Cryopreservation of oocytes: history, achievements and future
2023-11-08ShiyuZhaoandGangZhao
Shiyu Zhao, and Gang Zhao
Department of Electronic Engineering and Information Sciences, University of Science and Technology of China, Hefei 230027, China
Abstract: There have been increasing requirements for women’s fertility preservation due to oncological and nononcological reasons in recent years, and meeting these demands will be a hot topic in the coming years.Oocyte cryopreservation is a workable option for preserving women’s fertility, and great advances have already been made and much progress has been made in mammalian gene banking and human oocyte banks.In this paper, we systematically introduce the history of oocyte cryopreservation and vitrification technology and highlight the vitrification carrier.Furthermore, we summarize the fundamentals of oocyte vitrification and discuss the effects of vitrification on oocyte quality.Strategies to improve the effect of oocyte cryopreservation are also proposed.At the end of this review, we conclude oocyte cryopreservation and outline future perspectives.
Keywords: fertility preservation; oocyte; cryopreservation; vitrification
1 Introduction
Currently, 1 in 51 women is diagnosed with cancer by the time they are 39 years old[1].Although anticancer medicines have been able to increase the survival rate, one of the side effects is ovarian failure[2], since chemotherapy and/or radiotherapy treatments increase the likelihood of infertility[3,4].Even if young women are cured of cancer, they still struggle with reproductive issues.Additionally, there are some other circumstances in which reproductive functions are impacted,including women who have undergone multiple ovarian surgeries, received treatment for certain collagen diseases, and wished to postpone having children until the age of 35 because of social pressures such as professional development[5].Hence, addressing the urge for fertility preservation has significant social implications.
Cryopreservation of embryos and oocytes refers to the approaches and tactics that assist reproductive technologies(ART) used to achieve fertility preservation[6].Many advancements and improvements in embryo freezing have been made.The first successful research on human embryo freezing resulting in pregnancy was conducted in 1983[7].However, there have been difficulties occurring due to the scarcity of a clear definition regarding the status of cryopreserved embryos,which results in raising ethical and legal concerns regarding supernumerary embryo cryopreservation.For an embryo to be cryopreserved, a male partner or sperm donor must be present instead of absent.Therefore, it is only natural to raise moral and legal questions concerning what would happen to the orphaned embryo if the patient passes away or splits up[8].The moral, legal, and theological difficulties associated with embryo cryopreservation were resolved by oocyte cryopreservation,which is superior in many ways[9].Oocytes are also quite precious and worth cryopreserving.Finite oocytes are present at birth in women, and their number drops from a peak of 6-7 million during the gestation period to 1-2 million at delivery[10].At approximately age 51, the number of oocytes will continue to decline until it is less than 1000.Oocyte quality will also fall along with their quantity.Epidemiological studies revealed a decrease in female fertility at the beginning of thirty years[11].As a result, there is now a great deal of interest in the cryopreservation of oocytes[12], and significant progress has been made in this area.
The basic mechanism for achieving long-term preservation of oocytes is the apparent reduction in biochemical reactions in cells as the preservation temperature drops.This is accompanied by processes such as metabolic activity, active transport, enzymatic reaction, and diffusion descent[13,14].As a result, the cryopreserved oocytes can stay in a suspended animation state, preserving women’s fertility.
In this review, we introduce the great importance of oocyte cryopreservation and the indications for oocyte freezing.Then, we provide an overview of the history of oocyte cryopreservation and discuss the cryopreservation procedure (vitrification).The devices used for vitrification are also introduced.In addition, the fundamentals of vitrification and the influence of vitrification on oocytes are summarized.Approaches to improve the quality of oocyte cryopreservation are outlined, such as adding cryoprotectant and antioxidants,antifreeze proteins (AFP), and bioinspired ice-controlling materials, as well as applying engineering strategies (external physical fields and encapsulation).Finally, the possible future directions of oocyte cryopreservation are outlined briefly.
2 History of oocyte cryopreservation
Studies on oocyte cryopreservation have been conducted since the late 1940s and have led to findings that have both practical and theoretical significance[15].Slow freezing was the earliest technique used in oocyte cryopreservation, and the results of preservation showed improvements over time[16].In the slow freezing procedure, samples are usually cooled in a controlled manner (typically -1 °C/min) with low-concentration cryoprotectant (CPA).Ice crystals are inevitably formed during this process, but to a certain extent, intracellular ice is avoided due to the slow cooling rate and the consequent cell dehydration.Even if intracellular ice crystals are formed, the viability and functionality of the sample can still be preserved to a certain extent[17].As a time-honored technology,slow freezing can preserve biological samples and minimize structural and functional damage to cell membranes[18]and cytosolic contents[19]during cryopreservation.The sample was usually chilled in advance to reduce the cytotoxic effect of the CPA solution, and the next step was to slowly cool the sample to -5 °C or -7 °C and induce ice nucleation.Then, the sample was cooled to -80 °C at a controlled rate, immersed in liquid nitrogen (LN2), and held in the cryogenic state for longterm storage.
In 1949, glycerol was first discovered to act as a CPA to protect spermatozoa[20].Then, researchers started to evaluate the effect of glycerol on oocyte cryopreservation.In 1965,Burks et al.[21]recorded the morphology of oocytes after cryopreservation with glycerol, and their results showed that oocytes exposed to 35% glycerol at 5 °C and then frozen had the highest survival rate (96%) compared with other concentrations of glycerol.In addition to using glycerol as a CPA, dimethyl sulfoxide (DMSO) has also been used to cryopreserve oocytes.In 1976, Parkening et al.[22]compared the effect of DMSO and glycerol on mouse oocyte cryopreservation, and they found that DMSO appeared to be better than glycerol for mouse oocytes.They first obtained three normal mouse offspring from cryopreserved oocytes with a slow freezing procedure.In the same year, Tsunoda et al.[23]reported the freezing of unfertilized oocytes at a much lower temperature (-176 °C)and successfully achieved the in vitro fertilization of freezingthawed mouse and hamster oocytes.These freezing procedures were very similar.First, oocytes were chilled to 0 °C at a rate of 0.5 °C/min, and precooled DMSO was loaded to a final concentration up to 1.5 mol/L.After a few minutes, oocytes were cooled to -5 °C, and ice nucleation was induced by a cooled hypodermic needle.The next step was to cool the oocytes to -80 °C at 0.33 °C/min and finally immerse them in LN2for long-term storage[22].This slow freezing procedure was also applied to freeze human oocytes and achieved significant results.In 1986, Chen[24]successfully presented slow freezing of human oocytes and first achieved a twin pregnancy followed by insemination.The cryopreservation procedure involved reducing the size of the oocyte-cumulus complex first.After washing twice in PBS, chilled DMSO was gradually added up to 1.5 mol/L, then the temperature was decreased to -7 °C, and the seeds were induced and the temperature was decreased to -36 °C at 0.5 °C/min and rapidly cooled to -196 °C for storage.Although the slow freezing procedure made much progress, the pregnancy rates were as low as 1%-2% in the late 1990s after transplanting embryos from freezing oocytes[16,25].Because of the technical challenges and low success rates, a dependable protocol is lacking in this area[26].
