Spectral and Morphological Analysis of Graphene-Based Two-Dimensional Carbon Materials
2023-10-23DiaoYuxiaXiangYanjuanXinMudiHeWenhuiXuGuangtongQiuLimei
Diao Yuxia; Xiang Yanjuan; Xin Mudi; He Wenhui; Xu Guangtong; Qiu Limei
(Sinopec Research Institute of Petroleum Processing Co., Ltd., Beijing 100083, China)
Abstract: This paper describes the spectral and morphological analysis of graphene, N-doped graphene, and graphene modified with functional groups. The similarities and differences in the surface and microstructure are characterized by infrared spectroscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy. Compared with high-purity graphene, the introduction of functional groups leads to more defects in the two-dimensional structure. The quality of graphene, reflected by the intensity ratio of peak D and G modes in the Raman spectroscopy, is consistent with that observed by scanning electron microscopy and transmission electron microscopy. The infrared spectra of graphene-based two-dimensional carbon materials are different from that of high-purity graphene, and the absorption peaks of the functional groups are obvious. The X-ray photoelectron spectroscopy results illustrate the diverse chemical states of carbon, and the atomic ratio of carbon to oxygen directly reflects the quality of the graphene-based materials. The results of electron microscopy and spectroscopic characterization of graphene samples provide an excellent basis for a wide range of applications in graphene production and quality control.
Key words: graphene; carbon material; Raman; FTIR; XPS; TEM
1 Introduction
Since Novoselov et al. discovered graphene by mechanical stripping in 2004[1], this novel two-dimensional (2D)carbon material has attracted tremendous research interest in applications such as sensing, energy storage,optoelectronics devices, and smart materials[2-4]. Graphene exhibits ultrahigh electron mobility (~2 × 105cm2/Vs),a significant room-temperature Hall effect, high light transmittance (single-layer graphene has 97.7% visible light transmittance), high mechanical strength (fracture stress ~130 GPa, Young’s modulus ~1.0 TPa), a high specific surface area (2630 m2/g), and high thermal conductivity (~5000 W/mK). Graphene production has evolved from the original “tape pulling” mechanical exfoliation to liquid-phase exfoliation[5], chemical redox[6],silicon carbide (SiC)[7], and chemical vapor deposition[8].At present, the large-scale production of high-quality graphene is one of the main challenges for graphene industrialization.
To enhance the excellent properties of graphene,chemical modification of graphene via doping using functional atoms and groups is extensively employed,resulting in graphene with more and better physical and chemical properties. For example, the conductivity and electrochemical performance of graphene can be improved by nitrogen atom doping[9]. Hydroxylated graphene- and carboxylated graphene-loaded metals or metal oxides can effectively catalyze flue gas denitration[10]. Thiolated graphene oxide composites have been successfully applied in photochemical sensors and metal ion adsorption[11-12]. Aminated graphene improves the removal rate of heavy metal pollution[13], and graphene oxides with carboxyl, hydroxyl, and other functional groups on the surface improve the water solubility of graphene, which is conducive to the preparation of intercalation materials or surface modification[14]. The market prospects of graphene and its derivatives have further accelerated the industrialization of graphene.
In terms of graphene industrialization, the standardization of preparation and analysis methods has become the only way to promote the development of graphene.Various national standards relating to the analysis of graphene have been released, such as the determination of surface oxygen-containing functional groups by titration[15], determination of graphene layers by Raman spectroscopy[16], measurement of graphene material thickness by atomic force microscopy[17], and the determination of graphene layers by optical contrast methods[18].
Graphene derivatives have several advantages in the packaging and electrochemical sensing trades of the food industry. Different derivatives display unique properties according to the functional groups effected in the chemical environment of the carbon skeleton[19]. Although the characterization and performance of graphene derivatives have been widely reported, the differences in the structural and physicochemical properties between graphene and its derivatives have not been comprehensively compared. In this work, the surface functional groups, structures, and spectra of graphene and its derivatives are characterized by image analysis methods, such as scanning electron microscopy (SEM) and transmission electron microscopy(TEM), and spectral analysis methods, such as X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), and Raman spectroscopy.The structural differences and spectral characteristics of different types of graphene are then analyzed. The present investigation offers some qualitative and quantitative guidance and references for the identification of graphenebased materials. Furthermore, the structural characteristics provide a basic foundation for the establishment of structure-activity relationships for graphene derivatives.
