhou ,Hualu Zhou ,Liu Xiong,Qiang Wang,Qingjie Sun,Lei Dai,,*
a College of Food Science and Engineering, Qingdao Agricultural University, China
b Qingdao Special Food Research Institute, Qingdao 266109, China
c Department of Food Science, University of Massachusetts Amherst, Amherst, MA 01003, USA
d Institute of Food Science and Technology, Chinese Academy of Agricultural Science/Key Laboratory of Agro-Products Processing, Ministry of Agriculture and Rural Affairs, Beijing 100193, China
Keywords: Peanut protein Composition Extraction methods Modifications Applications Plant-based foods
ABSTRACT As naturally sourced proteins,peanut proteins have garnered significant attention from the food industry,owing to their numerous advantages,such as easy extraction,non-pungency,and high bioavailability.Furthermore,peanut proteins are highly digestible in the gastrointestinal tract and boast a high net protein utilization rate,making them an appealing protein source in food products and a promising alternative to animal protein.In this paper,the recent works on the extraction method,modification method,and application of peanut proteins were reviewed.Both advantages and disadvantages of current extraction and modification were discussed.Recently updated information about peanut protein research was summarized.Based on these,the prospection of peanut proteins research was presented,which may be instructive for future research in this field.Future research is still needed for accessible modification methods to develop the functional properties of peanut proteins.
1.Introduction
Peanuts(Arachis hypogaeaL.)are an important agricultural crop that is grown worldwide,being the third largest source of plant proteins in the human diet,which amounts to around 11% of the global protein supply[1].Peanut is widely planted in the warm climates of Asia,Africa,Australia,North America,and South America.According to statistics,the annual global production of peanuts is around 45 million metric tons[2].The major countries involved in peanut production are China,India,Nigeria,and the United States.
Peanuts contain around 24% to 36% crude protein on a dry weight basis,which is second only to soybean(36%to 51%)in edible oil crops[3].Peanuts contain 8 essential amino acids and are rich in unsaturated fatty acids and linolenic acid,as well as various other health-promoting components,such as vitamins,procyanidins,flavonoids,and resveratrol[4–6].Moreover,peanuts contain fewer intrinsic anti-nutritional factors than soybeans.The relatively high nutritional value,high yields,and low price of peanuts have led to increasing interest in the application of peanut proteins and other byproducts in foods.
Peanut proteins can be divided into two main categories: watersoluble proteins (~10%) and salt-soluble proteins (~90%) on the basis of their differential solubilities.The salt-soluble fraction consists of around 73% arachin (14S),6% conarachin I (2S),21% conarachin II(8S),and some other minor components [7,8].The main fractions exhibit unique physical and functional properties due to their different amino acid profiles,molecular weights,and structures(Table 1)[9,10].
The nutritional value of peanut proteins has been reported to be relatively high,with a biological value (BV) of 59 and net protein utilization of 51.Compared to soybean protein,peanut protein has higher digestibility,which can reach over 90%,making it easier to be absorbed by the human body[11,12].This is partly because the trypsin inhibitory factor of peanut protein is only around 20% of that of soybeans.The nutritional value of peanut protein has been reported to be similar to that of animal protein[13].For instance,the protein content of peanut protein powder(55 g/100 g)is 2.7 times that of beef,16.7 times that of milk,3.3 times that of lean meat,2.6 times that of chicken,and 3.8 times that of egg[14].Moreover,peanut proteins do not exhibit the pungent taste exhibited by soy proteins,which makes them easier to incorporateinto a wider variety of food products.Therefore,peanut protein has great potential as a functional ingredient for utilization in the food industry,as well as being a viable alternative to animal proteins.

Table 1 General properties of arachin and conarachin.
The composition,structure,surface characteristics,physicochemical properties,and functional properties of peanut protein components depend on the extraction process.Therefore,the extraction process is a very important step.In particular,attention should be paid to the effects of pH value,ionic strength,and temperature on peanut protein during extraction [15,16].In this way,peanut protein can produce better solubility,emulsification,foaming,gelling,water retention,oil retention,and other functional characteristics,to have a positive impact on the formation,appearance,texture,and stability of the food,conducive to its application in the food industry[17,18].One of the main challenges in this area is that peanut proteins are usually obtained as a byproduct of the conventional methods used to extract oils from peanuts.As a result,they are often damaged (denatured) during the oil extraction process,which causes a reduction or loss of their functional attributes.Indeed,many conventional peanut protein ingredients have limited application in foods because of their poor functional performance,e.g.,solubility or gelling [19].Therefore,researchers are exploring various conventional and novel physical,chemical,and/or enzymatic modification methods to obtain peanut protein ingredients with improved functionality[17,20,21].
Traditionally,peanut proteins have been used as functional ingredients in a wide variety of foods,including bakery,dairy,and meat products[22].More recently,native and modified peanut proteins have been used as functional ingredients in a variety of novel applications,such as for the development of bioactive delivery systems,the creation of Pickering emulsion stabilizers,the development of biopolymer films,and the production of plant-based meat analogs[23–26].Nevertheless,when using peanut proteins as functional ingredients in new products it is important to consider health concerns,such as their allergenicity and the presence of aflatoxin[9].
The objective of this review is to discuss conventional and novel peanut protein extraction and modification methods,as well as describe the potential applications of peanut protein ingredients in food products.This information may stimulate further research and development into the utilization of peanut proteins as plant-based ingredients in foods.
2.Extraction method
The extraction method used significantly affects the structural,functional,and physicochemical properties of peanut protein ingredients.The conventional extraction methods include the press method,leaching process,and alkali-soluble acid precipitation method[27].The press method is to extract part of the oil in peanuts,to achieve the effect of making peanut meal at the same time,which can be divided into hot pressing method and cold pressing method.Although up to 80%–90%oil can be removed by hot pressing method,most proteins in peanut meal will be denatured and the content of water-soluble proteins and functional properties will be decreased obviously due to hightemperature treatment.The general cold pressing process is processed in an environment below 60◦C.The nutritional components remain intact,but the oil yield is low,so it is often used at the same time as the leaching method.The leaching process includes a direct leaching process and press before the leaching process.The former is suitable for some oils with low oil content,while the peanut with high oil content is suitable for pressing before the leaching process.However,the production process of the leaching method is more complicated,the cost is higher,and the addition of volatile flammable organic solvents also increases the safety hazard.The technology is still in preliminary study due to surfactant residue and the lack of production equipment.Alkalisoluble acid precipitation method is usually used to prepare protein isolate,which can not only remove water-soluble sugar but also remove starch,cellulose,and other components.The protein content of the product can reach 90%,but a large amount of acid and base will be used in the industrial production process,resulting in a certain degree of environmental pollution.Therefore,various novel methods have been developed to increase the yield,reduce denaturation,and improve the functional properties of plant proteins[15,28].
2.1. Aqueous extraction processing
A significant fraction of the peanuts grown around the world are used for oil production because peanuts contain relatively high amounts of oil(45%–55%).Peanut oil is typically isolated from peanuts using conventional extraction methods,such as mechanical pressing and solvent(n-hexane) extraction [29].However,many of the peanut proteins are denatured as a result of high temperatures during pressing or due to exposure to the organic solvent.Consequently,they lose their desirable functional attributes.For this reason,the main by-product of peanut oil production (peanut cake) generated using traditional methods which contains>50%protein is mainly used as a feedstuff or fertilizer.
