补光对设施栽培樱桃果实成熟和糖分积累的影响
2023-10-27李都岳吴延军
李都岳 吴延军
DOI:10.13925/j.cnki.gsxb.20230114
摘 要:【目的】探究不同補光处理对樱桃果实的影响,筛选设施栽培樱桃最佳人工补光方案。【方法】以设施栽培的中国樱桃诸暨短柄与黑珍珠为材料,从果实硬核期到采收期间,分别使用不同功率的LED灯光源、白炽灯光源、商品补光灯光源进行补光,以自然光照作为对照,对果实内源激素、相关酶活性与基因表达进行测定。【结果】发现采用红蓝光比例6∶1的LED光源补光显著促进了果实成熟进程,能够使果实成熟过程中内源激素动态变化趋势较对照组提早出现,并且显著提高了成熟软化与糖合成基因的表达,使果实蔗糖合成酶活性比同期对照组提高18.75%以上。【结论】功率36 W的LED补光处理效果最好,显著促进了果实成熟进程,提高樱桃糖分积累量,值得在樱桃生产中应用和推广。
关键词:樱桃;补光;LED;成熟;糖分积累
中图分类号:S662.5 文献标志码:A 文章编号:1009-9980(2023)10-2183-12
Effects of supplementary light on ripening and sugar accumulation of cherry under protected cultivation
LI Duyue, WU Yanjun*
(Institute of Horticulture, Zhejiang Academy of Agricultural Science, Hangzhou 310021, Zhejiang, China)
Abstract: 【Objective】 Because of the subtropical monsoon climate in most areas of southern China, the protected cultivation is widely applied in cherry production to reduce the impact of the large precipitation in spring. There are still problems such as weak light intensity, short light duration and uneven light distribution in protected cultivation environment. Therefore, improving the light environment of facility cultivation through artificial light supplement technology has important practical significance for regulating the growth and development and improving yield and quality of cherry. In this study, different supplementary light measures were compared and the best scheme for protected cultivation of cherry was selected. 【Methods】 The Chinese cherry cultivars Zhujiduanbing and Heizhenzhu were used as materials in this study. Four different types of light sources were used: LED lamp (36 W, RB 6︰1), LED lamp (50 W, RB 6︰1), commodity plant growth lamp (12 W, RB 2︰1, G101) and incandescent lamp (36 W), with no artificial light as the control (CK). All the groups were treated with mulching the ground with reflective film. The distance between the lights was 3 m, and the height from the top of the tree was 50 cm. Time of light supplement was set to 05:00—10:00, 16:00—19:30 on sunny days and 5:00—19:30 on rainy days. The contents of endogenous hormones GAS, IAA and ABA, the percentage of soluble solids, and the enzyme activities of peroxidase (POD), sucrose synthase (SS) and sucrose phosphate synthase (SPS) in cherry flesh were measured from the color transition stage to the harvest stage. The expression levels of genes related to ripening, softening, and sugar synthesis in fruits were also measured in the harvest stage. 【Results】 Each supplementary light treatment caused the dynamic change of endogenous hormones in the fruit earlier than control, and promoted the fruit development and maturation process of the two varieties. The comprehensive effect of LED light treatment was the best, resulting in the changing process of endogenous hormones GAS and ABA in the fruit earlier than control. Supplementary light treatments also increased the TSS contents of two cherry cultivars at harvest time. LED light treatments had the most significant effect on improving TSS content, which made the TSS of Zhujiduanbing fruit reach 17.63%, and made the TSS of Heizhenzhu fruit reach 14.67%. The increase of sucrose synthase activity in the fruit of each supplementary light treatment was earlier than control, thus promoting the accumulation of sugar in the fruit. Among them, LED supplementary light treatments had the most obvious promotion effect. LED 36 W treatment made the sucrose synthase vitality of Zhujiduanbing fruit reach a peak 3 days before harvest, which was 69.17% higher than the control during the same period, and increased the sucrose synthase activity of Heizhenzhu fruit by 18.75% compared to control during harvest. Overall, LED 36 W treatment had the best effect, and gene expression levels were measured for this treatment. It was found that the expression of the ABA synthase gene NCED1 was significantly higher with LED 36 W treatment than that with control, which regulated the levels of endogenous hormones related to fruit ripening at the molecular level. The relative expression levels of polygalacturonase gene PG1 and xylanase gene XYL1, which can regulate cell wall degradation, were also significantly higher with this treatment than those with the control, indicating that this treatment could promote fruit softening by positively regulating the process of cell wall degradation. The determination of anthocyanin synthesis pathway genes PAL, CHS, ANS and transcription factor MYB10 in the pericarp during harvest showed that the expression levels of CHS, ANS and MYB10 in Zhujiduanbing pericarp treated with LED 36 W were significantly higher than the control. And in Heizhenzhu pericarp, the expression levels of all four genes with LED 36 W treatment were significantly higher than those with the control. LED 36 W treatment activated the synthesis of anthocyanins in cherry pericarp by activating high-level expression of synthesis pathway genes and transcription factors at the molecular level, thereby promoting the coloring of cherry pericarp. The relative expression levels of sucrose synthase genes SS1, SS6 and sucrose phosphate synthase gene SPSA1 in fruit during harvest were also analyzed. The results showed that with the two cherry cultivars, LED 36 W treatment significantly increased the expression level of SS1 gene in fruit compared to the control, indicating that SS1 played a major role in responding to supplementary light treatment. LED 36 W treatment positively regulated the synthesis of sucrose synthase by activating the expression of SS1, thereby promoting sugar accumulation in cherry fruits through sucrose synthase. 【Conclusion】 All supplementary light treatments promoted the dynamic changes of endogenous hormones during cherry fruit ripening, and increased the level of soluble solids content in the fruit. Among them, LED (36 W, RB 6∶1) supplementary light treatment could advance the endogenous hormone change process of two Chinese cherry cultivars by 3 to 9 days, increase the activity of sucrose synthase in the fruit by more than 18.75% compared to the control during the same period, and significantly improve the expression of genes related to fruit ripening and softening, sugar synthesis, and anthocyanin synthesis at the molecular level, which had the best effect on promoting cherry ripening and improving fruit quality. It is worthy of application and promotion in cherry practical production.
