Simulation of Solvent Extraction for Naphthenic Lubricating Base Oils
2023-10-23XuXiaolingWangTianqiTianQingmeiLiuYansheng
Xu Xiaoling; Wang Tianqi; Tian Qingmei; Liu Yansheng,2
(1. State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing)at Karamay, Karamay Xinjiang 834000, China;2. State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102200, China)
Abstract: Solvent extraction is the process of separating aromatics from vacuum distillates for the production of lubricating base oils. In this study, the authors use dimethyl sulfoxide (DMSO) instead of furfural as solvent, in light of its higher selectivity, to obtain extracts with a high aromatic content for naphthenic lubricating base oils. We systematically investigated effects of the solvent-to-oil (S/O) ratio and extraction temperature on the yield of the extract, efficiency of aromatic removal, and composition of the extracts and raffinates. The results showed that the aromatic content of extracts for naphthenic oils could reach a high value of about 80%. The solvent maintained a high selectivity for aromatics for naphthenic oils even under a high S/O ratio and a high extraction temperature. Moreover, the efficiency of aromatic removal for naphthenic lubricating base oils could be enhanced by increasing either the S/O ratio or the extraction temperature,although these measures had limited effects in practice. Following this, we used the non-random two-liquid (NRTL) model based on the pseudo-component approach to simulate the liquid-liquid equilibrium of the system of DMSO + naphthenic lubricating base oils, and determined the parameters of binary interaction through regression based on the data on phase equilibrium. The modeling results showed that the predicted yield, content of the solvent, and composition of the raffinates and extracts were in good agreement with those obtained in the experiments. This validates the reliability of the model used to represent the DMSO + naphthenic lubricating base oil system. Both the experimental data and the method of simulation reported here can help optimize the extraction of naphthenic lubricating base oils, and provide a better understanding of the corresponding process.
Key words: solvent extraction; naphthenic lubricating base oils; liquid-liquid equilibrium; thermodynamic model; DMSO
1 Introduction
Various types of lubricating base oils are typically produced by liquid-liquid extraction to separate aromatics and satisfy the requirements of high-quality products.The raffinates obtained from the process of aromatic extraction have a low aromatic content, and can be used to produce lubricating base oils[1-2]. Extracts with a high aromatic content have been previously used as extender oils, or plasticizers, and rubber extenders in many rubber products owing to their excellent compatibility with rubber[3]. However, the distillate aromatic extract (DAE)contains a high percentage of toxic polycyclic aromatics(PCAs)[4], the content of which in eco-friendly oils has been limited to less than 3% by the European Union[5].Eco-friendly processing oils are thus frequently produced by removing PCAs from the DAE by using secondary solvent extraction[6-7]. A DAE with a high aromatic content is the intermediate product, and requires the efficient enrichment of aromatic hydrocarbons in the extraction phase during the process of primary extraction.
Selecting a solvent with a high selectivity and efficiency for extraction is a fundamental problem in the process of liquid-liquid extraction. A considerable amount of research has been devoted to identifying the appropriate solvents to this end. The most commonly tested solvents include furfural[8-11],N-methyl pyrrolidone (NMP)[12-13],dimethyl sulfoxide (DMSO)[14-15], and sulfolane[15-16].
Furfural is among the most widely used solvents, and extracts obtained by the solvent extraction of paraffinic lubricating base oils with furfural have an aromatic content of around 80%[8-10]. However, the aromatic content of the extract obtained through solvent extraction by using furfural is not sufficiently high in case of naphthenic lubricating base oils. For example, one study reported a significantly high content of the saturate mostly consisting of naphthenes (approximately 38%)in the extract when the aromatic content of the extract was 58%[17]. These results indicate that furfural has an unsatisfactory selectivity for naphthenic lubricating base oils, where this makes it difficult to produce eco-friendly processing oils with a high aromatic content. Therefore,it is important to use more selective solvents to extract aromatic hydrocarbons from naphthenic lubricating base oils for the production of eco-friendly processing oils.