In the past few decades,aqueous extraction processing (AEP),has been developed to separate oils and proteins from oil-containing starting materials (Fig.1 [30] and Fig.2 [31]).This method uses water as an extraction solvent,rather than an organic solvent[15,32].In AEP,oil is extracted because it is insoluble in water and floats in hot water.Instead,proteins dissolve in water and can be recovered by acid precipitation or membrane separation processes [33].Li et al.(2016) reported that the yields of peanut protein and free oil achieved using AEP were around 84% and 35%,respectively [34].These relatively low oil yields hold back the commercial adoption of this method.Moreover,Liu et al.(2018)reported that peanut protein concentrates(PPCs)prepared using AEP had lower protein contents and solubilities compared to those fabricated by conventional organic solvent(n-hexane)extraction.These results suggest that AEP should be used in conjunction with other methods to increase its efficiency [32].Li et al.(2016) used a threecylindrical roll crusher to improve oil and protein yields using the AEP of peanuts.The yield of free oil extracted from roasted peanut(150◦C,20 min) using the AEP method was around 92.2% using the optimized processing conditions: solids-to-liquid ratio=1:5;pH=9;temperature=60◦C,and time=2 h [33].Liu et al.(2020) investigated a combination of AEP and membrane separation for peanut protein extraction.After sequential MF(microfiltration)and UF(ultrafiltration)processing of the aqueous phase obtained from AEP,the retention rate of proteins reached around 81%[28].In conclusion,the protein extracted by the AEP method has a high extraction rate and good nutritional value and functional characteristics [32].AEP does not require the use of enzymes and is conducive to further application in the food industry[34].
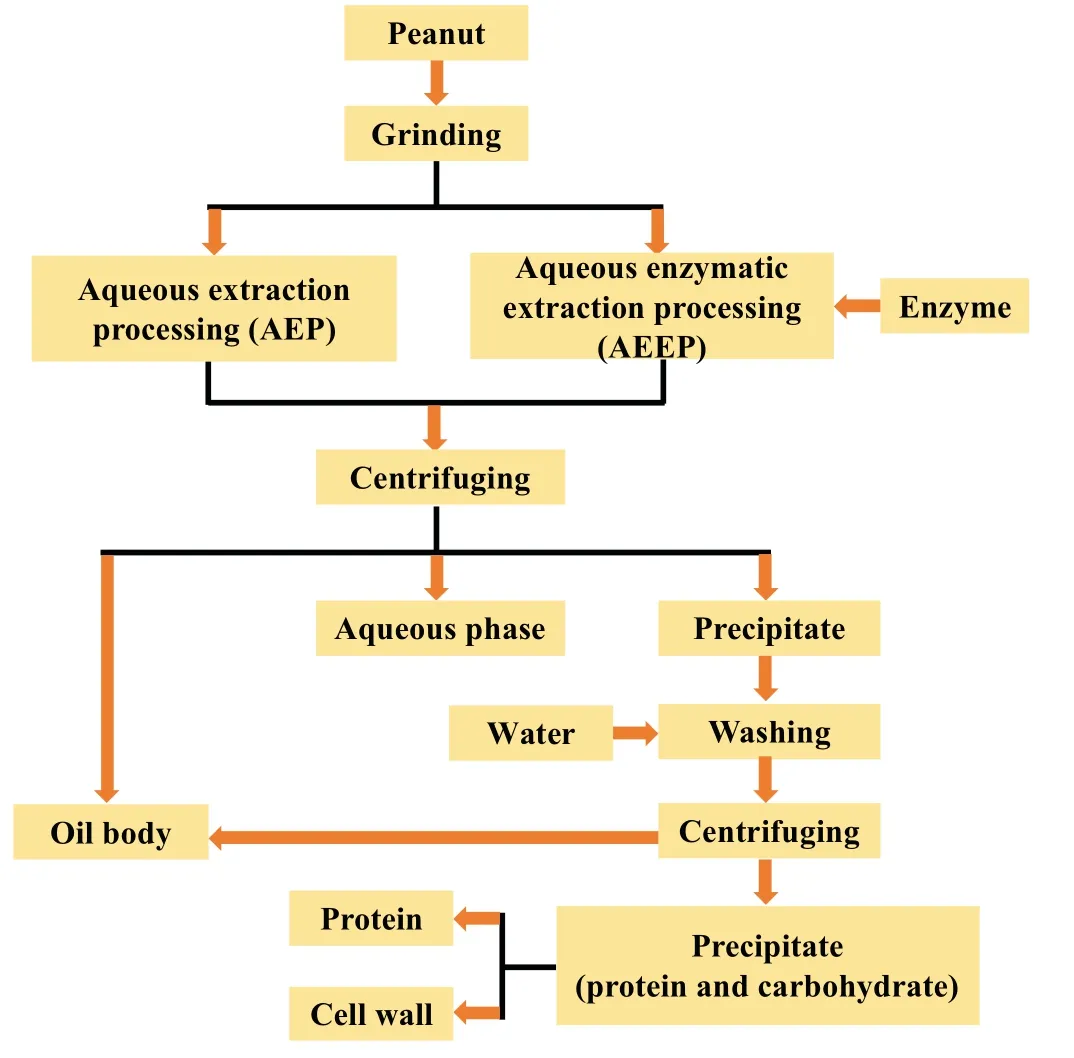
Fig.1. Process flow sheet for peanut protein and oil bodies from aqueous extraction processing (AEP) and aqueous enzymatic extraction processing(AEEP).Information from Li et al.(2017) [30].
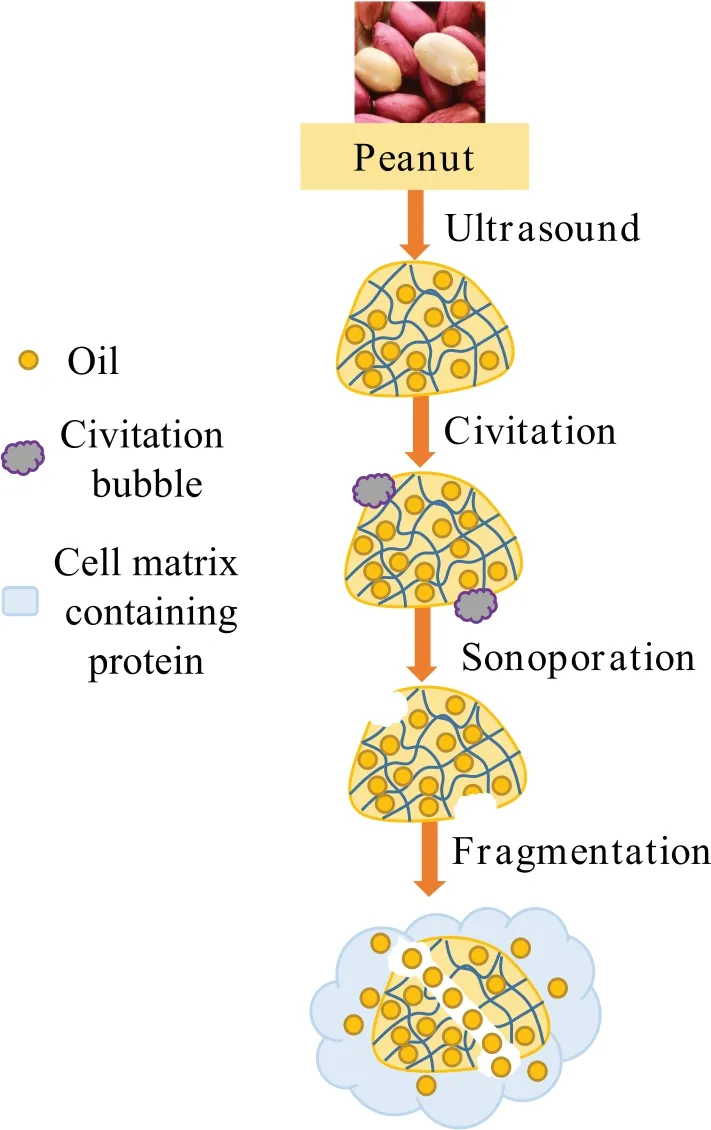
Fig.2. Mechanism diagrams of ultrasound-assisted alkaline extraction.Information from Wang et al.(2021) [31].