Key words: Cherry; Supplemental light; LED; Ripeness; Sugar accumulation
中国樱桃(Prunus pseudocerasus L.)是蔷薇科李亚科李属植物,起源于中国,是世界四大樱桃栽培种之一[1],果实富含多种维生素和矿质元素[2],深受消费者的喜爱。在浙江省等中国南方地区樱桃生产中,广泛采用设施栽培以减轻高温多雨环境对樱桃生长的影响[3]。传统设施栽培可以调控环境因子,但相对于自然条件还存在设施环境下光照度弱、光照时间短、光照分布不均[4]等问题。光照条件不足会导致作物营养生长受限[5]、果实发育缓慢[6]。因此,通过人工补光技术改善设施栽培的光照条件,对于调节作物的生长发育、提高产量、改善果实品质等具有重要的实践意义[7-9]。目前关于中国樱桃设施栽培中的补光技术研究尚未见报道。
研究表明,光照条件是影响植物的生长发育过程的重要环境因子[10],光照不仅为光合作用提供能量以促进植物营养生长,还影响植物生殖生长的整个阶段,参与调控作物品质形成等诸多方面[11-13]。目前,在蔬菜和柑橘等果树的设施栽培中,传统光源和LED新型光源的补光技术对有效延长光照时间、调控光质具有积极作用,能够促进果实的发育成熟,并提升其产量和品质[14-16]。此外,LED补光光源还能够影响作物的内源激素含量,显著提高葡萄、水稻等作物的产量和抗逆性[17-18]。光照环境差是影响浙江省樱桃生产的关键问题,当地春季梅雨季节降水量大,连续阴雨天气和避雨栽培所使用的薄膜等材料对设施透光率的影响,导致樱桃果实发育过程中自然光照条件不足,果实品质与产量受到影响。因此,开发合适的人工补光技术改善光照环境是樱桃生产中的迫切需求,该技术能有效提高设施内光照度,有利于促进果实发育成熟,增产增收。
诸暨短柄与黑珍珠是浙江省中国樱桃主栽品种[19],笔者在本研究中以诸暨短柄与黑珍珠為试验材料,选择传统白炽灯、商品补光灯“G101型激光植物生长灯”与红蓝光LED灯作为补光处理手段,探究不同的补光处理对果实成熟和糖分积累的影响,以期为开发设施栽培樱桃补光技术提供理论参考依据。
1 材料和方法
1.1 供试材料
试验于2022年4月在浙江省杭州市桐庐县樱桃园进行。供试品种为设施栽培的中国樱桃诸暨短柄和黑珍珠。诸暨短柄樱桃是由浙江省农业科学院等单位从中国樱桃地方品种中选育出的优株,1992年通过品种审定。黑珍珠樱桃是中国樱桃的芽变优株,1993年由重庆南方果树研究所选出。
1.2 试验设计
补光光源采用4种不同的补光灯类型:LED灯(36 W,RB 6∶1)、LED灯(50 W,RB 6∶1)、G101型激光植物生长灯(12 W,RB 2∶1)和白炽灯(36W),以不补光为对照(CK)。补光灯间距3 m,距离树顶高度50 cm,地表铺设反光膜。补光时间设置为晴天每天05:00—10:00、16:00—19:30,阴雨天每天05:00—19:30。
1.3 测定方法
于2022年4月13—21日两种供试材料果实转色期至采收期,间隔3~6 d取样,每次取样随机选取各处理3株植株共9个果实,用高效液相色谱法测定果肉GAS、IAA、ABA含量,3次重复,取平均值。用手持糖度计测定可溶性固形物含量。POD、SS、SPS酶活性的测定参照Solarbio公司的试剂盒说明书进行。
樱桃果皮与果实总RNA的提取采用优化的CTAB-LiCl法,合成cDNA第一链参照Beyotime公司的反转录试剂盒说明书进行。根据NCBI上查找到的同源性较高的樱桃的NCED1、PG1、XYL1、PAL、CHS、DFR、ANS、UFGT、MYB10、Riant、SS1、SS6与SPSA1,利用Primer 5.0软件设计特异引物(表1),使用实时荧光定量RT-PCR测定樱桃在采收期各基因的表达量。