Simulating the process of extraction is important for optimizing the relevant operating conditions. A consistent and representative thermodynamic model is needed to appropriately describe the liquid-liquid equilibrium(LLE) during the extraction operation. The accuracy of the model used to describe the properties of equilibrium depends not only on the thermodynamic basis, but also on the characterization of the lubricating base oils. Simplified methods of characterization are used for lubricating base oils, usually in terms of pseudo-components, owing to their complex composition. Previous studies have reported the use of the pseudo-component approach based on composition (in terms of saturates, aromatics,and polars) that can be used to accurately model LLE[8-10]. Moreover, such traditional thermodynamic models as the non-random two-liquid (NRTL) model[8-11]and the universal functional activity coefficient (UNIFAC)[18-19]model have been widely used to solve the problem of LLE. However, these methods have been mostly used to focus on the solvent extraction of paraffinic lubricating base oils, and few studies have reported simulations of aromatic extraction from naphthenic lubricating base oils.In this study, the authors use solvent DMSO, which has a higher selectivity than furfural, for aromatic extraction from naphthenic lubricating base oils. We examined the effects of the solvent-to-oil (S/O) ratio and the extraction temperature to formulate a system consisting of DMSO+ naphthenic lubricating base oil. Following this, we constructed a model of the system and simulated it by using data on LLE by considering a complex mixture of three pseudo-components (i.e., saturates, aromatics, and polars). The parameters of binary interaction of the NRTL model of the system were calculated based on regression.We also assessed the reliability of the NRTL model and the pseudo-component approach based on saturates,aromatics, and polars for solvent extraction in the DMSO+ naphthenic lubricating base oil system.
2 Experimental
2.1 Feedstock and reagents
Naphthenic lubricating base oils for the experiments were obtained from a refinery in Karamay (China). The properties of the feedstock are listed in Table 1. Its value of CNwas relatively high, and indicated a considerable amount of naphthenes in it, while the contents of nitrogen and sulfur were notably low. DMSO was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing,China), and was distilled before use to avoid oxidation.The distilled water was prepared by the authors in the laboratory.

Table 1 Properties of the naphthenic feedstock
2.2 Extraction experiments
A certain amount of feedstock and DMSO were placed in a 500 mL double-layer glass funnel during the process of extraction, and the temperature was controlled to within± 0.1 ℃ by recirculating silicone oil from a thermostatic bath. The mixture was agitated at 500 rpm for 1 h and allowed to settle for another hour to achieve complete phase separation. The raffinates and extracts were then collected in different sampling bottles, and the solvent was removed from each sample via rotary evaporation under vacuum (at about 3 kPa).
The obtained raffinates and extracts were accurately weighed by using an electronic balance to calculate their yields. The content of DMSO in the raffinates and extracts before the removal of the solvent was also calculated.The composition and properties of the raffinates and the extracts were then analyzed. The density and refractive index of the oils were determined by ASTM D1298, with an accuracy of ±0.00005 g/cm3, and ASTM D1747, with an accuracy of ±0.00002. The hydrocarbon composition of the oils was determined by means of ASTM D2007, and their viscosity was measured according to ASTM D445 by using a rotational viscometer (Anton Paar SVM3000) with an uncertainty of ±0.35%. The efficiency of aromatic removalEarwas calculated by the following equation:
whereYexdenotes the yield of the extract, andCar-exandCar-feedare the concentrations of the aromatics in the extracts and the feed, respectively.
2.3 Analytical methods
2.3.1 Density and refractive index of extracts
We determined values of the density (ρ20) and the refractive index of the extracts at 20 ℃ (n20), based on those at 70 ℃ owing to their poor fluidity at a lower testing temperature, by using the following equations,respectively[20]:
whereαVandαFdenote the coefficients of thermal expansion of the distillates with respect to their density and refractive index, respectively. The values ofαVandαFof the extracts were calculated from Eqs. (4) and (5),respectively.FRIis a function of the refractive index, and is defined in Eq. (6).
2.3.2 Analysis of carbon-type composition
The carbon-type composition of the studied mixtures was determined by ASTM D2140[21]based on the data on their density, refractive index, and viscosity. This enabled us to quantitatively classify the paraffinic and naphthenic oils.The carbon-type composition of the oils was expressed as the percentage of paraffinic carbons (CP), the percentage of naphthenic carbons (CN), and the percentage of aromatic carbons (CA). Moreover, the accuracy of the method used to determine the composition of oils with sulfur content no lower than 0.8 wt% was improved by applying sulfur correction:
Table 2 shows the liquid density (ρ), refractive index (n),and hydrocarbon composition (in the saturates, aromatics,and polars) of the feed, raffinates, and extracts. Each mixture is denoted by a letter (“R” for raffinate and “E”for extract), followed by the corresponding experiment number.