2.2. Aqueous enzymatic extraction
Various kinds of enzyme-assisted methods have been developed to improve the extraction of functional proteins from peanuts.Three phases were formed after centrifugal separation of the slurry formed using the AEP method:an oil-rich emulsion phase;a protein-rich liquid phase;and an insoluble fiber-rich solid phase[35].The formation of the oil-rich emulsion led to a relatively low free oil yield,which would limit the application of AEP in the food processing industry.However,if AEP is combined with a suitable enzymatic treatment,the oil can be released from this oil-in-water emulsion,thereby increasing the overall oil yield[36].
The principle of Aqueous enzymatic extraction processing (AEEP)extraction is firstly to mechanically destroy peanut cell structure,and then hydrolyze the internal macromolecular complex (lipoprotein,lipopolysaccharide,and cell wall polysaccharide) with enzymes to promote the release of oil and protein (Fig.1).Oil and protein can be separated according to the difference in density and affinity between oil and water components[28].
2.2.1.Cell wall degrading enzymes extraction
Cell wall degrading enzymes (such asViscozyme L.,cellulase,hemicellulose,pectinase,and xylanase)are used to breakdown the main cell wall components,thereby destroying the cell wall integrity,which improves the extraction of both oils and proteins from peanut seeds[28].Liu et al.(2020) investigated four cell wall degrading enzymes (Viscozyme L.,cellulase,hemicellulase,and pectinase) on the molecular weight distribution and yield of peanut proteins [28].The aqueous enzymatic extraction method did not change the molecular weight distribution of the peanut proteins compared to those in a commercial peanut protein powder.The peanut seeds treated byViscozymeL.at a solid-to-liquid ratio of 1:4(g/mL)and enzyme concentration of 1.35%at a hydrolysis temperature of 52◦C(90 min),resulted in a yield of peanut protein and oil bodies of around 79% and 48%,respectively.The functional properties,including foam stability,emulsifying activity,emulsifying stability,water holding capacity (WHC),and oil holding capacity (OHC) of the peanut protein powder obtained by aqueous enzymatic extraction were better than that of the commercial peanut proteins.
2.2.2.Proteases extraction
Proteases have also been used for improving protein and oil extraction from peanut seeds[34].In this case,the peanut proteins extracted by the aqueous enzymatic extraction method contained smaller molecules,which suggested that they had been hydrolyzed by the proteases.The hydrolysis of proteins reduces their molecular weight and alters their structure,which leads to changes in their physicochemical and functional properties when compared to intact proteins [36,37].In the case of aqueous enzymatic extraction,around 89%of free oil and 81%of protein hydrolysates were obtained when using 1.5%Alcalase 2.4 L(W/W)under fixed conditions(pH 8.5,60◦C,8 h)[34].Zhang et al.(2015)reported that enzymatic hydrolysis significantly decreased the molecular weight of peanut protein,the disulfide bond content,and the surface hydrophobicity,which reduced their ability to stabilize peanut proteinstabilized emulsions [36].Protease pretreatment of heat-denatured peanut flour with Alcalase 2.4 L (pH 10.0,45◦C),papain (pH 7.0,50◦C)or Protamex(pH 7.5,50◦C)for 30 min,resulting in a higher peanut protein concentrate yield (44.0%,35.2%,and 24.7%,respectively) at DH 1%than that obtained for the untreated peanut protein concentrate(13.2%) [38].The PPCs prepared by enzymatic pretreatment showed good functional properties (e.g.,increased solubility,emulsifying activity index,foaming capacity,and foaming stability),which was mainly attributed to the enzymatic degradation of the denatured and insoluble proteins in the original peanut protein samples.This study demonstrated that Alcalase pretreatment was a highly effective way of increasing the yield and functionality of PPCs.
AEEP is an innovative technology for simultaneously extracting peanut protein and oil from skinless peanut seeds that has the advantages of being organic solvent-free,having lower energy consumption,being more environmentally friendly,using milder processing conditions,being easy to control,and having good safety[30,37,39,40].However,the application of AEEP is also limited by the cost of enzymes involved in the extraction process.Therefore,it is necessary to reduce the amount of forming enzymes[34].
2.3. Ultrasound-assisted alkaline extraction
High-intensity ultrasonic processing involves exposing materials to intense high-frequency oscillating pressure waves,which generate cavitation,turbulence,and shear forces that can physically disrupt the materials.In particular,ultrasonic processing produces acoustic streaming effects,which result in the rapid formation and collapse of gas cavitation bubbles (Fig.2).The intense disruptive forces generated during this process can break down plant cell walls and membranes,which promotes deeper penetration of solvents into the cellular material,thereby improving mass transfer and increasing extraction efficiency[41,42].In addition,due to the mechanical effect and cavitation effect of ultrasound-assisted,subunits will dissociate or aggregate,and peptide bonds will be destroyed,thus interfering with non-covalent interactions between protein molecules and changing the structure and functional properties of proteins [43].For this reason,ultrasonic processing has been widely used to assist in the extraction of various food components from raw materials,including proteins,polysaccharides,and polyphenols [44,45].
Ultrasound is often used in combination with alkali treatments to extract peanut protein from defatted peanut flour or meal.Studies have shown that ultrasound-assisted alkali extraction enhances the extraction efficiency and improves the functional properties of the peanut proteins obtained [16,46].These effects can be attributed to the ability of ultrasound treatment to alter the molecular weight,aggregation state,and structure of peanut proteins.
Researchers investigated the effects of various processing parameters on the efficacy of ultrasound treatment,including defatted peanut meal/water ratio,ultrasonic power,pH,temperature,and time [8].The researchers reported that the protein yield achieved a maximum value of around 88%at a defatted peanut meal-to-water ratio of 1:20(W/V),an extraction pH of 6.8,an ultrasonic power of 30 W/g,a temperature of 50◦C,and a sonication time of 15 min.In another study,the authors reported that ultrasound-assisted alkali extraction significantly improved the extraction efficiency and emulsifying properties of peanut protein isolate (PPI),compared with only alkaline extraction.The maximum extraction efficiency of PPI was around 93%at an ultrasound intensity,temperature,and time of 3.17 W/cm3,35◦C,and 30 min,respectively.Moreover,the ultrasound-assisted alkali extracted protein isolates exhibited higher levels of hydrophobic amino acids and smaller molecular weight,which led to a higher emulsifying activity index and emulsifying stability index when they were used to prepare emulsions[16].