1.4 数据统计分析
采用Excel 2013进行数据初步处理,采用SPSS 18.0进行差异显著性分析(p<0.05)。
2 结果与分析
2.1 补光处理对果实成熟进程的影响
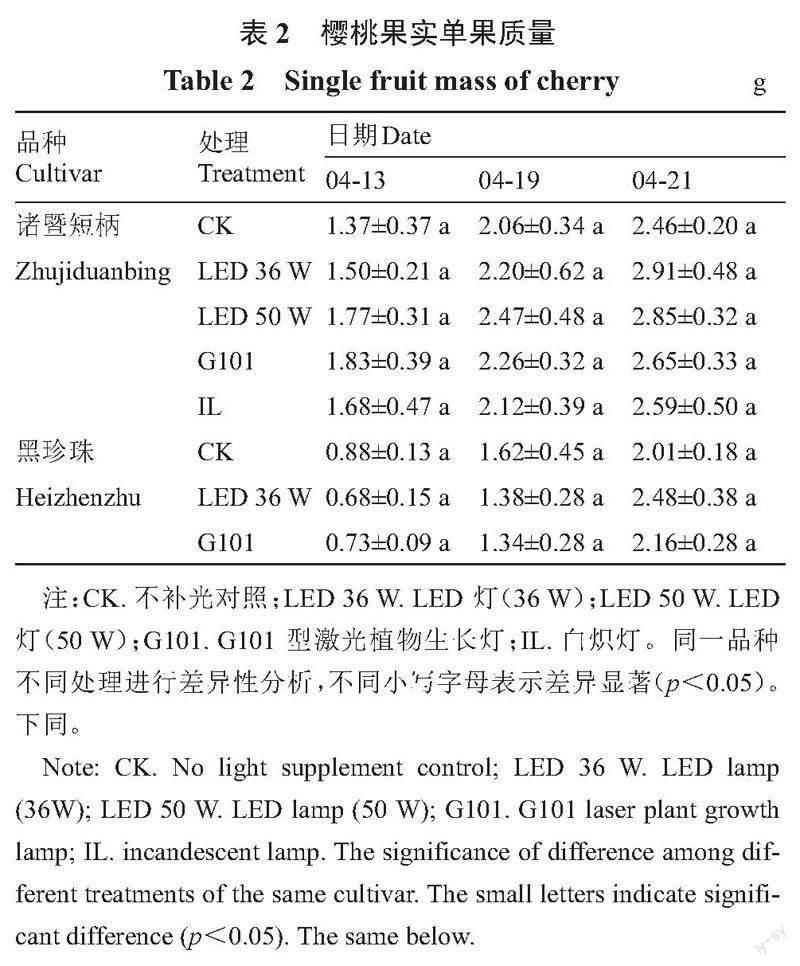
2.1.1 补光处理对单果质量变化的影响 补光处理对樱桃的果实单果质量增加具有促进作用(表2)。其中LED 36 W补光处理效果最好,使两个品种中国樱桃果实单果质量上升幅度大、采收期单果质量高。该处理下诸暨短柄单果质量在采收期达到2.91 g,比对照组高18.29%,黑珍珠单果质量在采收期达到2.48 g,比对照组高23.38%。
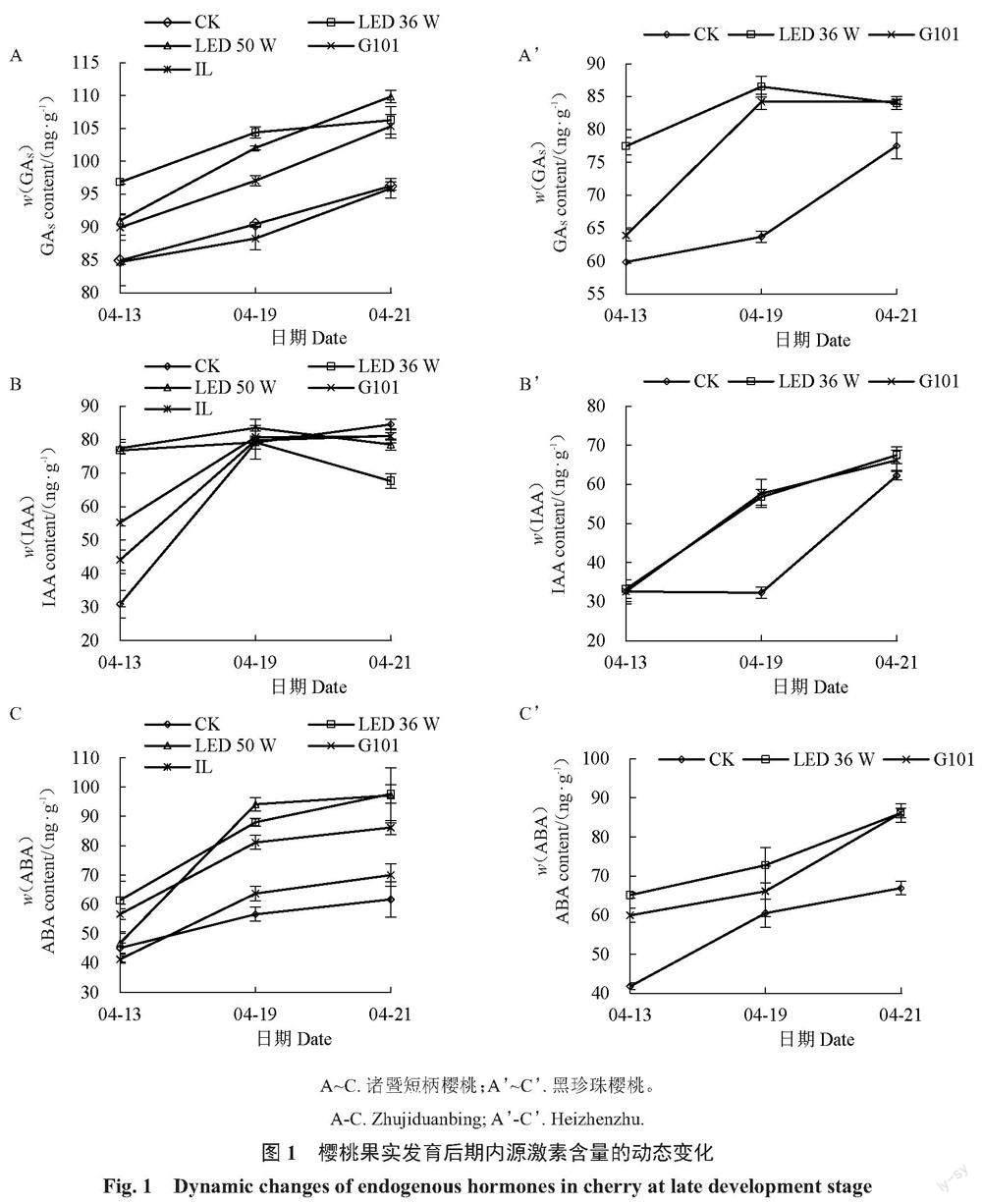
2.1.2 补光处理对果实内源激素含量的影响 内源激素含量是标志成熟进程的重要指标,研究中发现两个樱桃品种果实发育后期,果实内源激素GAS/IAA/ABA总体呈现上升趋势,补光处理对两个品种樱桃的内源激素含量动态变化进程具有不同程度的促进作用(图1)。在诸暨短柄果实中,内源GAs与ABA含量在采收前持续上升,两种LED补光灯与G101型激光植物生长灯处理的果实中GAS与ABA含量在采收前9 d内持续高于对照组。而LED补光处理的果实IAA含量上升在采收前9 d达到最高点,随后波动,比对照组和其他补光处理提前6 d左右。在黑珍珠果实中,LED补光灯与G101型激光植物生长灯处理的3种内源激素含量上升也均提前于对照组,其中GAS含量在采收前3 d达到高点并波动,比对照组早3 d以上。整体上,各补光处理使果实内源激素动态变化趋势提早于对照组,促进了两个品种的果实发育成熟进程,其中LED补光处理综合效果较好。
2.1.3 补光处理对樱桃成熟软化相关基因的影响 根据内源激素动态,选择促进果实成熟进程效果最明显、两个樱桃品种均具有的LED 36 W补光处理组,进行成熟软化相关基因检测,与对照组进行比较,发现LED 36 W补光处理在基因水平上促进了两个品种果实的成熟和软化(图2)。采收期果实中ABA合成酶基因NCED1在LED 36 W补光处理后表达量显著高于自然光照对照组,在分子层面调控果实成熟相关的内源激素水平。而调控细胞壁降解的多聚半乳糖醛酸酶基因PG1、木聚糖酶基因XYL1在该处理下相对表达量也显著高于对照,表明补光处理能够通过正向调控细胞壁降解进程促进果实的软化。
2.1.4 补光处理对果皮花青素合成的影响 果皮着色也是樱桃果实成熟过程的重要指标,LED 36 W补光灯处理对两种樱桃果皮花青素合成均具有明显促进效果,对果皮着色起积极影响(图3)。通过检测采收期果皮中花青素合成与转运途径基因PAL、CHS、DFR、ANS、UFGT、Riant与转录因子MYB10,发现在诸暨短柄果皮中,LED 36 W补光处理使CHS、ANS、Riant与MYB10表达量显著高于对照组。而在黑珍珠果皮中,该处理使PAL、CHS、ANS、Riant与MYB10表达量均显著高于对照组。LED 36 W补光处理通过在分子层面激活合成途径基因和转录因子的高水平表达,启动果皮中花青素的合成,进而促进樱桃果皮的着色。
2.1.5 补光处理对果实过氧化物酶活性的影响 过氧化物酶POD作为活性氧清除剂在果实成熟过程中发挥协调作用,与细胞内的活性氧生成之间保持着平衡,笔者在本研究中发现POD活性在两个品种樱桃果实发育后期呈现下降趋势,黑珍珠POD活性整体高于诸暨短柄(表3)。各补光处理下果实POD活性的下降不同程度地提早于对照组,两种功率LED补光灯处理的POD活性下降最早,在采收前9 d内持续低于同时期对照组。在诸暨短柄果实中,其他补光处理果实的POD活性下降到两种LED补光处理的水平在时间上滞后9 d左右,而在黑珍珠果实中,G101处理POD活性下降在前期比LED 36 W处理滞后6 d左右,在采收前3 d则加速下降,在采收期达到与LED 36 W处理相同的水平。这在酶活性动态变化层面体现出果实发育成熟的进程被补光处理所促进,其中两种功率LED补光处理的促进效果最明显。