Then, as an alternative to slow freezing, vitrification was introduced in oocyte cryopreservation and resulted in higher success rates.The definition of vitrification is that the viscosity of the solution is high enough to form a glassy state without crystallizing.It is the direct conversion of the liquid phase to the glass solid phase.When the temperature reaches the glass transition temperature (Tg), disorder-free molecules in the solution stop moving.Under this circumstance, almost all metabolic activities come to a halt, which leads to an interruption of life activity[27], and biological samples can remain in a suspended state during this special time.When the solution is transformed from a liquid state to a glassy state, the concentration of the solution does not change, avoiding the formation of inner ice and minimizing the negative influence of excessive sample dehydration.According to Stiles[28]proposed in 1930, producing a finely crystalline or amorphous substance by rapid freezing was feasible, which would restore the original state of the sample after thawing.Then, in 1937, the concept of cryopreservation cells at a rapid cooling rate was introduced by Luyet[29].In 1985, Rall et al[30]developed the vitrification procedure and applied it to cryopreserve mouse embryos.To date, the clinically preferred technique for the cryopreservation of oocytes is vitrification[31].The results on oocyte cryopreservation by vitrification, a promising and rapidly expanding cryopreservation alternative, have been generated in animals and humans.In 1989, Nakagata et al.[32]first reported the birth of normal young by in vitro fertilization (IVF) of vitrified mouse oocytes.In 1999,Kuleshova et al.[33]reported the birth of a healthy baby girl delivered by a 47-year-old recipient.After collecting human mature oocytes from IVF patients, oocytes were vitrified in highly concentrated CPA (40% v/v ethylene glycol + 0.6 mol/L sucrose + 10 mg/mL HSA) and placed in open pulled straws (OPS) at an ultrarapid cooling rate to -196 °C and warmed by transferring OPS into prewarmed sucrose solutions at 37 °C.This study suggested that oocyte vitrification could be used for patients who undergo in vitro fertilization(IVF), for oocyte donation, for patients who are at risk of infertility due to radiation and/or chemotherapy, and for those who want to delay having children for other reasons.Legal restrictions on embryo cryopreservation have sped up research on oocyte preservation[34]and the development of efficient vitrification devices[35,36].Fig.1 shows some significant developments in human and mouse oocyte cryopreservation.The details of the human and mouse oocyte cryopreservation procedures are given in Table 1.

Table 1.Various methods, carrier, CPA solution, dilution solution, survival and pregnancy of mouse and human oocyte cryopreservation.
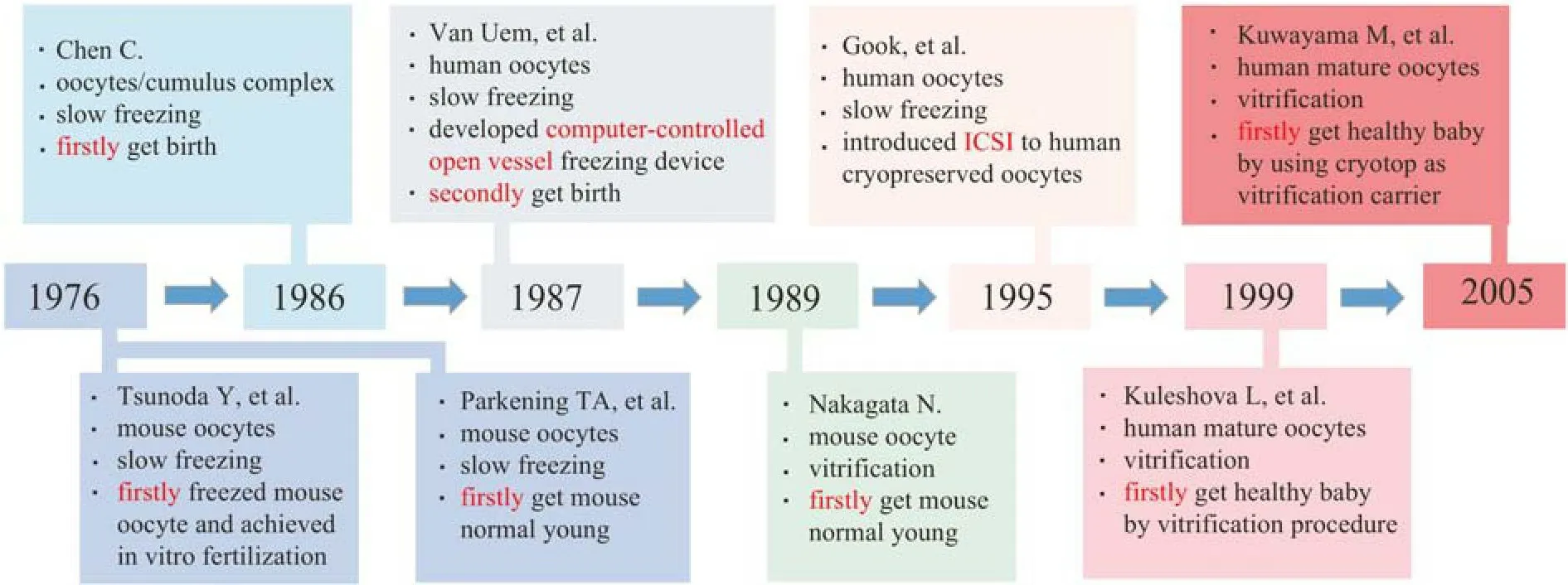
Fig.1.The history development of human and mouse oocyte cryopreservation.
In addition to OPS, researchers have developed many other carriers for oocyte vitrification and have made great progress.In 1996, Martino et al.[37]attempted to use an electron microscope grid as a vitrification carrier to vitrify bovine oocytes by immersing the grid directly into LN2.After warming,30% of bovine oocytes cleaved after IVF, and half of them developed into blastocysts, but oocytes vitrified with straws produced only 3% cleavage, and <1% developed into blsatocysts.In 2005, Kuwayama et al.[36]developed the Cryotop method and used this new carrier to cryopreserve MII human oocytes.The Cryotop method yielded the optimal result compared with the other two methods (plastic straws and openpulled straws).Since then, Cryotops have gradually become the most commonly used vitrification carrier for oocytes.In addition, other vitrification carriers, such as cryotips and cryolefs, are also used for oocyte vitrification.Fig.2 shows the shapes of Cryotop and other common carriers.A more detailed description of another different vitrification carrier will be given later.