The microstructure of the protein isolate obtained by ultrasoundassisted alkaline extraction technique was not changed,but the secondary structure was affected.Thus,the functional properties of peanut protein and protein digestibilityin vitrowere improved,and the yield was higher.It was an ideal choice to assist in the alkaline extraction of defatted peanut powder[46].However,the effectiveness of ultrasound in improving the functional properties of proteins depends on a variety of factors.To obtain the desired results,it is best to apply optimized ultrasonic treatment parameters,including frequency,amplitude,duration,system temperature,and optimal conditions related to the intrinsic properties of proteins [31].The possible synergistic effect of ultrasound-assisted processing on protein structural properties has great application potential in improving the texture of foods such as sausages[43].The different extraction methods of peanut protein were compared in Table 2[16,28,34].
3.Modifications
As mentioned in the previous section,peanut proteins exhibit a range of different functional properties depending on their type,structure,and aggregation state.According to their mechanism of action,the functional properties of proteins can be divided into three major groups:(i)properties related to the interaction between proteins and watere.g.,solubility,water absorption,water retention,and swelling;(ii) properties related to the ability of proteins to aggregate with each other and form structurese.g.,gelling,thickening,and film forming;and (iii)properties related to their ability to adsorb to surfacese.g.,emulsifying and foaming [29].
As mentioned earlier,the application of peanut proteins in foods is often limited by their poor functional performance,which may be due to their inherent molecular attributes or to changes in these attributes during extraction [19,20,22].For this reason,chemical,physical,and enzymatic modification methods have been developed to improve the functional properties of peanut proteins.The functional properties of proteins are ultimately determined by their molecular characteristics,such as molecular weight,surface hydrophobicity,electrical charge,and aggregation state.The essence of protein modification methods is to alter specific molecular characteristics of proteins to enhance their physicochemical properties and functional performance.In this section,we provide a brief review of different methods of altering the functional properties of proteins through physical,chemical,or enzymatic modification.
3.1. Physical modification
Controlled heat treatments are one of the most commonly used physical modification methods for improving the functional performance of peanut proteins in the food industry.However,conventional heat treatments often require extended reaction times and involves high energy consumption.For this reason,researchers have investigated several other physical modification technologies to improve peanut protein functionality,such as high-pressure processing,high-pressure homogenization,extrusion cooking,sonication,and electron beam irradiation[47].
3.1.1.Heat treatment
Heat treatments are commonly applied to alter the structure and properties of proteins,e.g.,to enhance their adsorption to interfaces and to promote the formation of gels or films.
Compared to unheated PPI,heat-treated PPI has been shown to exhibit better emulsifying properties,which can be attributed to the partial unfolding of the globular protein molecules [21].Compared to untreated samples,a combination of blanching and roasting improved foam stability and increased fat absorption capacity (FAC) and water absorption capacity (WAC).Moreover,this combined modification process gave more improvement in foam stability,FAC,and WAC than when roasting was used without blanching.Zhang et al.(2019) investigated the influence of heat-pressure treatment and heating(80◦C,30 min) on the structure and functional properties of PPI.They reported there was an increase in the surface hydrophobicity of the peanut proteins from around 117 to 253 after treatment,as well as an increase in their interfacial diffusion rate [21].Moreover,Hu et al.(2019) studied the influence of three commonly practiced thermal treatments on the properties of PPIs dispersed in aqueous solutions containing glucose:simulated pasteurization(80◦C),retort sterilization(100◦C),and UHT sterilization(121◦C).As the severity of the thermal treatment increased,there was a greater degree of protein unfolding and a greater extent of Maillard reaction [48].
3.1.2.High pressure processing
High-pressure processing (HPP) is a non-thermal method that involves exposing products to pressures of 100 MPa or higher,typicallyusing a fluid as the pressure-transmitting medium[49].HPP is involved in cavitation,shear,turbulence,and temperature rise processes [50].HPP includes high hydrostatic pressure (HHP),high-pressure homogenization (HPH),and dynamic high-pressure micro fluidization(DHPM)methods.Both liquid and solid foods can be packaged in flexible materials and treated by HHP.Therefore,the use of HPP does not depend on the state,size,geometry,and composition of food products and packaging materials.Compared to conventional heat treatments,the HPP method often has shorter processing times and reduced energy expenditure,as well as leading to food products with less thermal degradation[51].In recent years,HHP has been widely applied to modify the functional properties of food proteins by inducing their denaturation and/or aggregation [52].The effects of HHP on the properties of proteins depend on the type and concentration of proteins,the duration and intensity of the applied pressure,the operating temperature,and solution conditions.He et al.(2014) indicated that HHP treatment significantly improved the water-holding and oil-binding properties of peanut protein isolates,whereas in most cases,its heat-gelling properties were not improved with treatment from 50 to 200 MPa [53].These results were attributed to the fact that elevated pressures changed the conformation of the peanut proteins,which induced the exposure of more hydrophobic groups at the surfaces of the protein molecules,which led to protein aggregation.
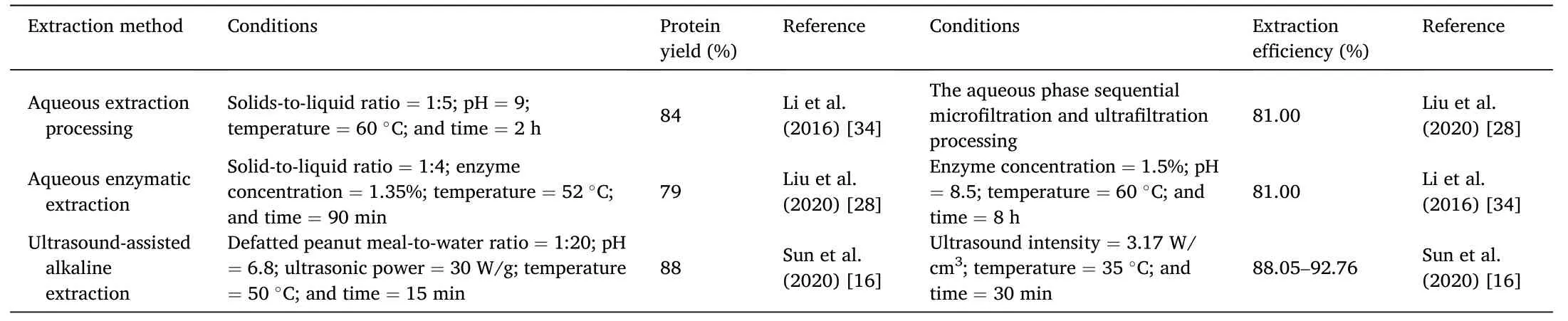
Table 2 Comparison of different extraction methods of peanut protein.
HPH has also been used to modify the molecular structure and physicochemical properties of food-grade animal and plant proteins such as whey,soy,and pea protein [54].This method has also been applied to peanut proteins.For instance,a pronounced increase in peanut protein solubility over the pH range from 4 to 7 was obtained after treatment at 40 MPa,which was attributed to a change in the structure of the proteins [55].Moreover,HPH has been shown to be capable of modifying the interactions between protein and polysaccharide molecules.For instance,Jiao et al.(2018) investigated the effect of HPH on the interactions between PPI and neutral(guar gum)or charged polysaccharides (chitosan and xanthan gum) in an aqueous medium [56].Interestingly,HPH treatment of PPI-polysaccharides mixtures delayed the gelation of these systems.The PPI-chitosan mixture formed a relatively weak gel network,whereas the PPI-guar gum and PPI-xanthan mixtures formed stronger gel networks.