2 Experimental
2.1 Chemicals
The samples used in this study were purchased from a well-known reagent manufacturer in the market, and the commodity information was marked as (a) graphite, (b)graphene nanoplatelet aggregates (G-nano), (c) highpurity graphene (hp-G), (d) hydroxylated graphene (GOH), (e) carboxylated graphene (G-COOH), (f) thiolated graphene (G-SH), (g) aminated graphene (G-NH2), and (h)nitrogen-doped graphene (N-doped G).
2.2 Characterization
The morphology and structure of the graphene-based carbon materials were characterized with a field-emission scanning electron microscope (FESEM) (Hitachi S4800) and a high-resolution transmission electron microscope (HRTEM) (JEM-2100, JEOL Ltd, Japan).The SEM micrographs were obtained at 5 kV and a working distance of 8 mm. The TEM was operated at an acceleration voltage of 200 kV. The XPS (Thermo Fisher ESCALAB 250xi+) was conducted with 150 W AlKaradiation and the charging effect was calibrated using C1s of graphene at 284.3 eV. The Raman spectroscopy(Via Qontor Raman microscope) was performed using a laser excitation wavelength of 532 nm and a scan range of 700–3400 cm–1. The FTIR spectra were measured with an infrared spectrometer (Nicolect560, Thermo Fisher,USA) at 400–4000 cm–1per spectrum with a resolution of 0.5 cm–1and an average of 32 scans.
3 Results and Discussion
3.1 FTIR
FTIR is extensively used to identify organic functional composition. For example, FTIR has been applied to the qualitative characterization of functional groups in graphene, especially the oxygen-containing functional groups. The characteristic FTIR spectra of graphene and its derivatives are depicted in Figure 1. The natural graphite has no obvious infrared transmission peak.The FTIR of G-nano and hp-G are the same as that of graphite, indicating that there are no significant functional groups in these graphene materials. The spectrum band at 3400 cm–1(from 3000–3700 cm–1) can be attributed to the stretching vibration mode of hydroxyl groups (O–H)[20]. For the G-OH, G-COOH, G-SH, and N-doped G samples, there is a weak and broadened spectral peak in this region. We believe that –OH, –SH, –NH, or adsorbed water molecules exist in these graphene derivatives,which results in the formation of hydrogen bonds on their surfaces. There is a weak absorption peak around 2920 cm–1for G-OH, G-COOH, and G-SH, which may be caused by antisymmetric and symmetric expansion of CH2. The peak at 1724 cm–1is due to C=O stretching vibration (carbonyl/carboxyl)[21]. The characteristic peak at 1598 cm–1corresponds to the in-plane vibrations of aromatic C=C stretching. As the degree of oxidation increases and the conjugation effect of adjacent atoms or groups is enhanced, the carbon sp2hybrid-type atoms in the structure of the graphene derivatives gradually decrease in number, and the C=C bonds in the structure gradually become rarer. The peak around 1374 cm–1can be attributed to the C–O stretching vibration peak of the carboxyl group, which appears in graphene with hydroxyl and carboxyl functional groups. The peak around 1072 cm–1is ascribed to the stretching vibration of C–OH. The region from 1000–1450 cm–1mainly consists of C–H inplane bending vibration, C–O stretching vibration, and C–C single bond skeleton vibration. The spectral signals in this fingerprint area suggest that the surface bonding of G–SH is more complex. Figure 1 illustrates that heteroatom-doped graphene has obvious –OH absorption peaks at high wavenumbers and C=O stretching vibration peaks. The thiol groups are indicated by the presence of the sharp C–SH bending peak at 818 cm–1[22]. The peak position of FTIR is an important indicator for identifying oxygen-containing functional groups.