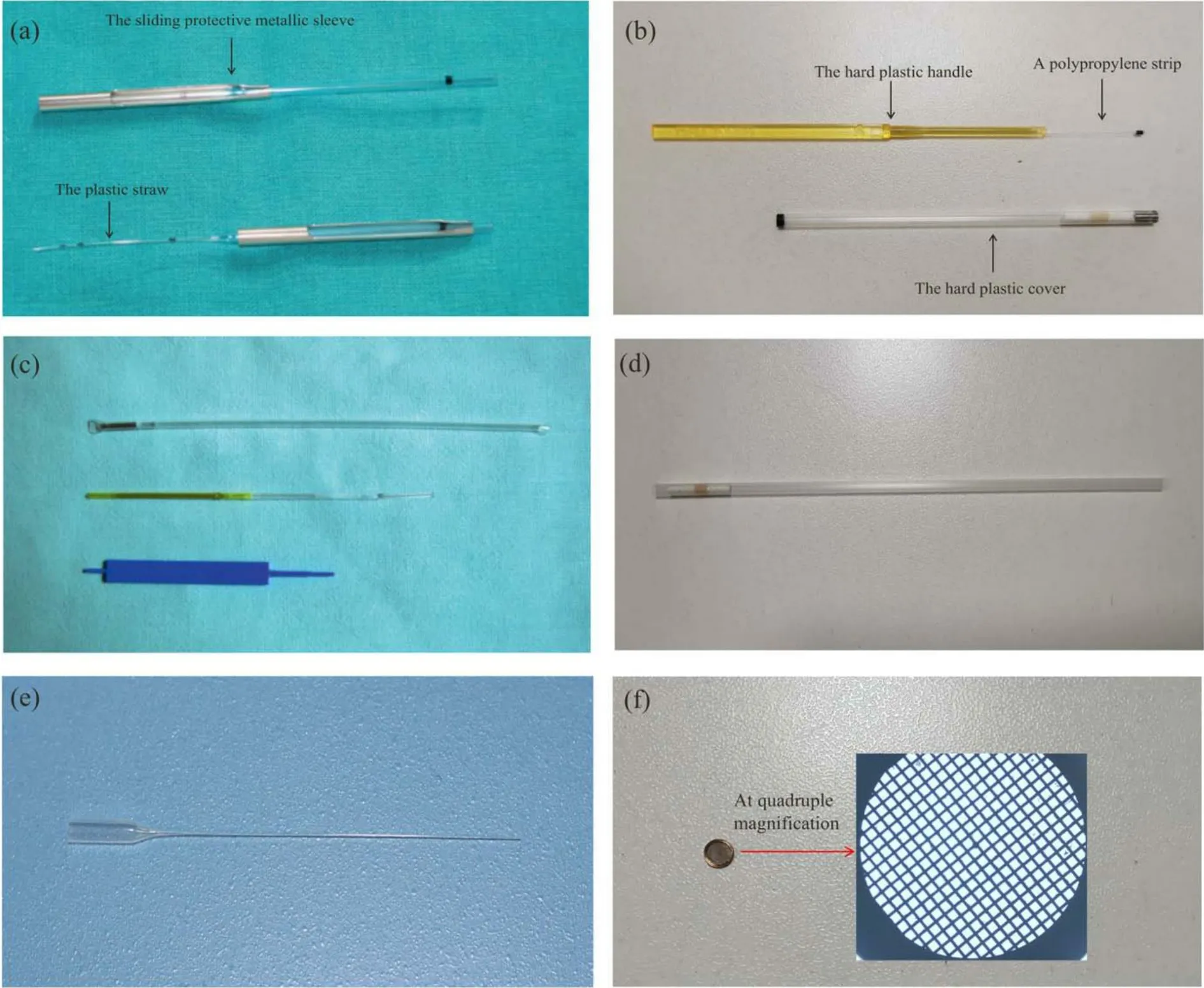
Fig.2.Different kinds of vitrification carriers.(a) Cryotip; (b) Cryotop; (c) high security vitrification kit.(a, c) Reproduced with permission from Ref.[38].Copyright 2012, University of Cambridge.(d) Plastic straw; (e) glass capillary; (f) electron microscope grid.
For slow freezing or vitrification, which one is more efficient for oocyte cryopreservation? To determine a more efficient method for oocyte cryopreservation, Cao et al.[39]compared various indicators in slowly frozen and vitrified human oocytes.The latter group had a higher oocyte survival rate,fertilization rate, division rate of developing to the embryo,and percentage of blastocyst development rate than the former group (91.8%, 67.9%, 42.3%, 33.1% vs.61.0%, 61.3%, 24%and 12.0%, respectively).Many other studies have also indicated that vitrification is more efficient than standard slow freezing for oocyte cryopreservation[16,40].
The introduction of intracytoplasmic sperm injection(ICSI) brought a turning point in improving the efficiency of oocyte cryopreservation.In 1995, Gook et al.[41]used ICSI for inseminating cryopreserved oocytes.Compared with traditional insemination, the ICSI group had a higher cleavage rate.This was the first time that ICSI was successfully applied to cryopreserved human oocytes and resulted in normal development to the hatching blastocyst stage.Moreover, ICSI was found to solve a fertilization problem caused by cryopreservation (cryopreservation can cause hardening of the zona pellucida)[42].
Despite the technology of oocyte cryopreservation being optimized, the blastocyst rate and clinical pregnancy rate are not as good as those of fresh oocytes[43].Fresh oocyte donation remains the “gold standard”, as national outcome data from the United States show that the use of fresh oocytes results in much higher live births per recipient cycle than the use of vitrified oocytes (51.1% vs.39.7%)[34].
Oocytes are very sensitive to cryopreservation; therefore,they present more technical challenges than other cells.The physical characteristics of oocytes make them extremely sensitive to freezing[7], and they are susceptible to cryoinjury during cryopreservation.The oocyte is one of the largest cells in the human body with a low surface area to volume ratio,which results in a slower dehydration rate and a higher possibility of forming intracellular ice compared to smaller cells[44].The osmotic shock caused by a high concentration of CPA solution, which can occur during the loading and unloading process, can lead to severe deformation of the oocyte membrane and can even result in immediate death.All these factors bring technical difficulties to oocyte cryopreservation.
3 Vitrification carrier
The vitrification technique requires a higher concentration CPA solution and a higher cooling rate than the slow freezing technique.However, an ultrahigh cooling rate can substitute for a low-concentration CPA solution[45]and can still form a vitrification state.A low concentration CPA solution is beneficial for reducing the toxic effect and osmotic shock to cells.To meet the requirements of extremely high cooling rates, various vitrification carriers have been designed.These carriers can be divided into two categories: open vitrification carriers and closed vitrification carriers.Open vitrification carriers include conventional plastic straw, glass capillaries,quartz microcapillaries, Cryoloop, Cryotop, electron microscopy grid (EMG) and Cryoleaf; closed vitrification carriers include high security vitrification (HSV) straw and closed pulled straws.The different characteristics of open and closed carriers are summarized in Table 2.Although open vitrification carriers are effective, doubts regarding the safety and sterility of open carriers have been raised due to the contact between sample and LN2[50], and there might be microbial contamination in LN2.Some suggestions have been proposed to avoid the potential risk, for example, vitrifying oocytes with nitrogen vapor or using sterilizing LN2,which has a lower density of airborne contaminants in the environment[51].Using a closed carrier to isolate oocytes and LN2is another option,such as using the sealed “straw in straw” closed carrier, but it can also raise concerns about the efficiency of oocyte vitrification because closed carriers have lower cooling rates than open carriers[52].Bonetti et al.[53]showed a higher survival rate and less severe ultrastructural changes in human oocytes with open carriers after vitrification and warming, and the ultrastructure of oocytes with closed vitrification carriers could not be preserved as well as that of oocytes with open carriers.Many researchers have considered that the vitrification of isolated samples in closed carriers might decrease the efficiency of vitrified oocytes because closed carriers decrease the cooling rate.Paffoni et al.[52]noted that oocytes using an open carrier (Cryotop) led to higher fertilization and cleavage rates than oocytes using a closed carrier (Cryotip) (73%,55.4% vs.57.6%, 34.5%).However, a controversial point is that some researchers believe that the closed carrier allows a prominently higher oocyte survival rate.Pujol et al.[54]vitrified oocytes with different methods.One group used an open carrier (Cryotop), and the other used a closed carrier (Rapidi).Their results showed that the closed carrier resulted in a better survival rate (94.5% vs.88.9%).The cooling rate of the Rapid-i carrier was -1220 °C/min, while the cooling rate of Cryotop was -23000 °C/min, possibly implying that a wide variety of cooling rates could be used to vitrify oocytes without affecting survival, at least in terms of survival rate[54].Researchers who used other closed carriers also achieved high oocyte survival[55,56].Further studies are needed to decide which system is the best for oocyte cryopreservation.There are also carrier-free vitrification methods, namely, dropletbased vitrification and solid surface vitrification.