2.2 补光处理对果實糖分积累的影响
2.2.1 补光处理对果实可溶性固形物(TSS)含量的影响 各补光处理不同程度地提高了两个品种樱桃果实采收期TSS含量,以红蓝光比例6∶1的LED补光光源效果最好(图4)。LED 50 W补光灯处理对诸暨短柄果实TSS含量的提高效果最明显,使果实采收期TSS含量达到17.63%,显著高于对照组的15.30%。而LED 36 W补光灯处理对黑珍珠果实TSS含量的提高效果最明显,使果实采收期TSS含量达到14.67%,显著高于对照组的12.03%。
2.2.2 补光处理对蔗糖合成酶(SS)活性的影响 果实糖分合成与蔗糖合成相关酶密切相关,SS活性在樱桃果实发育后期上升,并在采收前到达高点。各补光处理组果实SS活性在采收前3 d内均显著高于同时期对照组,促进了果实糖分积累(表4)。其中LED补光处理的促进效果最明显,LED 36 W补光处理使诸暨短柄果实SS活性上升较早,在采收前9 d开始高于对照组与其他补光处理,并快速升高,在采收前3 d达到高点,比同时期对照组高69.17%。而LED 50 W补光处理SS活性达到高点较晚,在采收期SS活性最高,比同时期对照组高21.14%。黑珍珠果实发育后期SS活性整体水平稍高于诸暨短柄,但到达高点较晚,在采收前整体保持上升。LED 36 W补光处理使黑珍珠果实SS活性上升较快,在采收期比对照组提高18.75%。
2.2.3 补光处理对蔗糖磷酸合成酶(SPS)活性的影响 在两个樱桃品种果实发育后期,SPS活性均没有表现出明显的变化规律,黑珍珠SPS活性整体高于诸暨短柄(表5)。LED 36W补光处理使黑珍珠果实SPS活性在采收前9 d内持续高于同时期对照组与其他补光处理,采收期SPS活性比对照组高35.79%。而在诸暨短柄果实中,各处理组在果实发育后期SPS活性均普遍保持较低水平。
2.2.4 对蔗糖合成相关基因的影响 根据果实TSS与蔗糖合成相关酶活性,选择促进果实糖分积累效果最明显、两个樱桃品种均具有的LED 36 W补光处理组,进行蔗糖合成相关基因检测,发现LED 36 W补光处理在基因水平上促进了两个品种的果实糖分积累(图5)。分析采收期果实中与糖合成相关的蔗糖合成酶基因SS1、SS6与蔗糖磷酸合成酶基因SPSA1的相对表达量,结果表明,在两个品种中,LED 36 W补光处理使果实中SS1基因表达量显著高于对照组,而SS6与SPSA1表达量在不同处理之间无明显差异,表明SS1在响应补光处理这一过程中起主要作用。此结果与前文对SS与SPS的研究结果相符,即补光处理主要通过激活SS1的表达正向调控SS的合成,进而通过SS促进樱桃果实的糖分积累。
3 讨 论
人工补光处理可以缩短园艺作物果实发育期,促进产品提早成熟上市,提高设施园艺生产经济效益[20]。在影响果实的生长发育与成熟进程的诸多因素中,内源激素起着重要的调控作用,赤霉素和生长素在果实品质形成过程中,促进了果肉细胞的生长和体积增大,脱落酸则对果实的成熟软化具有重要的调控作用。内源激素对果实发育的影响,在葡萄[21]、梨[22]等果树上已有研究,甜樱桃果实发育成熟过程中内源激素动态变化也有报道[23-24]。笔者在本研究中发现,两种中国樱桃果实发育后期内源激素动态变化趋势与前人在甜樱桃中的研究成果基本一致,GAS、IAA、ABA含量总体呈现上升趋势,并在采收前达到高点。