Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
2023-10-23YangJunfengZhouManliWangMeng
Yang Junfeng; Zhou Manli; Wang Meng
(1. Qingyuan Yakoo Chemical Co., Ltd, Qingyuan 510640, China; 2. Department of Polymer Science and Engineering, South China University of Technology, Guangzhou 510640, China)
Abstract: Gelation adversely affects the aqueous adiabatic terpolymerization of acrylamide (AM), sodium acrylate (NaAA),and 2-methacryloyloxyethyl trimethylammonium chloride (DMC). Here, the mixed dispersants sorbitan monooleate (Span 80) and polyoxyethylene sorbitan monooleate (Tween 80) were introduced to the terpolymerization system in an attempt to mitigate the gel effect. This enabled the preparation of high-performance amphoteric polyacrylamides, which were characterized by Fourier-transform infrared spectroscopy and thermogravimetric analysis. The influences of the dispersant type and content as well as the hydrophilic-lipophilic balance (HLB) on the gel effect were examined, and the mechanism underlying the suppression of the gel effect was considered. The obtained results indicated that the gel effect can be effectively mitigated using an aqueous adiabatic terpolymerization system containing mixed Span 80/Tween 80 dispersants at various contents. In particular, mixed dispersant contents of 0.6%-0.8% with HLB values of 5.0-6.0 afforded the optimal performance (e.g., high viscosity, fast aqueous dissolution time, and the like).
Key words: gel effect; dispersants; amphoteric polyacrylamide; terpolymerization
1 Introduction
Water pollution increasingly poses an unprecedented threat to the human living environment, and it is both a pressing need and of scientific interest to develop effective and efficient technologies for wastewater treatment. A variety of approaches have been explored in recent years, including adsorption[1], flocculation[2],oxidation[3], membrane filtration[4], and other methods.Among them, flocculation is widely used and has some practical benefits, including a simple process, high efficiency, and low cost[5-6]. Amphoteric polyacrylamides are widely utilized as flocculants because they consist of both anionic and cationic moieties at the molecular level and thus can adsorb a range of suspended substances,including both organic and inorganic species. In our research, we are particularly interested in synthesizing high-performance amphoteric polyacrylamides for flocculation-based wastewater treatment.
Vinyl monomers are vital ingredients for the preparation of amphoteric polyacrylamides via free radical copolymerization. However, when multivinyl monomers are employed, gelation often occurs in the course of polymerization, even at low monomer conversion[7-10].This gelation process adversely affects the progression of polymerization, thus making it difficult to obtain the desired final polymer. In some extreme cases, gelation may even lead to catastrophic reactor explosion because of the sudden, uncontrollable temperature rise associated with the exothermic nature of the reaction.
Numerous efforts have been devoted to preventing gelation, such as using a variety of chain transfer agents[11-16]. For example, Kong et al.[17]exploited the weak transfer of isopropyl alcohol (IPA) to control the radical polymerization of two asymmetrical divinyl monomers, namely, allyl methacrylate and furfuryl methacrylate. This successfully delayed the gelation in the later stages of the reaction, allowing polymers with high molecular weights (106–107Da) to be obtained under high monomer concentrations (10%–30%). For the purpose of manipulating the gel content, Fang et al.[18]utilizedn-dodecyl mercaptan (DDM) as a chain transfer agent during the monomer-starved seeded semi-continuous emulsion polymerization of butyl acrylate, dodecafluoroheptyl methacrylate, acrylic acid,and 2-hydroxyethyl acrylate. The results showed that increasing the DDM concentration from 0 to 0.1 to 0.2 parts per hundred parts monomer led to a remarkable decrease in gel content from 52.7% to 38.7% to 12.3%, respectively. Zoco et al.[19]found that a large amount of gel was formed during the seeded emulsion copolymerization process ofn-butyl acrylate and styrene in the absence of a chain transfer agent. By contrast, in experiments conducted in the presence of 1% DDM,gelation did not occur.