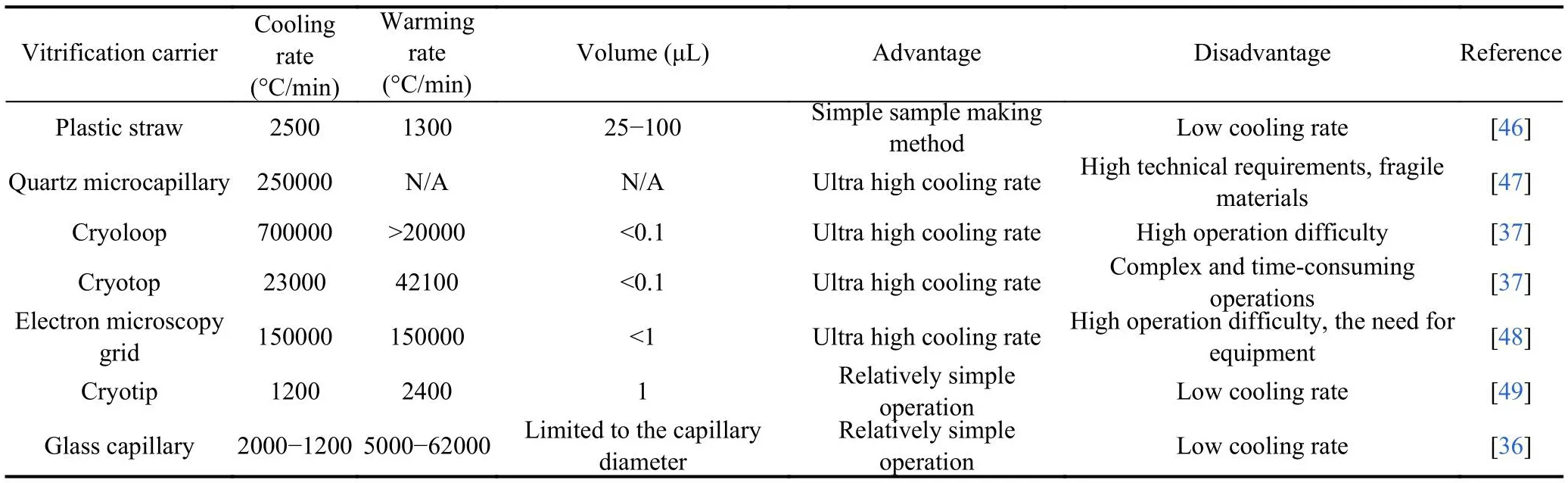
Table 2.Different types of vitrification carriers.
4 Fundamentals of oocyte vitrification
It is well known that vitrification is a more effective method for oocyte cryopreservation than slow freezing.In the vitrification procedure, oocytes must be exposed to CPA solution at first to sufficiently improve their intra- and extracellular viscosity.Only in this way can water molecules have no time to rearrange themselves into the crystalline phase at an ultrarapid cooling rate because of the high viscosity.Water solidifies quickly as soon as it is immersed in LN2.To achieve this vitrification state, oocytes need to experience several incubation steps to gradually increase the intracellular concentration of permeable vitrification solutions in advance.First, oocytes are transferred from the gamete solution to the equilibration solution (ES) containing a permeable component for a few minutes to allow the permeable molecules to flow into oocytes.Then, oocytes are transferred to the vitrification solution (VS) containing a higher concentration of penetration and nonpenetration vitrification solutions for a short time.Finally, oocytes are immersed in LN2for long-term storage.The ES step and VS step are important because they help to create an intracellular vitrification state and an extracellular vitrification envelope.VS contains the nonpermeable component sucrose, and high molecular weight Ficoll is usually used.Once in contact with CPA solutions, oocytes start to dehydrate, and the cytoplasm becomes concentrated, which will promote the formation of an amorphous state.After the vitrification process, oocytes are stored in LN2until they need to be warmed and fertilized.For the warming process, the vitrified oocytes are immediately transferred to a prewarmed (37 °C)solution (containing nonpermeating components at low concentration) and then gradually diluted, and the permeating and nonpermeating components are finally removed.After incubation in the warming solution for 1 min, oocytes were transferred to dilution solution 1 (usually 0.5 mol/L sucrose), then oocytes are transferred to dilution solution 2 (usually 0.25 mol/L sucrose).Finally, oocytes are transferred to wash solution (without nonpermeating and permeating components) for 3-5 min and returned to a nearly original volume by rehydration.At this step, the survival rate can be assessed.The typical temperature and volume changes of oocytes during vitrification are shown in Fig.3a and b, and the typical oocyte vitrification process is summarized in Fig.3c.

Fig.3.Fundamentals of oocyte vitrification.(a) Typical temperature profiles for oocyte vitrification[57].(b) Typical cell volume excursions for oocyte vitrification[57].(c) Typical human oocyte vitrification protocol.
Vitrification can avoid the formation of ice crystals, but it does not mean that there is no danger during oocyte cryopreservation.Especially in the dangerous temperature range(between -15 and -160 °C) in the warming process, small ice crystals (including intracellular ice) grow quickly into larger ice crystals[58].The intracellular ice formed in this term is generally considered lethal to cells[59].The degree of supercooling can lead to the formation of invisible small ice crystals during the cooling process.The growth of these small ice crystals is limited by the small number of nuclei, and nuclei are too cold to grow[60].Once warming starts, these small ice crystals can grow into large lethal crystals at the expense of smaller crystals (recrystallization).Devitrification is another phenomenon that can occur during the warming process,which is the formation of ice during the warming process but not the reversal of vitrification[61].The occurrence of recrystallization and devitrification is much faster during warming, so warming rates must be high enough to exceed the speed of recrystallization and devitrification[62,63].The formation and growth of ice crystals during freezing and rewarming is the main cause of the loss of vitality of biological samples.Ice crystals are inevitable during cryopreservation, so effective control and inhibition of ice crystals are the key to minimizing oocyte damage[64].Some methods to inhibit ice crystal formation have recently been described.