补光处理对两个品种樱桃的内源激素动态变化进程具有不同程度的促进作用,使激素达到高点的时间提前于对照组,表明补光促进了樱桃果实成熟进程。在前人对补光调控果实内源激素的研究中,发现夜间补光在提高葡萄果实IAA和GA3含量的同时,还能降低ABA含量[25]。而本研究中细化了内源激素测定时间点,以呈现激素水平的动态变化,在单一时间节点中补光处理对内源激素水平的影响与前人研究不完全一致。其原因可能包含了不同物种果实内源激素调控的差异以及取样测定时间节点不同等因素,有待进一步探究。
果实的成熟软化过程在基因层面受到分子网络的调控,研究主要围绕调控软化过程的植物激素ABA的合成途径基因,以及细胞壁降解相关酶类合成基因。NCED是ABA合成途径中的限速酶,对于果实的成熟具有重要的作用[26]。在番茄中沉默SlNCED1会延缓果实成熟进程[27],桃的NCED2/3基因也正向调控果实成熟[28]。笔者在本研究中发现,两个中国樱桃品种采收期果实中NCED1相对表达量在LED 36W补光处理后均显著高于自然光照对照组,表明其在分子层面调控了果实内源激素水平,促进了果实成熟进程。多聚半乳糖醛酸酶基因PG是可合成分解果胶或果胶酸酶类基因中的一种,而木聚糖酶基因XYL是合成降解半纤维素的木聚糖酶的主要酶类,二者通过促进细胞壁的降解直接调控果实软化过程[29]。在本研究中,两个品种中国樱桃果实采收期的PG1、XYL1相对表达量在LED 36W补光处理后均显著高于对照组,表明补光处理在基因调控层面还能通过调节细胞壁的降解促进樱桃果实的软化,与前人研究结果一致[29]。
花青素的生物合成也会受到光照环境的影响,补光处理会显著提高苹果等多种果树果实果皮中的花青素含量[30-31]。PAL、CHS、DFR、ANS、UFGT与MYB10是果皮花青素合成途径的关键基因和转录因子[32-34],调控谷胱甘肽S-转移酶(GST)合成的Riant基因则在花青素从内质网至液泡的转运过程发挥作用[35]。笔者在本研究中发现,这些花青素合成与转运途径基因不同程度地被LED 36 W补光处理激活高水平表达,与前人研究成果基本一致。糖积累也在果实发育和品质形成过程中起着重要的作用,SS与SPS是参与蔗糖合成的关键酶,在甜樱桃中也被证实参与糖分合成[36]。在本研究中,LED 36 W补光处理均显著地提高了果实SS活性和SPS活性。而对基因层面的研究则发现,调控SS合成的SS1基因在补光促进两种中国樱桃果实糖分积累这一过程中起主要作用,与在甜樱桃中对SS基因家族的研究结果基本一致。
笔者在本研究中发现,促进果实发育整体效果最好的处理是LED 36 W补光处理,该方法使樱桃果实成熟进程整体提早于对照组,并提高了果实糖分积累相关指标。同时,由于诸暨短柄果实成熟期稍早于黑珍珠,因而在内源激素和酶活性动态变化的过程中,同期两个品种存在差异,如何针对具体品种适配补光措施尚需进一步研究。
开发与设施栽培相配套的补光处理技术能够促进樱桃果实成熟与糖分积累,提升果实品质,值得进行深入研究并推广应用。
4 结 论
人工补光处理对设施栽培樱桃果实成熟与糖分积累具有促进作用。LED补光灯(36 W,RB 6∶1)处理能够促进果实内源激素变化进程,并在基因层面促进果实软化、果皮着色并提高果实糖分合成能力。该方案能够通过改善设施栽培中的光照环境有效提高果实综合品质,值得进行深入研究并推进其在浙江省樱桃产业中的应用。
參考文献References:
[1] 何文,张静,黄智林,陈清,汤浩茹,汤福义,王小蓉. 基于ITS序列对栽培中国樱桃遗传多样性及其群体遗传结构的分析[J]. 西北植物学报,2014,34(3):463-472.