DHPM is an emerging processing technology that has been shown to be an effective method of improving the solubility,emulsifying,and gelling properties of proteins.For instance,a study reported that a DHPM treatment at a relatively low pressure (≤120 MPa) reduced the degree of aggregation in PPI solutions [57].The disaggregation of the proteins resulted in an increase in the number of hydrophobic exposed to water,which promoted protein hydrolysis.In contrast,an increase in aggregation of PPI was observed after DHPM treatment at relatively high pressures(150–210 MPa).Gong et al.(2019) further explored the driving forces responsible for the structural alterations of PPI in aqueous dispersions caused by the DHPM treatment.It was proposed that the aggregation state of peanut protein molecules during microfluidic treatment depended on changes in the molecular interactions between them,such as hydrophobic,hydrogen bonding,electrostatic,and sulfhydryl/disulfide bond exchange interactions[47].At relatively high pressures,more extensive protein unfolding is induced,which leads to the exposure of more non-polar surface groups,thereby promoting aggregation through hydrophobic attraction.
The HPP combined with enzymatic hydrolysis and high-temperature cooking significantly improved the solubility and stability of the peanut protein solution.In addition,this modified peanut protein has great potential as a food additive for acidic drinks [50].
3.1.3.Cold atmospheric plasma
Cold atmospheric plasma (CAP) processing is an emerging nonthermal technology that is being considered as an alternative to traditional thermal technologies [58].A plasma is an ionized gas composed of electrons,ions,atoms,and molecules in their ground or excited states.Several researchers have recently examined the application of CAP processing for protein modification to improve their functional attributes [59].These studies have shown that CAP can change the tertiary and secondary structure of both plant and animal proteins within a few minutes,thereby affecting their physicochemical and functional properties of proteins.Ji et al.(2017) used CAP to alter the functional properties of peanut proteins[60].These authors showed that the CAP treatment significantly improved the solubility,emulsifying properties,and WHC of PPI,which was attributed to its ability to promote protein unfolding and disaggregation [60,61].CAP has also been considered a rapid glycosylation method for enhancing the efficiency of grafting carbohydrates to proteins [22].This application is discussed in more detail later in Section 4.2.
3.2. Chemical modification
Chemical modifications are also commonly used to increase the functional properties of proteins.The common chemical modification methods used for this purpose include acylation,phosphorylation alkylation,oxidation,glycosylation,and acid-alkali treatment [62].Beuchat (1977) investigated the impact of the acylation of peanut proteins with succinic anhydride on their functional properties (pH 7.4 to 8.0).It was reported that the major components of peanut protein were dissociated into subunits after acylation.Meanwhile,the functional properties of the proteins,such as water-retaining capacity,wateradsorption capacity,emulsion capacity,and apparent viscosity were significantly increased.Yu et al.(2015)reported that both the hydroxyl groups (-OH) in serine and threonine residues and the amino groups(-NH2)in the lysine residues of peanut proteins were involved in their reaction with polyphosphate.The emulsification activity index of the sodium tripolyphosphate(STMP)modified peanut protein produced was increased to 65 m2/g [63].Similar results were also reported by Sanchez-Resendiz et al.(2018) who found that a typical peak (1311 cm-1) within the zone of phosphate esters was presented in the FTIR spectrum of peanut proteins,which confirmed the modification of the primary protein structure.The phosphorylation of the peanut protein reached around 30% when 2% STMP was used under conditions of 35◦C,pH 12.5,and 3 h.In addition,compared to the control PPI,the modified peanut protein exhibited an increased emulsifying activity from around 79% to 95%,whereas the solubility of the peanut protein decreased by around a half[64].
Glycosylation is another commonly used chemical modification method for proteins.In particular,conjugating proteins with sugars and polysaccharides using the Maillard reaction has received considerable attention.Glycation is carried out between the amine groups of aminoacid side chains on protein molecules and terminal-reducing carbonyl groups on carbohydrates [65].Glycation is considered to be a safe and green chemical modification method because no hazardous substances are produced [65].This modification method has been shown to improve the solubility,emulsifying,thermal stability,gelling,and antimicrobial properties of proteins [66].Moreover,studies have reported that the glycated proteins have reduced allergenicity,which was attributed to structural changes in the proteins [67].In general,the glycation of proteins with carbohydrates is carried out by using either dry-or wet-heating treatments.The dry-heating method involves heating a powdered protein-carbohydrate mixture under controlled temperature and relative humidity conditions for several days or weeks,whereas the wet-heating method usually involves heating the mixtures in an alkaline solution for a few hours or days [67].For example,Liu et al.(2012)used dry-heating at 60◦C and 79%relative humidity for 7 days to obtain PPI-dextran conjugates,which were shown to have a higher water solubility from pH 4.5 to 6.0 than PPI or physical PPIdextran mixtures [65].In addition,the thermal stability,emulsifying,and foaming properties of the PPI were improved after conjugation with dextran.Similarly,Li et al.(2014) produced PPI-dextran and PPI-gum Arabic conjugates using the dry-heating method (60◦C,79% relative humidity,7 days).They showed that polysaccharide type influenced the molecular and functional properties of the glycated conjugates [67].A greater degree of glycation was observed in the PPI-gum Arabic conjugates than in the PPI-dextran ones.The formation of the conjugates resulted in changes in protein structure and surface hydrophobicity,which greatly influenced the functional properties of the proteins.In particular,the conjugated proteins have improved solubility and emulsifying properties.
However,the glycation of proteins with polysaccharides using dryor wet-heating treatment is often a time-consuming process.Therefore,some advanced techniques have been used to shorten the reaction time and improve the efficiency of graft reactions,including sonication,microwave,and CAP treatments.For example,Li et al.(2014)reported that PPI-dextran conjugates had a degree of grafting (DG) of around 36%after using simple wet-heating (80◦C for 24 h),but around 45% after using ultrasound-assisted wet-heating (200 W,80◦C for 40 min) [67].Chen et al.(2016) used ultrasound-assisted wet-heating to graft maltodextrin(MD)to PPI using the Maillard reaction.Oil-in-water emulsions stabilized by untreated PPI or PPI-MD conjugates produced using wetheating alone were unstable,whereas those stabilized by PPI-MD conjugates produced using ultrasound-assisted wet-heating (32% DG) had better stability,especially at pH values near the isoelectric point of the peanut proteins[68].
CAP treatments have also been used to accelerate the glycosylation process between proteins and carbohydrates[22].For instance,Ji et al.(2020)produced PPI-dextran conjugates with a DG of around 22%after only 1.5 min of CAP treatment [69].After glycosylation by the CAP treatment,the PPI exposed a greater number of reactive amino groups on its surface,thereby providing a larger number of grafting sites for the polysaccharide molecules to covalently attach to[19].
The pH-shift method has also been used to facilitate the grafting of carbohydrates to proteins.This method involves incubating proteins in extremely acidic or alkaline conditions,which results in partial protein unfolding due to the increased intra-molecular electrostatic repulsion[10,12,70,71].As a result of these conformational changes,proteins have enhanced chemical reactivity and functional attributes.For instance,subjecting PPI to an extremely alkaline environment (pH 10)for 1 h improved its solubility,reduced its aggregation,increased its free sulfhydryl group content,and increased its surface hydrophobicity,which improved the rheological and water-holding properties of gels formed from the protein [71].Similar results were also reported by Wang et al.(2020)[45].