5 Cryo-injury of oocytes
Although oocyte cryopreservation has made significant progress, the cryopreservation procedure still has potentially negative effects on oocyte quality.
5.1 Ultrastructural change
The ultrastructure of oocytes after cryopreservation was examined by electron microscopy (EM), and the results showed that the vacuoles emerged as empty entities with irregular rounding and a bordered membrane[65].The membrane of the Golgi and/or smooth endoplasmic reticulum (SER) may swell and coalesce to form vacuoles.The internal displacement of other organelles, such as mitochondria, may also be the origin of vacuoles[65-69].It is noteworthy that vitrification carriers may be related to the occurrence of vacuoles in vitrified oocytes, with different carriers exhibiting different thermal conductivities as they are modified by numerous materials and designs (such as closed and open carriers).Open vitrification carriers have a faster cooling rate than closed vitrification carriers.In addition, the thermal isolation of closed vitrification carriers requires a longer time to discharge the vitrified oocytes into the warming solution.Bonetti et al.[53]reported the ultrastructural characteristics of vitrified human oocytes with two different carriers (Cryotop and Cryotip), and they observed that using the closed Cryotip carrier group resulted in more severe ultrastructural characterization than using the open Cryotop carrier group.As a result, the presence of vacuoles, as a nonspecific response to cryoinjury[70], may indicate that there was cryodamage during the vitrification and warming processes.
Golgi-derived, membrane-bound cortical granules (CGs)are organelles that contain peroxidase, glycosaminoglycans,proteases, and acid phosphatases, and CGs are formed during the early stage of oocytes[66].CGs are evenly distributed in the cytoplasm during the germinal vesicle (GV) stage and spread outward during the MII stage.After fertilization, the contents of CGs are massively released and diffused into the perivitelline space (PVS), causing the subsequent hardening of the zona pellucida (ZP) so that supernumerary spermatozoa cannot easily penetrate oocytes[71,72].Cryopreservation induces the premature release of CGs, which alters the structure of the ZP, hardening the ZP, altering membrane permeability, premature oocytes, and reducing the fertilization rate of vitrified oocytes[71,72].In addition, the calcium content in human and mouse oocytes can be increased by using various CPA solutions.Larman et al.indicated that PROH could cause a protracted increase in calcium levels and then promote the release of CGs, consequently causing zona pellucida hardening and cellular degeneration[73-75].
For proper chromosomal segregation to occur throughout future embryo development, the alignment of chromosomes by the meiotic spindle is essential.The spindle of oocytes at the MII stage, however, is very sensitive to temperature fluctuations[76].Even cooling mouse oocytes to room temperature negatively affects the spindle and results in abnormal spindle conformation[77,78].In addition to being exposed to low temperatures, the spindle also suffers from ice crystals that form during the cryopreservation process[70].Abnormal spindles will affect the expulsion of the second polar body and the migration of prokaryotes[79].Equatorial chromosome alignment and normal bipolar spindle configuration were only seen in a small portion of vitrified-warmed oocytes (32.6%), which was lower than the fresh oocytes (59.1%), according to Coticchio et al[80].Additionally, activating lysosomal cathepsin B during vitrification may affect the function of the spindle assembly checkpoint in mouse oocytes[49].
5.2 Exposure to CPA solution
Both the permeating and nonpermeating CPA components must be present in high concentrations to achieve a vitrification state[81-83].However, high concentrations of CPA solution can cause osmotic stress and toxic effects and affect the structure and function of organelles and membranes[84,85], hence inducing detrimental consequences to oocytes[86].Some CPA components even damage DNA integrity[87].Three commonly used permeating CPA components for oocyte vitrification,ethylene glycol (EG), dimethyl sulfoxide (DMSO), and propylene glycol (PROH), have been evaluated for their genotoxic effects on oocytes[88].It was found that PROH significantly intensifies DNA damage in oocytes[87,88].Hu et al.[89]investigated the effects of DMSO and PROH on bovine oocyte vitrification.The damage in the PROH group was higher than that in the DMSO group, and the researchers suggested that PROH triggered more DNA methylation, which might be directly related to imprinting disorders.In addition, it is important to note that the permeability of PROH is lower than that of DMSO and EG[88], so the intracellular cryoprotective effect is lower than that of the other two CPA components for the same incubation time.
In the future, new effective CPAs should be developed for application to oocyte cryopreservation instead of using the above methods.
5.3 Epigenetic effect
Epigenetic modification, including DNA methylation and histone modification, can change the functional state of chromosomes and regulate gene expression.The vitrification process of oocytes, which involves exposing oocytes to protective agents and cooling them, can cause epigenetic changes[90-94].More research is needed to detect the influence of vitrification oocytes on epigenetic effects.
5.4 Decreased physiological function
Mitochondria are one of the most abundant organelles in oocytes, and their dysfunction has a negative effect on embryo developmental potential[95].Lei et al.[96]checked the function of mitochondria in human matured oocytes after vitrification and warming.Their results showed a sharp decrease in inner mitochondrial membrane potential (MMP) in MII oocytes compared with the control (1.019 vs.1.397).Zander-Fox and coworkers revealed that after 2 h of culture, vitrified mouse oocytes cannot recover the normal mitochondrial distribution and membrane potential state[97].
6 Approaches to improve the efficiency of oocyte cryopreservation
6.1 Approaches that have been taken in oocyte cryopreservation
6.1.1 CPA loading
CPA solution plays an important role in cryopreservation procedures.Although completely avoiding ice formation during cryopreservation is impossible, lowering the chilling injury of cryopreserved oocytes could be realized.
Today, the components of CPA solutions can be divided into two main categories: penetrating and nonpenetrating.The former probably shows cell toxicity at high concentrations and should be limited in their dosage in clinical applications[98-101].The commonly used penetrating CPA components for oocytes are DMSO, EG, PROH, and acetamide.The latter is not permeable, and sucrose is the most popular nonpenetrating CPA component used in oocyte cryopreservation.Nonpenetrating components can cause the dehydration of oocytes and reduce the required concentration of penetrating CPA components.Initially, Rall and Fahy[30]used DMSO,acetamide, PROH and PEG to vitrify mouse embryos.Then,Ali et al.[102]investigated different combinations of these CPA components, and their results confirmed that EG and glycerol had the least toxic effect on mouse morula compared with other components.These CPA components were widely used to cryopreserve mouse and sheep embryos without significantly affecting their viability, and then they were used to cryopreserve oocytes.In 2000, Chen et al.[103]vitrified human oocytes and concluded that the rates of survival, fertilization,and early embryo cleavage were improved with the above formula.