HE Wen,ZHANG Jing,HUANG Zhilin,CHEN Qing,TANG Haoru,TANG Fuyi,WANG Xiaorong. Genetic diversity and population genetic structure among local Chinese cherry varieties [Cerasus pseudocerasus (Lindl.) G. Don] based on ITS sequence[J]. Acta Botanica Boreali-Occidentalia Sinica,2014,34(3):463-472.
[2] 贾海慧,张小燕,陈学森,陈晓流,赵春芝. 甜樱桃和中国樱桃果实性状的比较[J]. 山东农业大学学报(自然科学版),2007,38(2):193-195.
JIA Haihui,ZHANG Xiaoyan,CHEN Xuesen,CHEN Xiaoliu,ZHAO Chunzhi. Survey of partial physiological index of cherry different cultivars[J]. Journal of Shandong Agricultural University (Natural Science Edition),2007,38(2):193-195.
[3] 洪莉,庞一波,何玲玲,陈令会,江景勇,王娇阳. 南方矮化甜樱桃优选品种比较试验初报[J]. 浙江农业科学,2015,56(12):1972-1975.
HONG Li,PANG Yibo,HE Lingling,CHEN Linghui,JIANG Jingyong,WANG Jiaoyang. Preliminary report on the comparative test of the selected varieties of dwarf sweet cherry in South China[J]. Journal of Zhejiang Agricultural Sciences,2015,56(12):1972-1975.
[4] 王孝娣,王莹莹,郑晓翠,宋杨,张震东,王海波. 人工补光对设施园艺作物生长发育影响的研究进展[J]. 北方园艺,2019(20):117-124.
WANG Xiaodi,WANG Yingying,ZHENG Xiaocui,SONG Yang,ZHANG Zhendong,WANG Haibo. Research progress on the effect of artificial supplementary light on the growth and development of facility horticultural crops[J]. Northern Horticulture,2019(20):117-124.
[5] 彭佃亮,迟文娟,信国琛,唐玉海,张敬敏. 不同时段补光对日光温室早春茬马铃薯光合特性、产量和品质的影响[J]. 中国瓜菜,2022,35(1):65-69.
PENG Dianliang,CHI Wenjuan,XIN Guochen,TANG Yuhai,ZHANG Jingmin. Effect of supplemental illumination in different periods on photosynthetic characteristics,yield and quality of potato in solar greenhouse[J]. China Cucurbits and Vegetables,2022,35(1):65-69.
[6] 王舒亞,徐威,唐中祺,王鹏,景涛,刘琪,马正宇,吕剑,郁继华. 不同补光时长对日光温室西葫芦生长、品质及产量的影响[J]. 中国瓜菜,2020,33(4):23-27.
WANG Shuya,XU Wei,TANG Zhongqi,WANG Peng,JING Tao,LIU Qi,MA Zhengyu,L? Jian,YU Jihua. Effects of different duration of light supplementation on growth,quality and yield of Cucurbita pepo in greenhouse[J]. China Cucurbits and Vegetables,2020,33(4):23-27.
[7] 陈善飞,陈晖,陈善忠,王正良. 大棚栽培草莓高压钠灯补光效果试验[J]. 中国果树,2015(3):49-51.
CHEN Shanfei,CHEN Hui,CHEN Shanzhong,WANG Zhengliang. Experiment on the effect of supplementing light with high-pressure sodium lamp for strawberry cultivation in greenhouse[J]. China Fruits,2015(3):49-51.
[8] 赵海亮,赵文东,孙凌俊,高圣华,马丽. 补光对延迟栽培‘巨峰葡萄生长发育及光合荧光特性的研究[J]. 中国农学通报,2015,31(1):99-103.
ZHAO Hailiang,ZHAO Wendong,SUN Lingjun,GAO Shenghua,MA Li. Study on supplemental lighting on the growing development and photosynthetic fluorescence characteristics of‘Kyoho grape under delayed cultivation[J]. Chinese Agricultural Science Bulletin,2015,31(1):99-103.
[9] 李洪艳,文仁德,张兰,李节法,刘金标,曹幕明,陈国品,韩佳宇,盘丰平,谢蜀豫,白先进. 补光处理对设施栽培巨峰葡萄冬季果生长的影响[J]. 中国南方果树,2016,45(5):93-94.
LI Hongyan,WEN Rende,ZHANG Lan,LI Jiefa,LIU Jinbiao,CAO Muming,CHEN Guopin,HAN Jiayu,PAN Fengping,XIE Shuyu,BAI Xianjin. Effect of supplementary light treatment on winter fruit growth of Kyoho grape under protected cultivation[J]. South China Fruits,2016,45(5):93-94.
[10] HERN?NDEZ R,KUBOTA C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs[J]. Environmental and Experimental Botany,2016,121:66-74.
[11] 张善平,冯海娟,马存金,李耕,刘鹏,董树亭,赵斌,张吉旺,杨今胜. 光质对玉米叶片光合及光系统性能的影响[J]. 中国农业科学,2014,47(20):3973-3981.