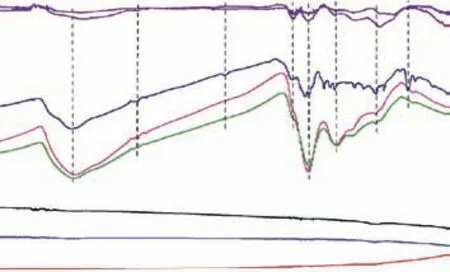
Figure 1 FTIR spectra of graphene-based 2D carbon materials
3.2 Raman spectroscopy
Raman spectroscopy is an important technique for characterizing sp2and sp3hybridized carbon atoms and for determining the graphene layers. Furthermore, the shape and size of the graphene can be confirmed by Raman data. The mechanically exfoliated graphene has a clear stacking mode and a large sample size; therefore, its Raman peaks exhibit a certain regularity with respect to the number of layers[23]. However, the microstructure of graphene sheets is significantly affected by the preparation method, resulting in significant differences in the Raman peaks. The single-layer graphene shows two typical characteristic peaks in the Raman spectrum[24]. One is the G-mode, which appears at approximately 1582 cm–1and is highly sensitive to strain effects in the sp2system.Consequently, it can be used to probe modifications on the flat surface of graphene. The other is the G'-mode,located at about 2700 cm–1, which reflects the interlayer stacking mode of C atoms and is strongly dependent on the frequency of the excitation laser energy. Therefore,this mode can be employed to determine the number of graphene layers. In addition, the D-mode signal related to the defects and impurities in graphene appears at about 1350 cm–1. Figure 2 presents the normalized Raman spectra of a series of commercialized 2D graphene materials. The intensity ratio of the D-mode and G-mode(ID/IG) accurately reflects the defect density in graphene.The G-mode and G'-mode of high-purity graphene are far more intense and sharper. In the series of materials analyzed, theID/IGratios are 0.18, 0.87, and 0.06 for the graphite, G-nano, and hp-G samples, which reflects the monolayer structure and low defect concentrations in these samples. For other samples with functional groups,the values are far larger. TheID/IGratios are 1.80, 1.88,1.66, 1.72, and 1.61 for G-OH, G-COOH, G-SH, G-NH2,and N-doped graphene, respectively, demonstrating the existence of defects in these modified graphene-based materials (Fig. 3a). The signals at 2700 cm–1disappear for graphene-based materials with functional groups on the surface. This may be caused by the increased layer spacing and the weakening of layer stacking in 2D materials caused by the successful doping of functional groups. The peak intensities of graphite, nano-G, and hp-G at the D-mode are weak, while those for graphene materials with functional groups on the surface or doped with heteroatoms are significantly stronger. These phenomena illustrate that the structural defects are significantly enhanced at the edges or within the layers.Accordingly, it can be concluded that the introduction of heteroatoms destroys the highly conjugated chemical structure on the graphene surface. The significant broadening of the G peak may be related to the hybrid transition from sp2to sp3. The D-mode height andID/IGpeak intensity ratio are consistently in good agreement.These structural results predict that graphene derivatives possess more chemically active sites and defects, which broadens the possible applications of graphene materials.The intensity ratio of the G'-mode and G-mode (IG'/IG) is related to the layers of graphene (Fig. 3b). The crystalline quality of graphene is reduced in the derivatives, as suggested by the increasedID/IGratio and the decreasedIG'/IGratio shown in Figure 3[25-26].

Figure 2 Raman patterns of graphene-based 2D carbon materials

Figure 3 (a) ID/IG and (b) IG'/IG in Raman spectra of graphene-based 2D carbon materials
3.3 XPS
The chemical states, chemical modification, heteroatom dopants, and quantitative elemental composition of graphene have been extensively investigated by XPS[27].Tables 1–3 list the quantitative XPS results for the elemental contents and C1s peak-fitting as acquired from the surfaces of graphene-based materials. The atomic ratio of carbon to oxygen (C/O) is often used to characterize the oxidation degree of graphene-based materials. Generally,graphene exhibits a C/O ratio of more than 20[28], while the corresponding value for graphene oxide is less than 3[29].Table 1 illustrates that the atomic ratio of C/O is greater than 30 for graphite, G-nano, and hp-G, and the ratio for the graphite sample is as high as 60. The full width at half maximum (FWHM) provides a useful indicator of chemical state changes. According to the FWHMs of the C1s peaks,the ordering degree of carbon gradually decreases from graphite to high-purity graphene.