The synthesis of amphoteric polyacrylamides can also suffer from gelation[20]. However, to the best of our knowledge, only a few studies[21-23]investigating the mitigation of these gelation processes have been published. In this work, we focused on controlling the gel effect for the aqueous adiabatic radical terpolymerization of acrylamide (AM), sodium acrylate (NaAA), and 2-methacryloyloxyethyl trimethylammonium chloride(DMC) by the pre-addition of single dispersants, a chain transfer agent, a surfactant, and, in sparticular, mixed dispersants (e.g., Span 80/Tween 80 mixtures). Various factors potentially affecting gelation, such as dispersant type, dispersant content, and hydrophilic-lipophilic balance (HLB), were thoroughly studied. It is anticipated that the results reported here will prove valuable for the preparation of high-performance amphoteric polyacrylamides with practical industrial applications in wastewater treatment.
2 Experimental
2.1 Materials
AM (SNF Flocculant, Taixing, China), DMC (Elf Atochem SA, Toulouse, France), and 2,2'-azobis(2-methylpropionamide) dihydrochloride (AIBA, Shanghai Hengyi Chemical Co., Shanghai, China) were used as received without further purification. NaAA (Lvcheng Chemical Manufacturer, Jinan, China) was purified to 98% using activated carbon. Potassium persulfate(K2S2O8), sodium bisulfite (NaHSO3), and IPA were obtained from Guangzhou Chemical Group Co.,(Guangzhou, China) and used as received. Sorbitan monooleate (Span 80), sorbitan trioleate (Span 85),polyoxyethylene sorbitan monooleate (Tween 80),polyoxyethylene sorbitan trioleate (Tween 85), and alkylphenol polyoxyethylene (OP-10) were obtained from Shandong Aokai Chemical Co., (Jinan, China) and used as received. Water was double distilled prior to use.
2.2 Adiabatic terpolymerization
Solutions of the three monomers (AM, NaAA, and DMC)of various concentrations were first added to a 500 mL flask equipped with a magnetic stirrer. Next, two aqueous solutions containing AIBA and additive were added to the flask. The resulting mixture was purged with nitrogen for 15 min. Magnetic stirring was then initiated and aqueous solutions of K2S2O8and NaHSO3were separately introduced into the flask using syringes. The flask was then surrounded with insulation and sealed with a rubber stopper equipped with a thermometer, and the reaction mixture was stirred at room temperature. As such, the resulting aqueous solution was subjected to adiabatic radical terpolymerization of AM, NaAA, and DMC in the presence of different dispersants under various reaction conditions as summarized in Table 1. A schematic diagram of the adiabatic terpolymerization reaction is presented in Figure 1.
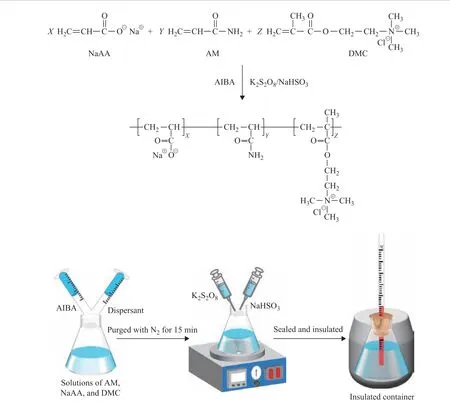
Figure 1 Adiabatic terpolymerization reaction of AM, NaAA, and DMC
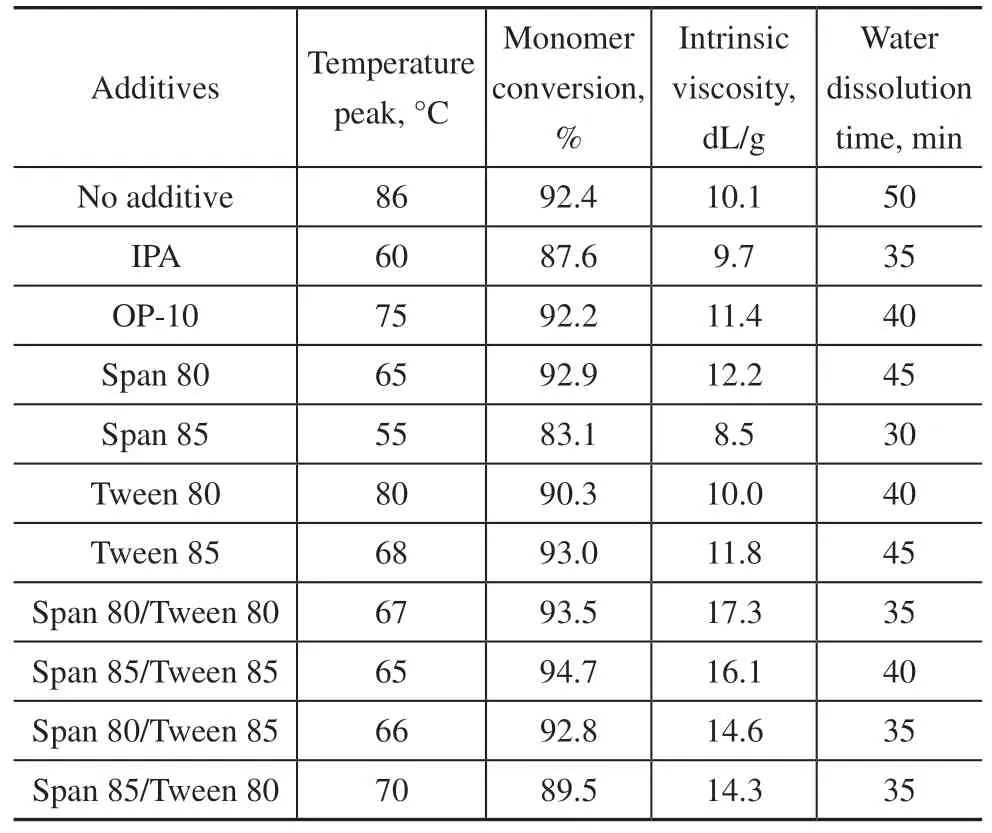
Table 1 Reaction parameters for synthesizing DMC/AM/NaAA terpolymers with various dispersants