Although it is a facile,convenient,and high-efficiency technology,chemical modification poses some risks to environment pollution,food safety,or human health,which should be considered.Moreover,chemical modification of proteins leads to the formation of new ingredients that may have to undergo time-consuming and expensive toxicity tests and regulatory approval.
3.3. Enzymatic modification
Enzymatic modification is one of the most precise methods of modifying the structure of proteins,thereby improving their nutritional and functional properties [18].Enzymatic modification of proteins can be divided into different categories depending on the changes in protein properties: (i) partial hydrolysis;(ii) cross-linking;(iii) surface modifications.
Partial enzymatic hydrolysis of proteins reduces their molecular weight,increases the number of ionizable groups,and increases the exposure of amino acids that were originally located in their interior,which can improve their solubility,emulsifying,foaming,and gelling properties [72].Commercial enzymes such as Alcalase,Neutrase,Papain,Protamex,and Flavorzyme have been used to hydrolyze proteins and extend their functionality.For example,Zhao et al.(2011) investigated the effects of limited enzymatic hydrolysis withAlcalaseon the conformation and functional properties of PPI[72].A limited degree of hydrolysis (DH<10%) improved the thermal stability,solubility,and gel-forming properties of PPI.In contrast,more extensive hydrolysis(DH>10%) decreased the emulsifying and foaming properties of the proteins,but increased their antioxidant and angiotensin-converting enzyme (ACE) inhibitory activities [73].Moreover,it was found that Alcalase and Flavorzyme were the most efficient proteases to produce PPI hydrolysates with high DH among the five commercial proteases tested (Alcalase,Neutrase,Papain,Protamex,and Flavorzyme) [74].Typically,peanut proteins are relatively resistant to enzyme hydrolysis due to their compact structures,and so hydrolysis only leads to limited improvement in their functional properties.For this reason,pretreatment methods are often used to enhance the protease accessibility of peanut proteins [75].These authors reported that extrusion pretreatment effectively improved the papain-hydrolysis efficiency of PPI.
Transglutaminase(TGase;EC 2.3.2.13),which can catalyze an acyltransfer reaction between the glutamine residues on one protein and the primary amines (especially theε-amino group of lysine) on other proteins,is commonly used to modify the functional properties of peanut proteins [20,76–79].Clare et al.(2007) reported that treating peanut proteins with TGase led to solutions with lower viscosity[76].Hu et al.(2011) found that the molecular and physicochemical properties of peanut proteins,including their surface hydrophobicity (H0) and secondary structure,were altered by TGase-induced conjugation[77].Feng et al.(2014)studied the impact of TGase-treatment on the properties of the arachin and conarachin-rich fractions of peanut protein [78].The TGase-treatment decreased the water-solubility of the arachin and coarachin-rich peanut proteins by around 66% and 37%,respectively.Zhang et al.(2020) reported that TGase-treated peanut proteins had better gelation and oil-binding properties than untreated ones,which was mainly attributed to changes in the conformation and aggregation state of the proteins [26].
Enzymes can also be used to add or remove specific functional groups on the surfaces of proteins,thereby modifying their functional attributes.For instance,enzymes have been used for deamidation [80] or acetylation [81].These changes would be expected to alter the solubility,gelling,emulsifying,and foaming properties of the proteins.The comparison of peanut modification methods is shown in Table 3[20,53,60,66,71].
4.Applications
4.1. Peanut protein nanoparticles
Protein nanoparticles are being investigated for their potential application as functional ingredients in foods [82].These particles typically have dimensions between about 10 and 500 nm depending on the proteins and processing methods used.A variety of applications of protein nanoparticles in the food industry have already been identified,including delivery systems for bioactive materials,stabilizers in Pickering emulsion,lightening agents to replace titanium dioxide,and additives in biodegradable films.
There is increasing interest in the preparation and characterization of peanut protein-based nanoparticles as functional food ingredients.A number of different fabrication methods have been developed for the preparation of peanut protein nanoparticles (PPNs) such as calciuminduced,ultrasound-assisted thermo-alkali-modified,and antisolvent methods.Calcium-induced PPNs with diameters ranging from 80 to 140 nm have been shown to exhibit good thermal-,dilution-,storage-,and gastrointestinal stability [83].Additionally,these PPNs exhibited no cytotoxicity towards normal model human cells and significantly increased the photostability of encapsulated resveratrol [84].PPNs prepared using an ultrasound-assisted thermo-alkali method were shown to be capable of incorporating curcumin at a relatively high encapsulating efficiency(around 83%)[85].
PPNs can also be utilized as colloidal particles to stabilize oil-inwater emulsions through a Pickering mechanism [86].In this case,the PPNs adsorb to the oil-water interface and form a layer of particles that is highly effective at inhibiting droplet coalescence.For instance,Seenriched PPNs formed by combined thermal/Na+-induced aggregation had partial wettability and a high surface charge,which made them highly effective at forming and stabilizing O/W Pickering emulsions[23].In addition,Pickering emulsions stabilized by PPNs were shown to improve the bioaccessibility and cellular uptake of a hydrophobic bioactive (5-demethylnobiletin) [86].Microgel particles formed from peanut proteins have also been successfully used to form and stabilize high internal-phase Pickering emulsions (oil>87%) [87].These emulsions may be used as a substitute for partially hydrogenated vegetable oils or as templates for producing porous materials.
Due to environmental concerns associated with the use of petroleumbased plastics,there has been increasing interest in the development of more environmentally-friendly packaging materials using biodegradable proteins and polysaccharides.However,these materials often do not have the physicochemical and functional properties required for commercial applications because of their poor mechanical and barrier properties.For this reason,there has been interest in incorporating nanoparticles into these materials to improve their performance.Peanut protein nanoparticles have been used for this purpose.For instance,the addition of PPNs was shown to significantly improve the tensile strength,water vapor barrier,and thermal stability of both protein and starch films [88].
4.2. Peanut protein-based film
As mentioned in the previous section,there is growing interest in the development of biodegradable packaging materials to replace petroleum-based plastics.A number of researchers have investigated the application of peanut proteins for this purpose.The processing conditions and physicochemical properties of peanut protein-based films are summarized in Table 4 [88–94].According to Table 4,casting is the most commonly used method to produce peanut protein-based films in research laboratories.The properties of these films are mainly influenced by heat treatment (temperature and time),pH,protein concentration,and the addition of plasticizers [88,90,92,93,95].Protein concentration has been reported to have a major influence on the optical properties of peanut protein films [91].The addition of glycerol (a plasticizer)has a major influence on the mechanical properties of peanut protein films [90,92].For instance,adding around 7.5% glycerol reduced the tensile strength about four-fold and increased the elongation at the break by about 387% [90].However,peanut protein films typically exhibit relatively poor stability in the presence of water and have much weaker mechanical properties than synthetic plastic films.Therefore,various methods have been used to improve their water resistance and mechanical properties,including blending with other components and crosslinking [90,94].For instance,Liu et al.(2017)reported that crosslinking peanut proteins with xylose led to the formation of films with enhanced mechanical properties and water resistance [95].The tensile strength and elongation at break increased by around 77%and 67%,respectively,while the water solubility decreased from around 97% to 43% after cross-linking.In addition,the incorporation of lipophilic materials(such as thymol)into peanut protein films has been shown to decrease their water vapor permeability,tensile strength,and elongation at break,but increase their total phenolic content,antioxidant capacity,and antimicrobial activity [94].