Table 2 Experimental results of the solvent extraction of naphthenic lubricating base oils by DMSO
3 Results and Discussion
The effects of both the extraction temperature and the S/O ratio on the efficiency of solvent extraction were firstly investigated by using DMSO with naphthenic distillates.Then the process of solvent extraction of naphthenic lubricating base oils was simulated by using the NRTL thermodynamic model based on the pseudo-component approach.
3.1 Effect of solvent-to-oil ratio
Experiments on the solvent extraction of naphthenic oils with DMSO were carried out at various S/O ratios ranging from 4 to 8, and an extraction temperature of 80 ℃. The effects of the S/O ratio on the yield of the extracts and the efficiency of aromatic removal areshown in Figure 1. An increase in the S/O ratio led to noticeable increases both in the yield of the extracts and the efficiency of aromatic removal due to an increase in the capacity of the solvent.

Figure 1 Effects of the solvent-to-oil ratio on (a) the yield of the extracts and (b) the efficiency of aromatic removal
Figure 2 presents the hydrocarbon composition (in terms of saturates, aromatics, and polars) and the carbon-type composition (in terms ofCA,CN, andCP) of the extracts at different S/O ratios. A slight decrease in the aromatic content occurred due to an increase in the contents of the saturate of the extracts. Consequently, the values ofCAof the extracts decreased slightly with an increase in the S/O ratio, and was accompanied by an observable increase in their values ofCP. Analyses of the hydrocarbon composition and carbon-type composition of the raffinates are shown in Figure 3. The contents of aromatics and polars of the raffinates both decreased with increasing S/O ratio, and a corresponding decline in the value ofCAof the raffinates was observed.

Figure 2 Effects of the solvent-to-oil ratio on (a) the component analysis and (b) the carbon-type composition of the extracts
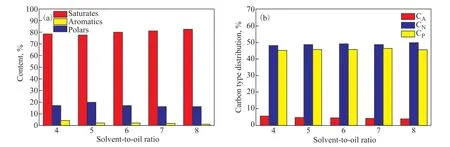
Figure 3 Effects of the solvent-to-oil ratio on (a) the component analysis and (b) the carbon-type composition of the raffinates
The aromatic content of the extracts remained high(80.69% to 74.74%) as the S/O ratio was increased from 4 to 8, which implies that the solvent maintained a high selectivity of aromatics for naphthenic oils even at a high S/O ratio. In contrast to the results of a previous study[17],those showed that the furfural extract had an aromatic content of 58%, with a content of the saturate of about 38% even under moderate operating conditions (S/O ratio of 2.5). These results validate the higher selectivity of DMSO compared with that of furfural, especially for naphthenic oils. The S/O ratio was higher when using DMSO than when using furfural owing to the lower solubility of the former solvent. In addition, it recorded an efficiency of aromatic removal of nearly 60% while maintaining a high aromatic content (74.74%) of the extract at an S/O ratio of 8.
3.2 Effect of extraction temperature
Figure 4 shows the effects of the extraction temperature on the yield of the extracts and the efficiency of aromatic removal at an S/O ratio of 8. Similar to the effect of the S/O ratio, both the yield of the extracts and the efficiency of aromatic removal prominently increased with the extraction temperature, where this can be attributed to an increase in the capacity of the solvent.
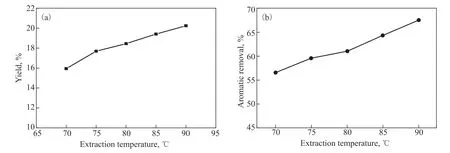
Figure 4 Effects of the extraction temperature on (a) the yield of the extracts and (b) the efficiency of aromatic removal
Analyses of the hydrocarbon composition and the carbontype composition of the extracts and the raffinates are presented in Figures 5 and 6, respectively. A slight decline was observed in both the aromatic content and the value ofCAof the extracts in Figure 5, indicating a slight decrease in the selectivity of the solvent with increasing extraction temperature. On the contrary, both the aromatic content and the value ofCAof the raffinates decreased with increasing extraction temperature. Moreover, the aromatic content of the extracts ranged from 79.57%to 74.49% as the extraction temperature was varied from 70 ℃ to 90 ℃, which reflects the high selectivity of aromatics for naphthenic oils at different extraction temperatures.