Table 1 Elemental composition, C/O molar ratio, and relative C species content deduced from XPS results
Figure 4 depicts the C1s spectra of graphene derivatives. All spectra comprise a dominant asymmetric C=C line centered at a binding energy of about 284.4 eV, which is related to the sp2-hybridized π-conjugated carbon atoms. By means of XPS peak-differentiation-imitating analysis, the chemical states of C are classified as sp2graphite carbon (284.4 eV),sp3carbon (284.8 eV), hydroxyl or epoxy carbon species such as C–O, C–S, and C–N (285.8–286.4 eV), carbonyl carbon C=O (287.8 eV), and carboxyl carbon O–C=O (289.5 eV). Figure 4 exhibits the C1s deconvolution spectra for the samples. The content of C=C indicates that the majority of C species exist in the elemental form for the graphite, nano-G,and hp-G samples. The considerably high C–C content of these samples is caused by surface contamination, which is inevitable in XPS studies. This phenomenon suggests that the majority of C atoms in the samples that have not been modified by heteroatoms contain more sp2-hybridized π-conjugated structures and fewer sp3-hybridized carbon atoms connected with oxygen, which is consistent with the C/O ratios. On the contrary, the C=C peak in the graphene decreases by at least 10% compared with that of graphite.We speculate that, in the process of 3D graphite exploding to 2D graphene, a higher specific surface area makes it easier for the carbon species to be affected and oxidized by oxygen.Table 2 indicates that the C/O ratios of graphene derivatives are significantly reduced to 5–7, which is slightly higher than the indicator of 3 for graphene oxide. We believe that the introduction of functional groups destroys the ordered structure of the graphene lattice. Generally, in the functional process, oxidants or reducing agents are required, such as H2O2, NaBH4, NaNO3, and Na2SO3[30], and these inevitably introduce some trace impurities, such as Na, Cl, N, and S.A considerable amount of S is detected on the surface of G-SH, and a relatively high level of N is present on the surface of G-NH2and N-doped G. These results indicate that S and N have been successfully introduced into the graphene lattice, which agrees with the Raman analysis.By comparing the C1s spectra of graphene derivatives with graphite, nano-G, and hp-G, the C species connected with heteroatoms increase significantly, indicating the successful introduction of functional groups. This is consistent with the C/O ratios. Compared with the carboxylated graphene, the binding energies of C–N/C–S in the –SH, –NH2, and N-doped graphene are lower than that of C–O. This is because of the difference in electronegativity between S, N, and O.