ZHANG Shanping,FENG Haijuan,MA Cunjin,LI Geng,LIU Peng,DONG Shuting,ZHAO Bin,ZHANG Jiwang,YANG Jinsheng. Effect of light quality on photosynthesis and photosystem of maize (Zea mays L.) leaves[J]. Scientia Agricultura Sinica,2014,47(20):3973-3981.
[12] 沈红香,沈漫,程继鸿,李娜娜,张婧. 不同光质补光处理对郁金香生长和开花的影响[J]. 北京农学院学报,2007,22(1):16-18.
SHEN Hongxiang,SHEN Man,CHENG Jihong,LI Nana,ZHANG Jing. Effect of supplemental lighting with different light quality on growth and bloom of tulip[J]. Journal of Beijing University of Agriculture,2007,22(1):16-18.
[13] 姜仲书,张光伦,江国良,刘伟,张微慧. 金冠苹果树冠内光质构成及其与果实品质的相关性[J]. 果树学报,2008,25(5):625-629.
JIANG Zhongshu,ZHANG Guanglun,JIANG Guoliang,LIU Wei,ZHANG Weihui. Study on light component and its correlation with fruit quality in canopy of Golden Delicious apple tree[J]. Journal of Fruit Science,2008,25(5):625-629.
[14] KANG S,ZHANG Y T,ZHANG Y Q,ZOU J E,YANG Q C,LI T. Ultraviolet-a radiation stimulates growth of indoor cultivated tomato (Solanum lycopersicum) seedlings[J]. HortScience,2018,53(10):1429-1433.
[15] 苏娜娜,邬奇,崔瑾. LED光质补光对黄瓜幼苗生长和光合特性的影响[J]. 中国蔬菜,2012(24):48-54.
SU Nana,WU Qi,CUI Jin. Effects of supplemental lighting with LED light quality on growth and photosynthetic characteristics of cucumber seedlings[J]. China Vegetables,2012(24):48-54.
[16] ZHANG L C,MA G,YAMAWAKI K,IKOMA Y,MATSUMOTO H,YOSHIOKA T,OHTA S,KATO M. Regulation of ascorbic acid metabolism by blue LED light irradiation in citrus juice sacs[J]. Plant Science,2015,233:134-142.
[17] 刘萍,张粟,蒋世翠,黄丹丹,李成宇,张士秀. LED补光对水稻秧苗素质及其生理特征和产量的影响[J]. 土壤与作物,2021,10(1):67-78.
LIU Ping,ZHANG Su,JIANG Shicui,HUANG Dandan,LI Chengyu,ZHANG Shixiu. Effects of light-emitting diodes on the seedling quality,physiological characteristics and grain yield of rice[J]. Soils and Crops,2021,10(1):67-78.
[18] BARTUCCA M L,GUIDUCCI M,FALCINELLI B,DEL BUONO D,BENINCASA P. Blue:red LED light proportion affects vegetative parameters,pigment content,and oxidative status of einkorn (Triticum monococcum L. ssp. monococcum) wheatgrass[J]. Journal of Agricultural and Food Chemistry,2020,68(33):8757-8763.
[19] 吴延军. 浙江省樱桃产业发展现状及建议[J]. 落叶果树,2021,53(3):6-9.
WU Yanjun. Development status and suggestions of cherry industry in Zhejiang Province[J]. Deciduous Fruits,2021,53(3):6-9.
[20] 张红艳. 植物生长灯在温室番茄生产中应用效果初探[J]. 辽宁农业科学,2013(1):72-73.
ZHANG Hongyan. Preliminary study on effects of plant-growth lamp on tomato in greenhouse[J]. Liaoning Agricultural Sciences,2013(1):72-73.
[21] 高江曼,孟莹,刘庆,王童孟,劉美迎,李汶冰,惠竹梅,张振文. 赤霞珠葡萄生长发育过程中内源激素的变化及其与果实成熟的关系[J]. 食品科学,2017,38(7):167-175.
GAO Jiangman,MENG Ying,LIU Qing,WANG Tongmeng,LIU Meiying,LI Wenbing,XI Zhumei,ZHANG Zhenwen. Changes in endogenous hormones during the development of Vitis vinifera L. cv. Cabernet Sauvignon and their relationship with berry ripening[J]. Food Science,2017,38(7):167-175.
[22] 齐开杰,赵碧英,张虎平,郭成宝,孙永平,张绍铃. 两个梨品种果实发育过程中果肉及种子内源激素的变化[J]. 中国南方果树,2016,45(1):18-22.
QI Kaijie,ZHAO Biying,ZHANG Huping,GUO Chengbao,SUN Yongping,ZHANG Shaoling. Changes of endogenous hormones in sarcocarp and seeds during fruit growth and development of Niitaka and Yali[J]. South China Fruits,2016,45(1):18-22.
[23] 刘仁道,何瑞生,范理璋. 内源激素与甜樱桃营养生长的关系[J]. 北方园艺,2001(6):20-21.
LIU Rendao,HE Ruisheng,FAN Lizhang. Relationship between endogenous hormones and nutritive growth of sweet cherry[J]. Northern Horticulture,2001(6):20-21.
[24] 刘丙花,姜远茂,彭福田,隋静,赵凤霞,王海云. 甜樱桃果实发育过程中激素含量的变化[J]. 园艺学报,2007,34(6):1535-1538.
LIU Binghua,JIANG Yuanmao,PENG Futian,SUI Jing,ZHAO Fengxia,WANG Haiyun. The dynamic changes of endogenous hormones in sweet cherry (Prunus avium L.) pulp[J]. Acta Horticulturae Sinica,2007,34(6):1535-1538.