6.1.2 Antioxidant
Cryopreservation leads to a significant accumulation of reactive oxygen species (ROS) in oocytes, which injures the physiological function of the endoplasmic reticulum and mitochondria due to oxidative stress[44].Excessive oxidative stress may be the reason for the quality reduction of vitrifiedwarmed oocytes[104].There are three main sources of ROS: superoxide radicals (O2•-), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH)[41].H2O2selectively reacts with other molecules and binds to some enzymes, such as glutathione,producing water and oxygen[105].H2O2can also produce•OH.•OH can interact with anything around it, oxidizing amino acids and damaging protein conformation[106].The endoplasmic reticulum can release a large number of calcium ions(Ca2+), and then Ca2+spreads into the cytoplasm when CPA solution such as DMSO is present.The subsequent increase in Ca2+uptake by mitochondria further promotes the formation of ROS, and low temperature can subsequently enhance this process in the presence of CPA solution[107].Light, especially blue light used in laboratory conditions, can also cause oxidative damage by stimulating cells to produce H2O2[108,109], and microscopy takes over up to 95% of the damaging radiation[110].The main endogenous nonenzymatic antioxidants in oocytes include cysteine (CYS) and cysteamine(CSH)[44].Nevertheless, the intracellular synthesized GSH decreased after vitrification, even when exogenous CYS or CSH was loaded, as compensation could not restore the blastocyst development rate of bovine oocytes[111].Therefore, it is necessary to supplement with the proper exogenous antioxidants during oocyte cryopreservation to reduce cryo-oxidative injuries, since endogenous nonenzymatic antioxidants cannot totally abate the cryopreservation-inflicted ROS alone[44].Melatonin (MLT) and its metabolites can regulate the expression of antioxidant enzyme genes such as catalase and gluta-thione peroxidase, acting as potent direct scavengers of free radicals and indirect antioxidants[112].According to previous studies, adding 10-9mol/L MLT to the vitrification solution can improve the quality of frozen bovine oocytes, lower the level of oxygen free radicals in bovine oocytes, and improve the developmental capacity[113].Wu et al.[114]discovered that when 10-7mol/L MLT was given throughout the CPA solution loading stage, the percentage of oocytes matured from the GV stage to the MII stage was significantly improved, and other indicators, such as mitochondrial membrane potential and ROS level, also noticeably increased and nearly returned to the normal fresh state compared to the untreated group.Applying MLT to human oocytes has also achieved remarkable outcomes.Zhang et al.[43]revealed that MLT could enhance the efficiency of vitrification in human oocytes by reducing oxidative stress and sustaining the permeability of the oolemma.A 10-9mol/L MLT addition could preserve the normal morphology (uniform cytoplasm and no vacuoles) of human oocytes, improve the survival rate and protect mitochondrial function after cryopreservation.MLT inhibits the process of early apoptosis by directly reducing ROS levels and improving MMP after warming to protect mitochondrial function in a nonreceptor-mediated manner instead of a receptormediated one.To reduce oxidative damage and improve the quality of oocytes after cryopreservation, additional antioxidants, such as resveratrol[115]and coenzyme Q10[104], can also be loaded into oocyte CPA solution.Therefore, adding more antioxidants during cryopreservation is a good strategy to increase the frozen quality of oocytes.
6.1.3 Antifreeze protein or mimic
In nature, there are many organisms living in the cryosphere[116].These hardy creatures include Alaskan tree frogs, polar fish, desert beetles, and more.To protect themselves from cold environments, they developed unique approaches, that is, ice-binding protein (IBF).It is known that IBPs produced by these organisms have a special ability to bind ice crystals and block ice growth[117].Therefore, it can protect these creatures from freezing damage.The IBP typically functions in a noncolligative way, with protein specificity depressing the freezing point.IBP, also known as antifreeze glycoprotein (AFGP), was originally found in the bodily fluids of polar marine fish in 1969 by DeVries and his colleagues[48].They isolated and purified it initially and found that this protein can lower the freezing point of fish fluids.When ice crystals formed inside the fish, IBP quickly bound to the crystal, thus preventing the addition of other water molecules from being added to the plane of crystals, thereby inhibiting the growth of ice crystals[118].After that, a variety of AFGPs were found in insects[119,120], plants[121]and microorganisms[122].According to certain theories, AFGPs consist of two different faces, the ice-binding face (IBF) with affinity to the ice plane and the nonice-binding face (NIBF) (Fig.4a)without affinity to the ice plane[123,124].Molecular dynamics show that IBF preferentially attaches to the ice plane, whereas the disordered free water layer on the NIBF prevents the growth of ice crystals (Fig.4b)[124].IBF prefers binding ice surfaces, which alters the curvature of the ice plane, influences the rate of ice growth and depresses the freezing point,causing so-called adsorption-inhibition.AFGPs can reduce the freezing temperature (Tf) but hardly affect the ice melting temperature (Tm), resulting in a gap betweenTfandTm, which is the so-called thermal hysteresis (TH) phenomenon(Fig.4c)[128].Living organisms can take advantage of AFGP to control TH so that they can protect themselves from fatal ice growth and survive when the ambient temperature is within the TH gaps[123].Different sources of AFGPs have different THs, as shown in Fig.4d.AFGPs can shape ice (Fig.4e-g)[129]and regulate special ice morphology for the capacity of binding to the basal or prism plane of ice.After binding, the free water molecules are prevented from adhering to the ice plane,and ice growth rates are also decreased.Ice recrystallization inhibition (IRI) activity is another characteristic of AFGPs.Ice recrystallization, a kind of Ostwald ripening, is the process in which smaller ice crystal sizes gradually increase over time.This phenomenon causes osmotic stress, leading to cell dehydration and functional damage.Ice recrystallization may also threaten cell survival by producing fatal damage during the thawing process of cryopreservation[13,130,131].Based on the aforementioned special properties, AFGPs have emerged as a promising CPA component for cryopreservation.O’Neil et al.[132]found that supplementation with 1 mg/mL antifreeze glycoproteins can significantly enhance the fertilization rates of mouse oocytes that have been cryopreserved.Jo and colleagues[133]confirmed that type III AFP had a protective effect on the cryopreservation of mouse oocytes, strengthening oocyte resistance to freezing damage.According to their findings, when type III AFP was added to CPA solution, the survival rate of the type III AFP-treated groups (500 ng/mL) significantly exceeded that of the untreated groups (97.1% vs.91%).The cleavage and blastocyst formation rates following the IVF procedure were also higher in the AFP-treated group than in the non-AFP-treated group (91.5% and 81.4% vs.69.8% and 67.1%, respectively).Natural AFGPs are produced primarily to protect their microenvironments from cryoinjury, and while they do have unique ice crystal control and modifying properties, they are not necessarily suitable for cryopreserving all biological samples.AFGPs also have some drawbacks, including high production costs, potentially negative effects on immunogenicity and toxicity, and induction of the formation of harmful needle-shape ice[134].Therefore, it is essential to design and find new materials that can effectively control ice, such as AFGPs.Currently, significant processes have been made in designing and synthesizing AFGP mimics, and they have been used well in cryopreservation.It was discovered that L-proline oligomers share a similar polyproline II helix structure as AFGP8 and can also inhibit ice recrystallization as AFGP8.In 2020, Qin et al.[127]employed L-Pro8as a CPA component to cryopreserve mouse oocytes.Their results revealed that L-Pro8can inhibit the growth of ice.The amount of DMSO and EG required for oocyte cryopreservation can be significantly reduced by adding 50 mmol/L L-Pro8.The survival rate of oocytes after cryopreservation with or without L-Pronis shown in Fig.4i.The mitochondrial function of the frozen-thawed oocytes with the addition of L-Pro8was also improved, as shown in Fig.4j.Fig.4h shows the IRI activity of L-Pro, L-Pron, DMSO and EG in PBS, and we can easily see that L-Pro8possesses strong IRI activity compared to DMSO and EG.Ectoine is a natural component found in extremophilic bacteria that can adapt to extremely harsh environments as well as osmotic stress conditions.This property is related to the prior exclusion of ectoine to the interface of a lipid monolayer of the cell membrane[135], and ectoine can play a protective role.As a bioinspired permeating CPA that does not interfere with normal cellular activities[136], Choi et al.[137]used ectoine as a bioinspired CPA component to replace DMSO and vitrified human oocytes.The survival results confirmed that there was no significant difference between the ectoine group (94%)and DMSO (97%) group.Overall, a wide range of bioinspired materials have been developed to improve the efficiency of oocyte cryopreservation.