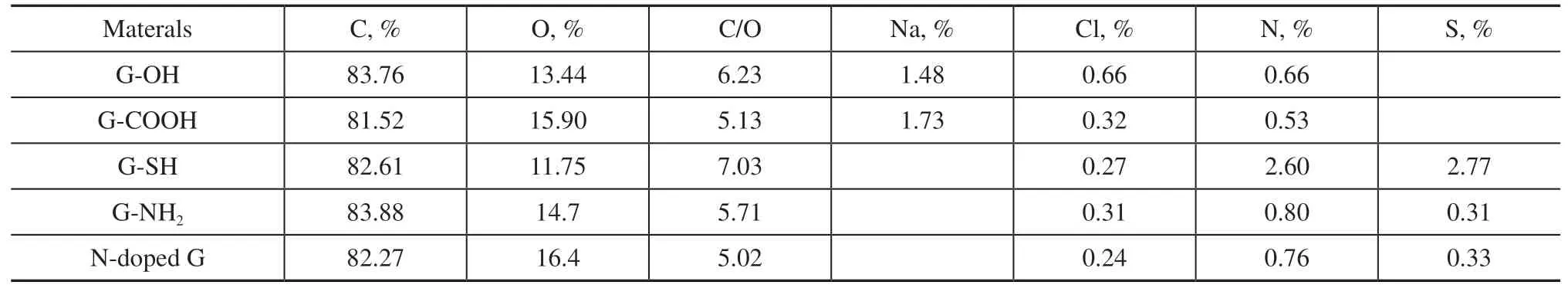
Table 2 Elemental composition of graphene derivatives from XPS results
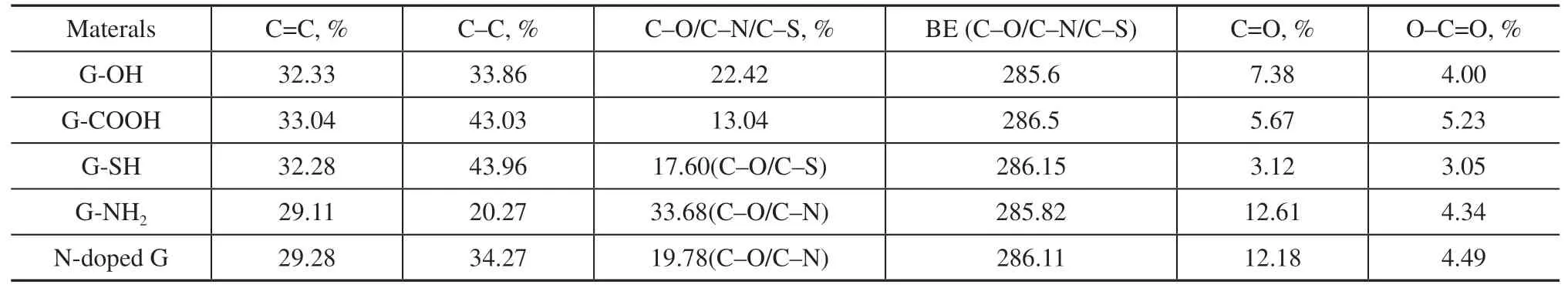
Table 3 Relative content of C species in graphene derivatives as deduced from the deconvolution results of C1s XPS spectra

Figure 4 C1s XPS spectra of graphene and derivatives
3.4 Image characterization
FESEM images reveal that the graphene-based materials consist of randomly aggregated, crumpled, and foldednanosheets. Figure 5 shows SEM images of this series of graphene-based materials. Natural graphite has a typical thick lamellar structure that is closely stacked between layers. Graphene flakes of various sizes overlap with each other in the graphene-based materials. The graphene sheet structure remains unchanged on the surface for samples with functional groups. Among them, the surface folds of G-OH are significantly decreased, and the sheet of N-doped G and G-NH2appears to be thickened in the SEM images.

Figure 5 SEM images of eight graphene-based 2D carbon materials: (a) graphite, (b) G-nano, (c) hp-G, (d) G-OH, (e)G-COOH, (f) G-SH, (g) G-NH2, (h) N-doped G
Atomic force microscopy is widely used to characterize low-dimensional materials and has been extensively applied to characterize few-layer graphene[31].Electron microscopes also enable a judgment of the quality of graphene. TEM is the intuitive identification method for graphene layers, allowing the layers to be estimated from high-resolution TEM images at the edges or folds.The thickness of single-layer graphene is 0.335 nm. The graphene layers stacked as single-, double-, or multilayer structures can be more accurately determined by electron diffraction[32]. As shown in Figure 6, the graphene exhibits good light transmission in the TEM experiments and good stability under electron beam irradiation. The aggregates of graphene nanochips have more folds and smaller lamellar sizes. High-purity graphene has obvious folds and thin layers, indicating that there are fewer layers. The appearance of these folds can reduce the surface energy of graphene and increase the stability of the 2D graphene lattice. To support the pore size distribution, the N2adsorption-desorption isotherms are shown for the graphite,G-nano, and hp-G samples in Figures 6(e) and 6(f).

Figure 6 TEM images of (a), (b) G-nano, (c), (d) hp-G, (e) N2 adsorption isotherms measured at 77 K of graphite, G-nano,and hp-G and (f) the pore size distributions
The pore size distributions for G-nano and hp-G exhibit characteristics of micropores, mesopores, and macropores compared with the graphite. This result is consistent with that observed by electron microscopy[33].