During the aqueous adiabatic terpolymerization, samples were removed at various reaction times for kinetic analysis using sampling syringes via a needle-tubing connector and immediately frozen in liquid nitrogen to stop the reaction. The samples were dissolved in distilled water, precipitated in acetone, dried in a vacuum oven at 45°C, and then weighed to determine the monomer conversion. The thermometer inserted in the reaction flask via the rubber stopper, which was able to reach the center of the reaction solution, provided the temperature profile over the course of the terpolymerization. When the reaction solution became very viscous, magnetic stirring was no longer effective. Once the reaction solution had attained a maximum temperature (temperature peak), the terpolymerization system was continued for approximately 2 h for maturation of the reaction. The gelled product was removed from the flask, cut into pieces, and then dried to constant weight under vacuum at 45°C. The monomer conversion ratioCRwas estimated according to the following formula:
where [M] is the weight of the dried product and [M0] is the total weight of the three monomers initially added to the flask before polymerization.
2.3 Viscosity and water dissolution time determination
The intrinsic viscosity of the terpolymer samples was measured using a method described in our previous paper[24], and it can be regarded as a reflection of the sample molecular weight. The water dissolution time of the samples was measured as the period of time required for a slowly added powder sample (0.1 g) to completely dissolve in distilled water (100 mL). The dissolution tests were conducted in glass beakers placed in a water bath set at 25°C under constant stirring at 200 r/min.
2.4 Characterization of terpolymers
Powder samples of the terpolymers were mixed with IR-transparent KBr powder and compression molded into pellets, and their Fourier-transform infrared (FTIR)spectra were recorded in the transmission mode on a Nicolet 760 Magna-IR spectrometer (FTIR Instruments,Shanghai, China). Thermogravimetric analysis (TGA)was conducted directly on the terpolymer samples using a TGA 2050 system (TA Instruments, Delaware, U.S.A.),with the TGA furnace being purged by nitrogen gas flowing at 20 mL/min. The TGA tests were performed over the temperature range from 0°C to 800°C at a heating rate of 20°C/min.
3 Results and Discussion
3.1 Adiabatic terpolymerization in the presence of dispersants
The aqueous adiabatic terpolymerization for synthesizing amphoteric polyacrylamides can be initiated by the K2S2O8/NaHSO3redox system at room temperature and then promoted by AIBA at approximately 40 °C[24]. Table1 compares the experimental results obtained after the addition of different additives to the terpolymerization system primarily composed of AM, NaAA, and DMC at the same monomer ratio (i.e., DMC/AM/NaAA =30:60:10 in mol%) and total monomer concentration(i.e., 30%). No obvious differences in the intrinsic viscosity were observed between the terpolymer samples prepared using a single dispersant or chain transfer agent(IPA) or OP-10 or without any dispersant. However, the terpolymer samples prepared using a single dispersant,OP-10 or IPA apparently exhibited lower temperature peaks of terpolymerization compared with the sample synthesized without any dispersant.