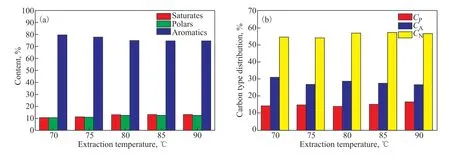
Figure 5 Effects of the extraction temperature on (a) the component analysis and (b) the carbon-type composition of the extracts
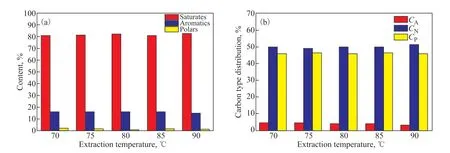
Figure 6 Effects of the extraction temperature on (a) the component analysis and (b) the carbon-type composition of the raffinates

Figure 7 Comparison between the predicted and experimental values of (a) density and (b) refractive index of thenaphthenic mixtures
We can infer from the above that DMSO maintained a high selectivity of aromatics for naphthenic oils even at a high S/O ratio and a high extraction temperature.
3.3 Consistency of LLE data
The consistency of the experimentally obtained data on LLE was verified by Hand’s[22]and Bachman’s[23]equations:
whereaandbare parameters of Hand’s equation, andmandnare those of Bachman’s equation. The superscriptsαandβrepresent the phases of the extract and the raffinate, respectively. The subscripts 1, 2, 3, and 4 are mass fractions of the saturate, aromatic component, polar component, and solvent, respectively. The regression coefficient (R2) and parameters of the equations are listed in Table 3.

Table 3 Parameters of Hand’s and the Bachman’s equations for the studied system
Table 3 shows that the regression coefficients of Hand’s and Bachman’s equations were both close to one, which indicates a high consistency of the experimentally obtained data on LLE for the DMSO + naphthenic lubricating base oil system.
3.4 Simulation of the process
Sound knowledge of the composition of fractions of petroleum and the selection of an appropriate thermodynamic model are necessary for studying the LLE. The pseudo-component approach proposed by Grieken et al.[8-10]has been frequently used to determine the composition of complex mixtures. The NRTL thermodynamic model has also been recommended in the literature[8-11]as it can accurately simulate the process of extraction of paraffinic lubricating base oils. We thus used the pseudo-component approach and the NRTL model to simulate the extraction experiments on naphthenic lubricating base oils.
3.4.1 Properties of pseudo-components
The pseudo-component approach used here assumed that the petroleum mixtures comprised three pseudocomponents—saturates (S), aromatics (A), and polars(Po)—of the lubricating base oil system during the process of solvent extraction[8-10]. The density and refractive index of the mixtures were determined from the following rules of mixing:
whereρ20andn20are the density and the refractive index at 20 ℃, respectively, andXS,XA, andXPodenote the mass fractions of the saturates, aromatics, and polars,respectively.
We then regressed the average properties of the pseudocomponents to predict the density and refractive index of the naphthenic oils during solvent extraction by using the above-mentioned mixing rules (Eqs. (12) and (13)). The optimum values oftheaverage properties (ρ20andn20)of the saturates, aromatics, and polars were obtained by minimizing the objective function (OF, Eq. (14)), which was defined as the sum of squared deviations between the experimental (Pexp) and the simulated values (Pcal) for each property of the studied mixtures:
The optimized average properties of the pseudocomponents calculated based on the experimental data are listed in Table 4. The deviations in the values of density and refractive index between the experiments and the simulations were calculated by using the average absolute deviation (AAD) and the maximum absolute deviation(MAD). The limits on Eqs. (12) and (13) wereρPo>ρA>ρSandnPo>nA>nS, respectively. Table 4 shows that the AADs ofρ20andn20, obtained by using the rule of mixing in Eqs. (12) and (13), were 0.0027 g/cm3and 0.0049,respectively. Similarly accurate predictions ofρ20andn20of the naphthenic distillates were obtained during solvent extraction in our previous studies[24-25]. Therefore, the pseudo-component method provided accurate predictions of the density and refractive index (as shown in Figures 7 and 8), which is reflected in the sound agreement between its results and those of the experiments. This provides a solid foundation for simulating the process of solvent extraction.