Fig.4.The ice regulation properties of AFGPs and the application of AFGP mimics.(a) The model of IBF and NIBF of AFGP.(b) The side view of IBF from TmAFP contact with ice plane by molecular dynamics.(a, b) Reproduced with permission from Ref.[124].Copyright 2016, the National Academy of Sciences of the United States of America.(c) Thermal hysteresis (TH).Reproduced with permission from Ref.[125].Copyright 2014, Elsevier.(d)The relationship between TH and AFGPs from different origins[126].(e) The shape of a single ice crystal in Tris buffer.(f) The shape of a single ice crystal in buffer containing MpdAFP along the a-axis.(g) The shape of a single ice crystal in buffer containing MpdAFP along the c-axis.(e-g) Reproduced with permission from Ref.[124].Copyright 2016, the National Academy of Sciences of the United States of America.(h) The IRI activity of different CPAs.(i) Survival rate of mouse oocytes.(j) JC-1 staining to measure the MMP of vitrified oocytes.(h-j) Reproduced with permission from Ref.[127].Copyright 2020, American Chemical Society.
6.1.4 Magnetic field
The static magnetic field (SMF) can act as a CPA component to protect cells from chill injury and can reduce the concentration of other CPA components[138,139].Studies have shown that the phospholipid bilayer of the cell membrane can align under the influence of SMF[140], and SMF can result in a reduction in membrane fluidity and an improvement in membrane rigidity, even if the applied magnetic induction is less than 1 T[141].There have been some advances in using SMF to cryopreserve oocytes.Exposure to SMF after the last VS procedure seemed to improve the quality of vitrified-warmed mouse oocytes[142]because both the mitochondrial function and ultrastructure of oocytes improved, as well as the embryo cleavage rate.
Electromagnetic fields can influence the size of ice crystals, preventing fatal cryodamage to cells and tissues[143,144].Sun et al.[145]studied the connection between the electromagnetic field frequency at 1-200 kHz and the rate of ice crystal growth.Their results showed that the field’s effectiveness in preventing freezing injury was frequency dependent.Ice size and freezing time were reduced as frequency increased.The smallest ice crystals were produced when the frequency was set to 50 kHz.An external electromagnetic field prevents the formation of ice nucleation and destroys the interaction between water molecules, influencing the mobility of water since it can directly impact the water freezing kinetics and further regulate the formation and growth of ice crystals during cooling, which can avoid the formation of fatal large crystals.
The warming process is significantly accelerated by applying an external magnetic field.The quality of cryopreserved samples is significantly influenced by the warming rate[58].The occurrence of recrystallization and/or devitrification during the warming process will result in fatal cryoinjuries if the warming rate is insufficiently rapid for cryopreserved samples.An external magnetic field can significantly enhance the thawing efficiency.Magnetic nanomaterials such as iron oxide nanoparticles (IONPs) can be added to CPA solution before the sample is cooled and transferred into LN2.The sample can be rewarmed in an alternating magnetic field with a radio frequency (RF) coil, where IONPs are excited by magnetic hysteresis and produce rapid and uniform heating,defeating the convective (boundary) warming rate that fails to cryopreserve the sample by forming crystallization and/or cracking[146,147].
6.1.5 Laser field
The near-infrared (NIR) laser with nanoparticles that possess photothermal conversion performance[148,149]can act as a heating platform to achieve an ultrarapid heating effect and decrease cryoinjury by avoiding devitrification and recrystallization during the warming process[150].In recent years, nanoparticles with high photothermal conversion efficiency have been on the rise in the biomedical engineering field, and their applications include cancer treatment, drug release, bioimaging and so on[150-152].Although laser light is usually attenuated at the surface of the system[153], nanoparticles (NPs) with high photothermal conversion efficiency can absorb many orders of magnitude of typical lasers.The absorbed energy can be transferred to the heat energy of NPs or re-emitted at the same frequency or at a shifted frequency[154,155].Fig.5a shows the laser warming device, and Fig.5b shows the possible mechanism by which lasers improve the efficiency of oocyte cryopreservation.
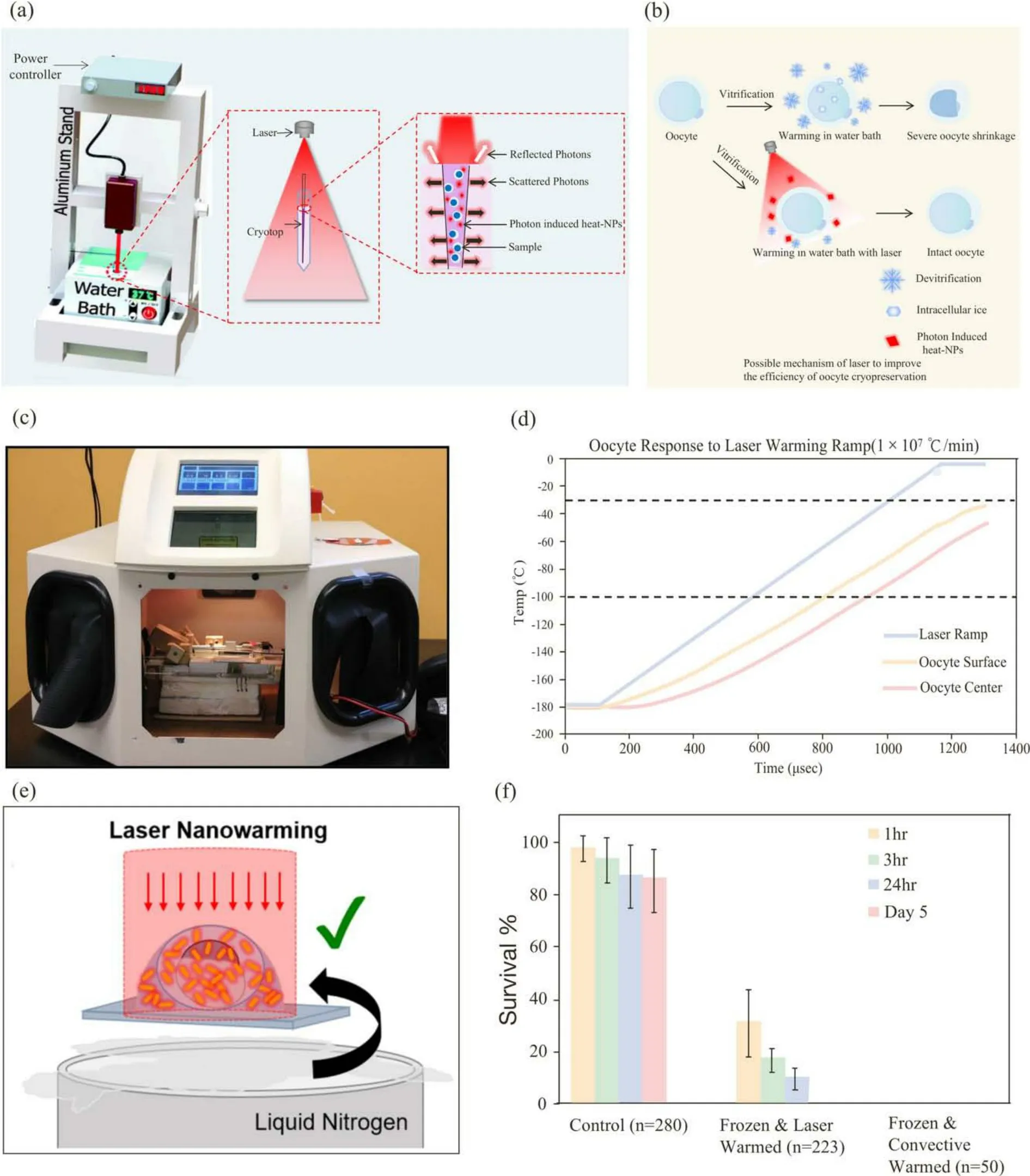
Fig.5.Laser heat suppressed ice recrystalization and devitrification for cryopreservation.(a) Laser warming device.Reproduced with permission from Ref.[148].Copyright 2018, the Royal Society of Chemistry.(b) The possible mechanism of laser improving the efficiency of oocyte cryopreservation.(c) Overview of laser chamber.(d) Thermal response of oocytes when subjected to laser-induced warming rates of 1 × 107 °C/min.(c, d) Reproduced with permission from Ref.[156].Copyright 2014,Elsevier.(e) Laser nanowarming with gold nanorods.(f) Comparison of the survival rate of cryopreserved zebrafish embryos.(e, f) Reproduced with permission from Ref.[157].Copyright 2014, Elsevier.