It is interesting to observe that when the mixed dispersants (HLB = 6.0) were added prior to the terpolymerization reaction, the intrinsic viscosity and water dissolution rate were significantly increased,while the temperature peaks of terpolymerization were noticeably decreased, compared with the sample prepared without any additives. It is also noteworthy to point out that the mixed Span 80/Tween 80 and Span 85/Tween 85 dispersants studied here exhibited better control over the gel effect for their respective adiabatic terpolymerization reactions (e.g., lower temperature peaks) than any of the single dispersants. Overall, the Span 80/Tween 80 system displayed the optimal performance and reagent cost, so we selected this system for further examination in the present study.
3.2 Effect of dispersant content and HLB
Keeping the monomer concentration constant, we thoroughly studied the influence of the addition of various amounts of mixed Span 80/Tween 80 dispersants on the gelation process. As shown in Figure 2, the monomer conversion increased with increasing dispersant content.Increasing the mixed dispersant content appeared to improve the uniformity of the chain radical distribution,thus promoting the underlying terpolymerization.

Figure 2 Effect of mixed dispersant content on monomer conversion and polymer intrinsic viscosity
Figure 2 also shows that the terpolymer samples displayed the maximum intrinsic viscosity at a mixed dispersant content of 0.6%-0.8%. For the terpolymerization systems with a constant monomer concentration, the uniformity of the chain radical distribution increased with increasing dispersant content and the radical life became longer,thus increasing the polymer molecular weight. However,the chain radicals were more inclined to undergo chain transfer reactions with the dispersant within the terpolymerization system. When the dispersant content exceeded 0.8%, these chain transfer reactions with the dispersant became more pronounced. In turn, the chain propagation reaction was blocked, the molecular weight of the product decreased, and the intrinsic viscosity also decreased.
Figure 3 shows the influence of the HLB value (Span 80/Tween 80) on the polymerization. The total mixed dispersant content of the terpolymerization system was kept constant. An HLB range of mixed dispersants close to that of the dispersed materials results in a welldispersed polymerization system[25]. Therefore, the closer the HLB value of the mixed dispersants was to that of the amphoteric polyacrylamide, the better the dispersion effect was, thus indicating the enhanced effectiveness of the mixed dispersants to sterically interfere with the polymer hydrogen-bonding interactions. For these reasons,the monomer conversion and polymer intrinsic viscosity reached their maximum values in the HLB range of 5.0–6.0.

Figure 3 Effect of mixed dispersant HLB value on monomer conversion and polymer intrinsic viscosity
3.3 Analysis of terpolymerization kinetics
The monomer conversion as a function of the reaction time for various dispersant contents is plotted in Figure 4. It can be seen that the rates of monomer conversion(i.e., the slopes of the curves) increased slowly in the early stage of the reaction, while increased sharply after approximately 18 min, which shows that the onset of the gel effect during the terpolymerization is actually a gradual phenomenon. The rates of monomer conversion in the early stages of terpolymerization were relatively similar, irrespective of whether the mixed dispersants were added to the polymerization system. However,at the acceleration stage of terpolymerization, the rate of monomer conversion was lower in the presence of the mixed dispersants. Meanwhile, the onset time of the gel effect was delayed by the addition of the mixed dispersants. In particular, the mixed dispersant content of 0.6% had a marked effect on mitigating the gel effect.The underlying mechanism by which the gel effect was controlled during terpolymerization can be explained as follows. The mixed dispersant molecules can adsorb to the growing terpolymer chains during terpolymerization.When the mixed dispersants were pre-added at approximately 0.6%, the binding forces of the mixed dispersant molecules to the terpolymer chains overcame the intermolecular hydrogen-bonding interactions between the terpolymer chains, as shown in Figure 5,thus inducing good spatial dispersion of the latter within the reacting terpolymerization system[26]. However, when the mixed dispersants were pre-added at a high content of 1.0%, the excluded volume effect may come into play. In other words, the excess mixed dispersants could act as excess micelles to “expel” the terpolymer chains,which would in turn tend to form complex aggregates(e.g., physical gels) owing to intermolecular hydrogen bonding and possibly physical crosslinking between the aggregates. This scenario is analogous to polyacrylamide complexes formed with nonionic surfactants[27]and watersoluble polymer complexes formed with sodium dodecyl sulfate[28].