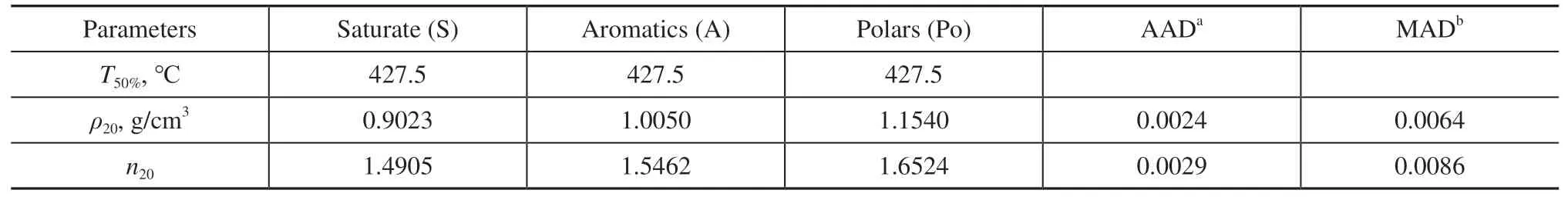
Table 4 Physical parameters of each pseudo-component of the naphthenic lubricating base oils, and deviations in the density and refractive index between the experiments and the calculations
3.4.2 Thermodynamic model
We used the NRTL model to simulate the process of solvent extraction. Each mixture was considered to be composed of the solvent and the three pseudo-components(i.e., DMSO + S + A + Po). The NRTL equation is based on the concept of local composition, which considers only binary interactions. The coefficient of activity, which includes binary interactions, was determined by the following equations[26]:
whereγiis the activity coefficient of liquid,xiis the molar fraction of componenti,αijis a non-random parameter related to the tendency of speciesiandjto be randomly distributed, andτijstands for the parameters of interaction between pairs of moleculesiandj.τijwas proportional to the reciprocal of the extraction temperature according to Eq. (17) in terms of the binary parametersaijandbij[27].Nis the number of components of the system.
Considering thatαijis a characteristic parameter of the solution that usually ranges from 0.2–0.47[28], which shows that it was not very sensitive to the process of the simulation, all values ofαijwere set to 0.30 (except for the value denoting the interaction between the solvent and saturates, which was set to 0.20) to reduce the calculation time and improve the stability of the NRTL model.
The experimentally obtained data on the LLE in Table 2 and the average properties of the pseudo-components obtained in Table 4 were used to determine the adjustable parameters of the NRTL model by performing data regression in Aspen Plus version 11 based on the generalized least-squares method[29]. Values of the parameters of binary interaction for naphthenic mixtures obtained by the NRTL model are shown in Table 5.
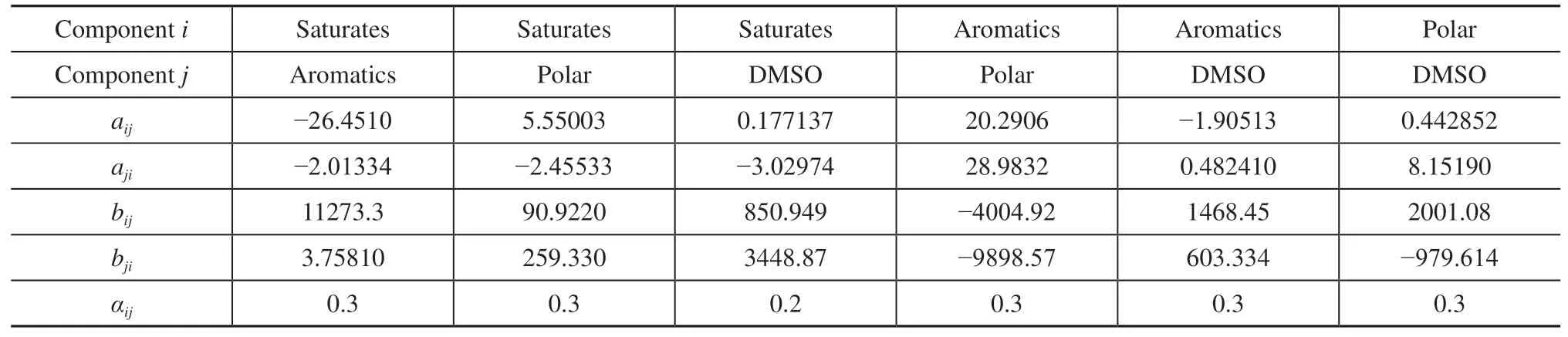
Table 5 Parameters of binary interaction of the NRTL equation
We simulated the one-stage process of extraction based on the average properties of the pseudo-components required for the feed stream and the previously determined parameters of the NRTL model by using Aspen Plus. Aflowchart of the process of extraction in Aspen Plus is shown in Figure 8. Streams of the feed and the solvent at room temperature (25 ℃) were pumped into the heat exchanger to be heated up to the extraction temperature.The decanter D1 was modeled as a liquid-liquid extractor module in Aspen Plus, and operated at 1 atm and 80 ℃according to the experimental data. The raffinate and the extract phases then entered the flash drums REC-COL1 and REC-COL2, respectively, for the removal of the solvent.