The speed of warming rates is more important than the cooling rate during cryopreservation[158].In 2014, Jin et al.[156]added carbon black into mouse oocyte CPA solution.Carbon black is a black body that absorbs all wavelengths, and μmsized carbon black particles absorb the energy of laser IR and are abruptly heated.This carbon black could transfer this heat to the surrounding solution, which in turn heats oocytes.Using the laser field, the rewarming rate of mouse oocytes could be rapidly increased (1.0×107°C/min), which significantly improved the survival rate of oocytes after freezing.The outcome of the calculations showed that oocytes can be quickly and evenly recovered by laser-induced heat, as shown in Fig.5d.Fig.5c shows the laser chamber in which the Cryotop holder was placed.
The gold NPs can highly generate heat when the laser wavelength matches the surface plasmon resonance energy of gold NPs[157].Once the laser is resonant, it generates vibrations in the gold NPs, which causes heat dissipation so that the temperature of the system increases rapidly.Zebrafish embryos can achieve a vitrifying stable state in LN2by cooling them with ultrafast speed, but it was difficult to successfully rewarmed because zebrafish embryos have a large volume (800 μm diameter).The convective warming was too slow for zebrafish embryos, so it easily led to failure[156].Nanowarming platforms with laser fields can provide a faster warming rate by using laser heat to accelerate the warming process of zebrafish embryos.In 2017, Khosla et al.[157]successfully cryopreserved zebraish embryos by loading 2 pmol/L gold rods into the zebrafish embryo and surrounding CPA solution and rewarmed this embryo with a 1064 nm laser pulse, improving the rewarming rate to 1.4×107°C/min.Experimental results showed that a warming speed of 1.4×107°C/min can effectively suppress ice growth.Fig.5e shows an overview of the laser rewarming process.Fig.5f shows the results of cryopreserved embryos after warming by laser or traditional water convection.
6.2 Approaches that have the potential to improve the efficiency of oocyte cryopreservation
As “living cell factories”, cell-loaded hydrogel beads/fibers and coreshell structured capsules/fibers have proven to be powerful tools for 3D cell culture, cell-based therapy, tissue engineering and cryopreservation[45].Hydrogels can be used in a 3D microenvironment for cell culture because their physical and chemical properties are similar to those of the native extracellular matrix (ECM)[159,160].For example, alginates can be cross-linked to form a hydrogel network by divalent cations.Due to their advantages, such as compatibility, mild cross-linking conditions, and low cost, hydrogels have received increasing attention[13].Moreover, hydrogels can also be used in the cryopreservation field.Once encapsulated, extracellular ice will be inhibited, and chilling damage will be reduced.Researchers found that using hydrogels to encapsulate cells can support vitrification and prevent the occurrence of devitrification during the thawing process.In 2022, Tian et al.[161]cryopreserved encapsulated mouse preantral follicles combined with nanowarming technology and increased viability by 33%.Therefore, we believe that it may be an effective method to encapsulate oocytes with hydrogels for cryopreservation.
7 Conclusions and perspective
Oocyte cryopreservation can light the hope of young women who may lose reproductive function for treatment reasons and/or surgery for cancer.There are some other situations that may benefit from oocyte cryopreservation, such as building oocyte banking for donation, helping women who are not ready to try pregnancy but are afraid of declining ovarian reserve, and preventing ovarian hyperstimulation syndrome(OHSS).It can also solve some cases, including the inability of the male partner or the lack of available semen aspiration techniques.Currently, oocyte cryopreservation is an effective technology with widespread indications and provides many conveniences to human ART, but it is clear that cryopreserved oocytes are still not as effective as fresh oocytes.In addition, although short-term safety data are proven to be secure, long-term data on children born from cryopreserved oocytes are still ongoing.We should be careful in every cryopreservation procedure and try our best to improve its consistency, efficiency, and safety as soon as possible.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82172114) and the Anhui Provincial Natural Science Foundation for Distinguished Young Scholars(2108085J37).
Conflict of interest
The authors declare that they have no conflict of interest.
Biographies
Shiyu Zhaois a doctoral candidate of the Department of Electronic Engineering and Information Sciences, University of Science and Technology of China, under the supervision of Prof.Gang Zhao.Her research focuses on oocyte cryopreservation.
Gang Zhaoobtained his Ph.D.degree from the University of Science and Technology of China.He is currently a Professor at the University of Science and Technology of China.His current research focuses on cryobiomedical engineering, micro- and nano-technologies, and biosensors.
杂志排行
中国科学技术大学学报的其它文章
- 中文概要
- An abnormal multidrug-resistant and hypervirulent Klebsiella pneumoniae clinical isolate without rmpA or rmpA2
- Dynamics in the assembly of the 30S ribosomal subunit investigated by coarse-grained simulations
- Construction of an M1 macrophage-related lncRNA signature for predicting the tumor immune microenvironment
- Modulating miRNA binding sites within circRNA for enhanced translation efficiency
- The function, structure and dynamic organization of centromeres and kinetochores