[25] 时晓芳,曹雄军,林玲,郭荣荣,周思泓,韩佳宇,成果,张瑛,谢太理,王博,马广仁,白先进. 阳光玫瑰葡萄冬果膨大期补光对内源激素和果实品质的影响[J]. 中国南方果树,2021,50(2):122-127.
SHI Xiaofang,CAO Xiongjun,LIN Ling,GUO Rongrong,ZHOU Sihong,HAN Jiayu,CHENG Guo,ZHANG Ying,XIE Taili,WANG Bo,MA Guangren,BAI Xianjin. Effects of light supplement on endogenous hormones and fruit quality of Sunshine Rose grape during winter fruit expansion[J]. South China Fruits,2021,50(2):122-127.
[26] IUCHI S,KOBAYASHI M,TAJI T,NARAMOTO M,SEKI M,KATO T,TABATA S,KAKUBARI Y,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase,a key enzyme in abscisic acid biosynthesis in Arabidopsis[J]. The Plant Journal,2001,27(4):325-333.
[27] JI K,KAI W B,ZHAO B,SUN Y F,YUAN B,DAI S J,LI Q,CHEN P,WANG Y,PEI Y L,WANG H Q,GUO Y D,LENG P. SlNCED1 and SlCYP707A2:Key genes involved in ABA metabolism during tomato fruit ripening[J]. Journal of Experimental Botany,2014,65(18):5243-5255.
[28] WANG X B,ZENG W F,DING Y F,WANG Y,NIU L,YAO J L,PAN L,LU Z H,CUI G C,LI G H,WANG Z Q. Peach ethylene response factor PpeERF2 represses the expression of ABA biosynthesis and cell wall degradation genes during fruit ripening[J]. Plant Science,2019,283:116-126.
[29] 陈丽萍,韩明丽,赵根,李艳冬. 植物多聚半乳糖醛酸酶研究进展[J]. 上海蔬菜,2013(2):11-13.
CHEN Liping,HAN Mingli,ZHAO Gen,LI Yandong. Research progress of plant polygalacturonase[J]. Shanghai Vegetables,2013(2):11-13.
[30] TAKOS A M,JAFF? F W,JACOB S R,BOGS J,ROBINSON S P,WALKER A R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples[J]. Plant Physiology,2006,142(3):1216-1232.
[31] ZHAO Y,DONG W Q,WANG K,ZHANG B,ALLAN A C,WANG K L,CHEN K S,XU C J. Differential sensitivity of fruit pigmentation to ultraviolet light between two peach cultivars[J]. Frontiers in Plant Science,2017,8:1552.
[32] 王碩,郑秀文,王琪,冯慧智,刘冠,张萌萌. 果树中花青素合成及其分子调控机制研究进展[J/OL]. 分子植物育种,2022:1-10. [2022-07-29]. https://kns.cnki.net/kcms/detail/46.1068.S.20220728.1710.014.html.
WANG Shuo,ZHENG Xiuwen,WANG Qi,FENG Huizhi,LIU Guan,ZHANG Mengmeng. Research progress on anthocyanin synthesis and its molecular regulation mechanism in fruit trees[J/OL]. Molecular Plant Breeding,2022:1-10. [2022-07-29]. https://kns.cnki.net/kcms/detail/46.1068.S.20220728.1710.014.html.
[33] 魏海蓉,谭钺,宗晓娟,朱东姿,陈新,徐丽,王甲威,刘庆忠. 不同色泽甜樱桃果实花色苷积累与其相关酶活性之间的关系[J]. 植物生理学报,2017,53(3):429-436.
WEI Hairong,TAN Yue,ZONG Xiaojuan,ZHU Dongzi,CHEN Xin,XU Li,WANG Jiawei,LIU Qingzhong. Relationship between anthocyanin accumulation and the activities of anthocyanin biosynthesis enzymes in different color of sweet cherry fruits[J]. Plant Physiology Journal,2017,53(3):429-436.
[34] HE F,MU L,YAN G L,LIANG N N,PAN Q H,WANG J,REEVES M J,DUAN C Q. Biosynthesis of anthocyanins and their regulation in colored grapes[J]. Molecules,2010,15(12):9057-9091.
[35] 李丽仙,王烁,陈莹,邬滢涛,王雅倩,房月,陈学森,田长平,冯守千. 甜樱桃PavMYB10.1促进PavRiant表达和花青苷积累[J]. 园艺学报,2022,49(5):1023-1030.
LI Lixian,WANG Shuo,CHEN Ying,WU Yingtao,WANG Yaqian,FANG Yue,CHEN Xuesen,TIAN Changping,FENG Shouqian. PavMYB10.1 promotes anthocyanin accumulation by positively regulating PavRiant in sweet cherry[J]. Acta Horticulturae Sinica,2022,49(5):1023-1030.
[36] 齐希梁,刘聪利,宋露露,李明. 蔗糖合成酶PavSS1调控甜樱桃果实成熟的功能分析[J]. 果树学报,2021,38(2):168-178.
QI Xiliang,LIU Congli,SONG Lulu,LI Ming. Functional analysis of sucrose synthase PavSS1 regulating sweet cherry fruit ripening[J]. Journal of Fruit Science,2021,38(2):168-178.
收稿日期:2023-03-31 接受日期:2023-07-30
基金项目:浙江省农业新品种选育重大科技专项(2021C02066-9)
作者簡介:李都岳,女,助理研究员,博士,从事樱桃新品种选育与栽培技术研究。Tel:18810518803,E-mail:mlea@qq.com
通信作者Author for correspondence. Tel:0571-86058831,E-mail:wyjwjht@163.com