4.3. Peanut protein hydrolysates
At present,protein hydrolysates are usually obtained using either enzymatic or chemical hydrolysis,but enzymatic hydrolysis is usually preferable from a food safety standpoint.Bioactive peptides generated by enzymatic hydrolysis of food proteins have been reported to exhibit a range of beneficial effects,including antioxidant,antihypertension,antithrombotic,immunomodulatory,opioid agonists or antagonists,mineral binding,antithrombotic,anticancer,and antimicrobial activities[96].Low molecular weight polypeptides can be absorbed directly from the intestine and have little allergenicity.In addition,some polypeptides have also been reported to elicit an intense umami taste,which means they can be used as taste modifiers.

Table 3 Comparison of modification methods of peanut protein.
Hydrolysates obtained from peanut proteins have also been reported to display bioactive properties[89].Researchers have characterized the peptides produced after hydrolysis of peanut proteins by commercial proteases,such as Alcalase,Protamex,papain,and Flavorzyme[96–100].The type of proteases and hydrolysis conditions used,the degree of hydrolysis,and the properties of the peanut protein hydrolysates (PPHs)produced are summarized in Table 5[10,89,97–99,101–106].
Many PPHs have been reported to exhibit antioxidant properties.For instance,Chen et al.(2007) reported that PPHs produced by Alcalase had relatively strong antioxidant activities.As an example,PPHs had an 82% scavenging effect in a DPPH radical assay when used at a concentration of 20 mg/mL,which was close to the effect obtained when using 0.5 mg/mL of BHT (84%)– a widely used synthetic antioxidant [97].Quist et al.(2009)compared the antioxidant activities of PPHs produced using either Alcalase or pepsin-pancreatin [98].The Alcalase gave a higher hydrolysis efficiency compared to the pepsin–pancreatin system.The molecular weight of peptides is one of the key factors determining their antioxidant activity.Peptides produced by Alcalase treatments were reported to have relatively low molecular weights (Mw<3 kDa)and strong antioxidant abilities[102].These researchers identified three antioxidant peptides with amino acid sequences of Thr-Pro-Ala (286 kDa),Ile/Leu-Pro-Ser(315 kDa),and Ser-Pro(202 kDa),respectively.A peanut peptide (p8) with an amino acid sequence of Lys-Leu-Tyr-Met-Arg-Pro (808.8 Da) was reported to exhibit strong antioxidant and angiotensin-converting enzyme (ACE) inhibiting activity [103].In another study,a peanut peptide was reported to exhibit strong antithrombotic activity[104].Su et al.(2011)found that in comparison with commercial proteases including Alcalase,Protamex,and papain,a crude protease extract (CPE) prepared fromAspergillus oryzaeHN 3.042 showed more extensive hydrolysis with around 81% protein recovery and 43% degree of hydrolysis (DH) [99].Su et al.(2012) showed that peanut protein isolate was a good source of umami peptides.Two novel umami and umami-enhancing peptides from PPHs were created by CPE hydrolysis and then purified and identified: Ser-Ser-Arg-Asn-Glu-Gln-Ser-Arg (963.9 Da) and Glu-Gly-Ser-Glu-Ala-Pro-Asp-Gly-Ser-Ser-Arg(1091.1 Da) [101].However,these two peptides had no structural homology with peanut proteins,which suggests that there was some restructuring during hydrolysis.The umami flavor of PPI peptides can be enhanced by using a combination of enzymatic hydrolysis and Maillard reaction [105].The hydrolysate of peanut protein has the effect of umami and enhancing umami.It is a potential good precursor material for umami peptides in the food industry [107].
In addition,the optimum conditions for the preparation of peanut protein hydrolyzed products by alkaline enzyme and trypsin were:solidto-liquid ratio of 1:26.22 and 1:30(W/V),the enzyme-substrate ratio of 6.00%and 5.67%,pH of 8.41,the temperature of 56.18◦C and 58.75◦C,respectively.The obtained hydrolysate with antidiabetic activity can be used as a natural substitute for synthetic antidiabetic drugs in food and pharmaceutical products[108].
4.4. Peanut protein-based meat analogues
The United Nations predicts that the global population will reach around 9.7 billion by 2050,which will lead to an increase in animal meat consumption.Nevertheless,the overconsumption of animal meat leads to climate and environmental pressures,decreased animal welfare,and negative impacts on human health.Because of these concerns,there has been considerable interest in the development of meat alternatives with similar appearances,textures,mouthfeels,and flavors as real meat[24,42,109,110].In particular,there is great interest in developing plant-based meat analogs,which are constructed from plant-derived ingredients [20,25].Currently,extrusion technology is commonly used to produce plant-based meat analogs with meat-like fiber structures.Based on the moisture content,extrusion technology can be divided into low-moisture extrusion(LME)and high-moisture extrusion(HME) (Fig.3 [111,112]).LME (moisture<30%) is mainly utilized to produce more or less expanded plant-based meat analog products that can be further processed into products like burgers,sausages,nuggets,and meatballs.HME (moisture>50%) is mainly used to manufacture products with well-defined fibrous structures that are dense and strong,which makes them more suitable for the development of whole-muscle meat analogs.Hence,HME is now considered one of the best options for developing plant-based meat substitutes [42,111].

Fig.3. The flow chart of plant protein-based meat analogue through low moisture extrusion (LME,A) and high moisture extrusion (HME,A) using a twin-screw extruder.Information from Liu &Hsieh (2008) [111] and Chen,Wei,&Zhang (2011) [112].