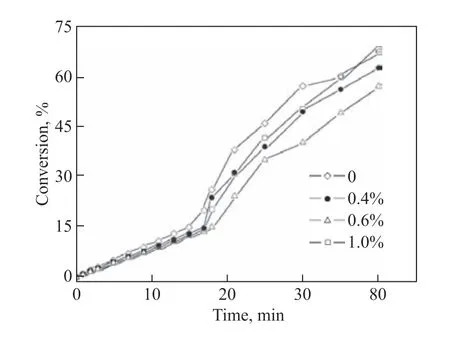
Figure 4 Effect of Span 80/Tween 80 content on monomer conversion as a function of reaction time
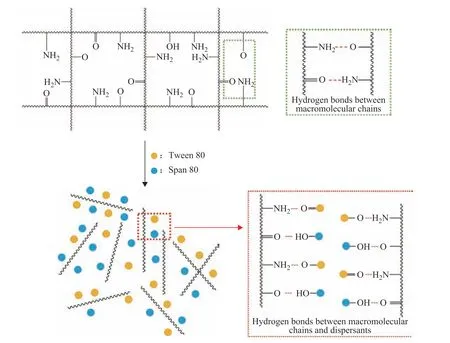
Figure 5 Schematic illustration of the binding forces between the mixed dispersant molecules and terpolymer chains
It is well known that the occurrence of gel effect is usually accompanied by a sudden change in polymerization temperature. As shown in Figure 6, the reaction temperature of the terpolymerization system was monitored as a function of time. Similar to the kinetic curves of the monomer conversion with respect to time(Figure 4), Figure 6 also revealed that the gel effect during polymerization was rather gradual, such that it was difficult to identify a specific time for the onset of gelation. Nevertheless, on the basis of the reaction times at which the temperature peaks were observed for the adiabatic terpolymerization systems containing the mixed dispersants at varying contents, it was still found that the addition of the dispersants efficiently retarded the gel effect during polymerization. It can also be discerned from Figure 6 that the rate of temperature increase was reduced upon the pre-addition of the mixed dispersants.The reaction times corresponding to the temperature peak increased from 70 min to 75, 78, and 83 min as the dispersant content was increased from 0 to 0.4%, 0.6%,and 1.0%, respectively. In addition, the temperature peaks for the terpolymerization systems containing any mixed dispersant content were reduced by approximately 10°C compared with the dispersant-free system. Similar to the kinetic curves of the monomer conversion with respect to time (Figure 4), the temperature peak decreased most markedly when the mixed dispersant content was 0.6%.The experimental results also showed that the addition of Span 80/Tween 80 prior to the terpolymerization decreased the possibility of a violent reaction and the gel effect resulting from the temperature increase.

Figure 6 Effect of Span 80/Tween 80 content on the reaction temperature as a function of time
Figure 7 shows the FTIR spectrum of the DMC/AM/NaAA terpolymer sample obtained using a mixed dispersant content of 0.4%. The broad absorption band at 3300–3500 cm-1and the sharp absorption band at 1675 cm-1were ascribed to the amide groups (–CONH2) in the AM units. Meanwhile, the sharp absorption bands at 1323 and 1559 cm-1(–CO2Na) and 1458 cm-1[–N+(CH3)3] were assigned to the NaAA and DMC units, respectively[24].

Figure 7 FTIR spectrum of the terpolymer sample obtained using a Span 80/Tween 80 content of 0.4%
The influence of the Span 80/Tween 80 mixed dispersant content on the molecular structure of the terpolymers was next investigated. Figure 8 presents the partial FTIR spectra in the 3000–4000 cm-1region for the terpolymers obtained using mixed dispersant contents of 0, 0.4%, 0.6%, and 1.0%. The broad absorption band corresponding to N–H stretching at approximately 3440 cm-1generally became sharper and shifted to higher wavenumber as the mixed dispersant content was increased. It is again worthwhile to note that the terpolymer sample obtained using a mixed dispersant content of 0.6% displayed the largest displacement of the characteristic peak (3450 cm-1). This suggests that the dispersant (0.6%) mediated an excellent separating effect in terms of the intermolecular crosslinking of the amphoteric polyacrylamide.