Figure 8 Flowchart of the process of solvent extraction
3.4.3 Results of simulations
The predictions of the model for naphthenic oils were compared with the experimental data to assess its accuracy. The predicted yield and solvent content fitted well with the experimental values, regardless of whether raffinates or extracts were considered, as shown in Figure 9. The simulated and the experimentally obtained compositions of the mixtures are compared in Figure 10,which shows that they agreed well. This further confirms the accuracy of the model.

Figure 9 Comparison between the (a) experimentally obtained yield and (b) solvent content, and their simulated values
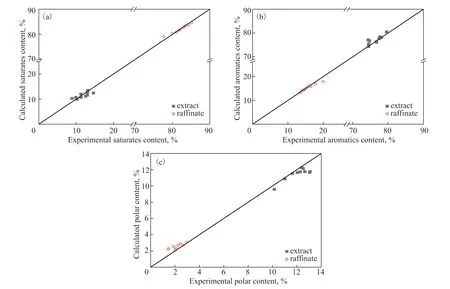
Figure 10 Comparison of the (a) content of the saturates, (b) aromatics content, and (c) polar content between the experiments and the simulations
The calculated errors between the simulations and the experiments in the yield, solvent content, and composition of the mixtures are presented in Table 6. The average absolute deviations of the yield and solvent content were 0.45% and 0.09%, respectively. No significant deviation was observed in the composition of the mixtures, with average absolute deviations of 0.83% for saturates, 0.67%for aromatics, and 0.49% for polars. We can conclude that the results of the simulations were in very good agreement with the experimental values. This verifies the accuracy of the NRTL model combined with the pseudocomponent method for the extraction of naphthenic lubricating base oils.

Table 6 Analysis of error between values simulated by the NRTL model and the experimental values
To further confirm the quality of the model, we compared the experimental values of the corresponding properties and yields of two phases with those obtained from the simulations at an extraction temperature of 80 ℃, and S/O ratios of 3/1 and 10/1, as shown in Table 7. Although the model exhibited a slightly lower accuracy at low and high S/O ratios, its results were generally consistent with the experimental values. Therefore, the proposed model can accurately describe the process of the DMSO-based extraction of naphthenic distillates.

Table 7 Comparison between the experimentally obtained composition of components and solvent content, and their simulated values obtained by the NRTL model
4 Conclusions
The experimental results of this study show that the aromatic content of extracts obtained through solvent extraction by using DMSO for naphthenic lubricating base oils was high, at about 80%. This confirms the higher selectivity of DMSO than furfural. The solvent maintained a high selectivity of aromatics for naphthenic oils even at a high S/O ratio and a high extraction temperature. In addition, the efficiency of its aromatic removal for naphthenic lubricating base oils could be enhanced by increasing either the S/O ratio or the extraction temperature, although their effects are limited in practice. An appropriate experimental setup involves a compromise between the solubility of the species and the selectivity of the solvent.
We used the pseudo-component approach and the NRTL model to simulate the extraction of naphthenic oils. The average absolute deviations inρ20andn20obtained by using the average properties of the pseudo-components were 0.0027 g/cm3and 0.0049, respectively, and verified the accuracy of predictions of the physical properties of different mixtures (feed, raffinates, and extracts) of naphthenic lubricating base oils. We also simulated the process of extraction based on the experimentally obtained properties of the pseudo-components and parameters of the NRTL equation. The results showed that the predicted yields and the solvent contents of the two phases, as well as the compositions of the raffinates and the extracts were in good agreement with the experimental values, where this verified the reliability of the model for the system of DMSO +naphthenic lubricating base oil. The average absolute deviations in the yield and solvent content between the simulations and the experiments were 0.45% and 0.09%,respectively, while those in values of the saturates,aromatics, and polars were 0.83%, 0.67%, and 0.49%,respectively. The proposed model can thus be used to design and optimize units of extraction for the DMSO +naphthenic lubricating base oil system.
Acknowledgments:The work was financially sponsored by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2022D01F37).
杂志排行
中国炼油与石油化工的其它文章
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