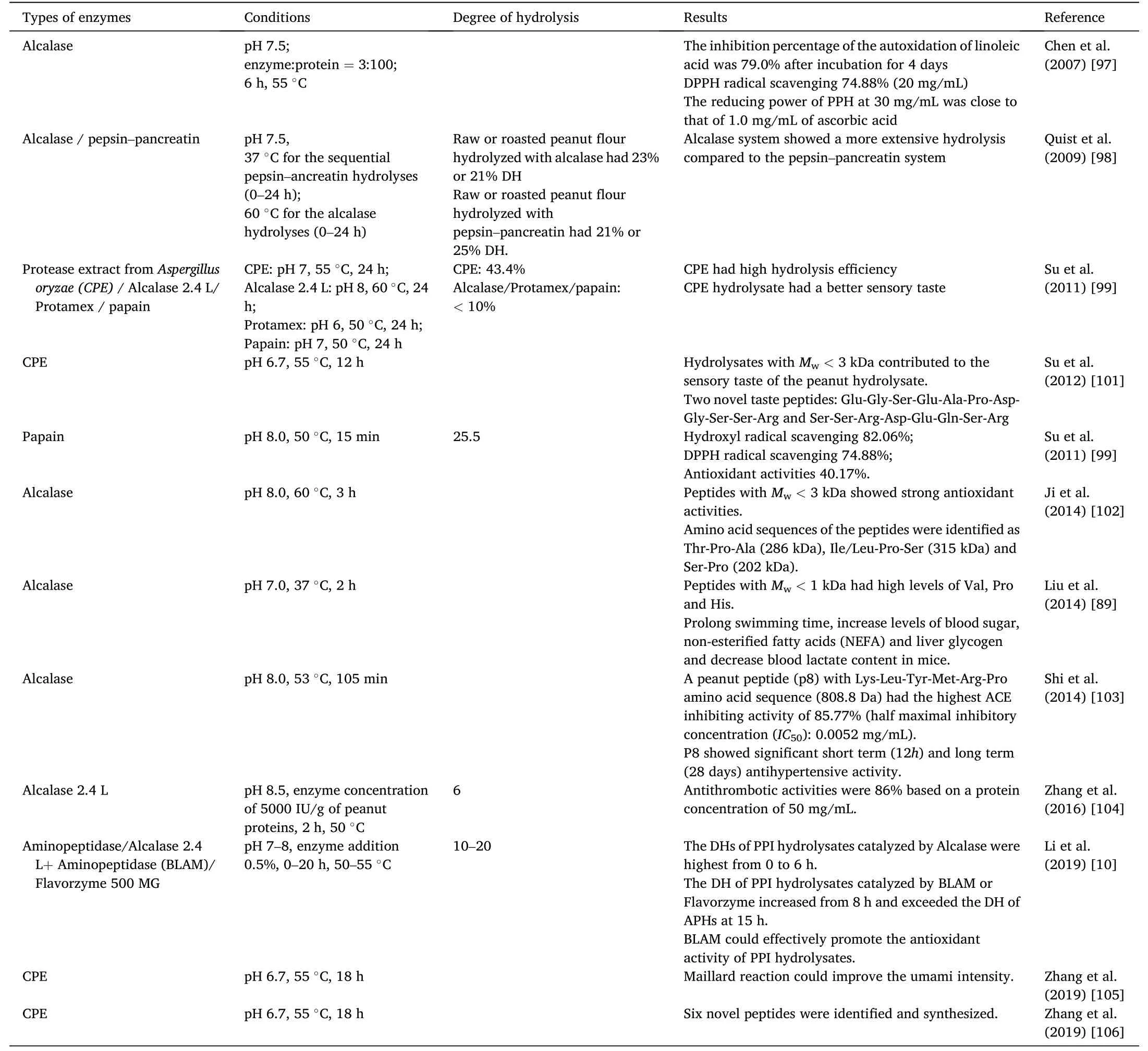
Table 5 Hydrolysis conditions of peanut protein hydrolyzed by protease.
A number of researchers have examined the potential of peanut proteins as functional ingredients in plant-based meat analogs.Early studies on textured peanut protein focused on the formation of thermoplastic extrudates using LME[113–115].For instance,Aguilera et al.(1980)successfully texturized defatted peanut flour(DPF)using singlescrew thermoplastic extrusion [113].The extruded products were functionally comparable to those of textured soy protein.Similar results were also reported by Chen et al.(2005) [114].
Recently,researchers and entrepreneurs have been exploring the use of HME to produce peanut-protein meat products.For HME,extrusion temperature,screw rotation speed,feed rate,and material moisture content have a great influence on the properties and interactions of the ingredients,the formation of fiber structures,and the quality of the final product [116].Rehrah et al.(2009) produced peanut-based textured meat analogs using a single-screw under HME conditions and reported that the optimum extrusion conditions were a protein content of 60%–65%,a moisture content of 50%–55%,a temperature of 160–165◦C,and a screw-speed of 80–90 rpm [115].Peanut-based meat analogs have good physiochemical and sensory properties,which were reported to be similar to or better than those of soy-based meat analogs.
Typically,the gel-forming properties of peanut proteins are worse than those of soybean proteins,so it is more difficult to form fibrous structures using HME.It has been reported that hydrophobic and hydrogen bonding interactions are the main forces in peanut protein gels(in contrast to the disulfide bonds formed in soybean protein gels),which results in a lower tensile strength [106,110,117].For these reasons,there has been interest in combining peanut proteins with other proteins (e.g.,soybean protein or wheat gluten) or non-protein ingredients(such as starches and gums)to improve the nature of the meat analogs formed.Zhang et al.(2018)reported that both soybean protein and wheat gluten addition improved the textural attributes of texturized peanut proteins [117].Moreover,it was reported that adding polysaccharides (such as carrageenan,alginate or starch) promoted the formation of meat-like fibrous structures in peanut protein during the extrusion process[26].Furthermore,the addition of TGase was reported to increase the forces holding the fibrous structure together in peanut protein-based meat analogs,thereby improving their appearance and mechanical properties [25,118].
Most previous research on peanut protein-based meat alternatives has focused on the production of fibrous structures that mimic the appearance and textural properties of real meat products.At present,there has been little research on the sensory attributes,digestibility,nutrition,and safety of these products.Therefore,the main objective of future research should be the development of delicious,healthy,and sustainable peanut protein-based meat alternatives.
5.Conclusions and future directions
Peanuts are one of the most important oilseeds in the world and contain both oil and proteins.Peanut meal is normally a byproduct of the edible oil industry,which contains a relatively high protein content(47%–55%).Nevertheless,due to its high denaturation and poor functionality,peanut protein obtained from the traditional oil-making process is typically only used in animal feedstocks,which leads to a huge waste of valuable natural resources.New gentle methods have been developed for the extraction of high-quality proteins from peanuts,but most of them are not currently suitable for large-scale industrial production.Therefore,more research is needed to develop efficient technologies for the commercial separation of high-quality oils and proteins from peanuts.
The main hurdles in the commercialization of peanut protein are its relatively low solubility and poor functionality.Peanut protein with improved functional properties can be obtained using physical,chemical,enzymatic,and combined modification methods.As mentioned earlier,each of these methods has its advantages and drawbacks.However,many of these modification methods are also not suitable for large-scale industrial applications.Consequently,there is a need to develop modification methods that are low-cost,efficient,environmentally friendly and suitable for industrial applications.
At present,studies on the application of peanut proteins in foods mainly focus on the production of delivery systems,edible films,bioactive peptides and meat substitutes.However,compared with soy protein,peanut protein is still underutilized.Therefore,the development of new value-added products from this inexpensive resource needs further study.
CRediT Authorship Contribution Statement
Song Cui:Writing–original draft,Investigation,Conceptualization,Validation.David Julian McClements:Visualization,Writing–review&editing.Xingfeng Xu:Investigation,Formal analysis.Bo Jiao:Investigation,Validation.Liyang Zhou:Investigation,Software.Hualu Zhou:Investigation,Validation.Liu Xiong:Investigation,Supervision.Qiang Wang:Investigation.Qingjie Sun:Investigation.Lei Dai:Conceptualization,Writing–review &editing,Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province [grant number ZR2020QC218];Key R &D plan of Shandong Province [grant number 2019YYSP005];Major Science and Technology Projects of Shandong Province [grant number 2019JZZY010722];Qingdao Municipal Science and Technology Benefit People Project [grant number 20-3-4-34-nsh];and Breeding Plan of Shandong Provincial Qingchuang Research Team [grant number 2021-Innovation Team of Functional Plant Protein-Based Food].
杂志排行
Grain & Oil Science and Technology的其它文章
- Encapsulation of lipases on coordination polymers and their catalytic performance in glycerolysis and esterification
- Drying kinetics of soy protein isolate-corn starch film during preparation and its moisture adsorption characteristics during storage
- Effect of pressure cooking on phenolic compounds of quinoa
- Plant phenolic extracts for the quality protection of frying oil during deep frying: Sources,effects,and mechanisms