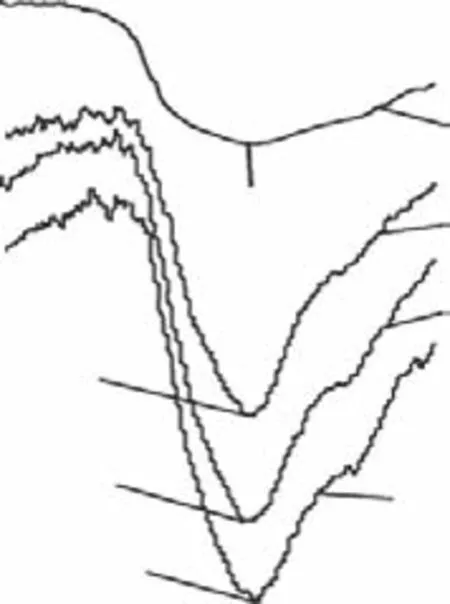
Figure 8 Partial FTIR spectra of the terpolymer samples obtained using various Span 80/Tween 80 contents
To examine whether the addition of the mixed dispersants could possibly provide satisfactory control over the gel effect during terpolymerization, the thermal stabilities of the various terpolymer samples were examined by TGA. Figure 9 presents the measured TGA curves in terms of instantaneous sample weight (as the weight percentage) as a function of temperature. It can be seen that the thermally induced weight loss (i.e., thermal degradation) primarily occurred within the temperature range of 200–400°C for all terpolymer samples. The TGA curves for the terpolymers obtained using different mixed dispersant contents were all shifted to lower temperature compared with the dispersant-free system. These results suggest that the addition of the mixed dispersants to the terpolymerization system lowered the inherent thermal stability of the polyacrylamide terpolymers. Furthermore,it can be inferred that the intermolecular hydrogen bonding in the amphoteric polyacrylamides became weaker because of the improved spatial dispersion effect induced by the addition of the mixed dispersants. Table 2 shows the temperatures at which the terpolymer samples displayed their maximum rates of thermal degradation(Tdm). It can be seen that the terpolymer sample obtained using a mixed dispersant content of 0.6% exhibited the lowest peak temperature (354 °C). In accordance with the above FTIR spectroscopy results, this again suggests that this sample had the weakest intermolecular hydrogen bonding and thus that this mixed dispersant content afforded the optimal dispersion effect.

Figure 9 TGA curves of the terpolymers obtained using various Span 80/Tween 80 contents

Table 2 Temperatures of maximum weight loss (Tdm) for the terpolymers obtained using various mixed dispersant contents
4 Conclusions
The amphoteric polyacrylamides prepared from terpolymerization systems containing mixed dispersants(Span 80/Tween 80 and Span 85/Tween 85) exhibited higher intrinsic viscosity and better water solubility than those obtained from other systems. The effect of mixed dispersant addition on the polymerization kinetics was monitored and comparatively assessed.Various terpolymer samples were characterized by FTIR spectroscopy and TGA. The obtained results revealed that the addition of the mixed dispersant Span 80/Tween 80 effectively suppressed the gel effect during terpolymerization and reduced the peak temperature of terpolymerization. Pre-addition of the mixed dispersant Span 80/Tween 80 to the terpolymerization system at various contents resulted in high-performance terpolymers with higher intrinsic viscosity and faster aqueous dissolution compared with single dispersants or the chain transfer agent IPA. In particular, the pre-addition of the mixed dispersants at a content of 0.6%–0.8% and HLB values of 5.0–6.0 afforded a polyacrylamide terpolymer with the optimal performance (e.g., the highest viscosity and the fastest aqueous dissolution) owing to the potent suppression of the gel effect during terpolymerization.
Overall, the reported results provide an advantageous approach for the preparation of high-performance amphoteric polyacrylamides of practical interest to the field of wastewater treatment. Furthermore, the discussion of the underlying mechanism of the mixed dispersants should prove valuable as a theoretical reference for the future development of highly effective dispersants suitable for preparing amphoteric polyacrylamides.
Acknowledgment:We’d like to acknowledge the Project of Science and Technology Program of Guangzhou City of China (Project Nol.201604020004) for the financial support to this research.
杂志排行
中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Application and Regeneration of a Non-Aqueous System of Cu/HCl and DMF for the Oxidation of Hydrogen Sulfide in Natural Gas
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