High-resolution TEM can easily identify the folded or curled graphene edges. Each layer of graphene corresponds to a dark line in the high-resolution lattice image. By counting the number of dark lines in the edge area, we can determine the individual graphene layers and atomic scale structural details. Figure 7a is a highresolution image of a typical area of G-nano aggregated in Figures 6a and 6b. A curly mass structure and large layered structure, with a thickness of more than 20 layers,can be observed at the edge. Figure 7b is a high-resolution image of a typical area of hp-G in Figures 6c and 6d.

Figure 7 HRTEM images of (a) G-nano and (b) hp-G
It is clear that the two graphene sheets consist of 4 and 8 layers, respectively. This characterization technique provides a relatively simple and fast approximation of the structure of multilayer graphene. For structures with only one or two layers, the edge structure is often not clear enough to be accurately judged by such images.Although it is possible to determine whether the graphene has a single- or double-layer structure from the intensity change of each diffraction point in a selected area of electron diffraction[34-35], this method is only suitable for bulk samples.Figure 8 shows TEM images of several commercial graphene derivatives. The edges of the graphene sheets are blurry compared with those of the hp-G sheet. Therefore, the introduction of functional groups and structural changes can be characterized by electron microscopy and spectroscopy.
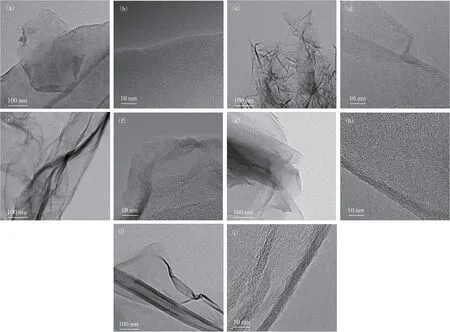
Figure 8 TEM images of (a), (b) G-OH, (c), (d) G-COOH, (e), (f) G-SH, (g), (h) G-NH2, (i), (j) N-doped G
4 Conclusions
This study comprehensively investigated a series of commercial graphene and graphene-based 2D carbon materials. We found that graphite is a compact lamellar structure with a certain thickness. SEM images show that the typical graphene is in the form of thin yarn with a low layer contrast and good peeling effect. The micromorphology of G-nano is porous and exhibits irregular clusters, whereas graphene structures have more than 10 layers because of aggregation in certain places. Highpurity graphene is a large film with wrinkles on its surface. High-resolution electron microscopy found that the number of graphene layers mostly ranges from 3–5.Raman spectroscopy results show thatID/IGis at least 0.06 in hp-G, close to the ratio of graphite, indicating that there are few surface defects in the lamellar structure.
Compared with hp-G, the graphene derivatives are relatively thick. TheID/IGratios of the graphene-based 2D carbon materials studied here are generally between 1.6–1.9, which may indicate sp3hybridization or lattice defects.The quality of graphene detected by Raman spectroscopy is consistent with that of the electron microscopy. XPS results show that the surface C1s absorption peak of the graphene derivatives is lower than that of graphene.The fitting of C1s peaks on the surface of the graphene derivatives shows that there are many oxygen-containing functional groups. XPS can be used to obtain the type and number of oxygen-containing functional groups on the surface of graphene. FTIR and XPS can be combined to obtain information on the type and quantity of oxygencontaining groups. It can be concluded from the results that electron microscopy and spectrum characterization are effective methods for identifying the quality of commercial graphene from different sources.
Acknowledgments:This work was financially supported by the Research Program of China Petrochemical Corporation(SINOPEC 420043-9 and 122074).
杂志排行
中国炼油与石油化工的其它文章
- Ultra-deep Removal of Metal Ions from Coal Tar by Complexation: Experimental Studies and Density Functional Theory Simulations
- A Metal-free Polyimide Photocatalyst for the Oxidation of Amines to Imines
- Effect of CeO2 on Activity of Catalysts CuO/ZnO/Al2O3/CeO2 for Synthesis of Methanol
- C9H10O2:0.5ZnCl2/SG as a High-Efficiency Catalyst for Desulfurization of Model Oil
- Effect of Mixed Dispersants on Suppression of the Gel Effect during Aqueous Adiabatic Terpolymerization of AM, NaAA, and DMC
- Pyrolysis Mechanism of a Cyclotriphosphazene-Based Flame-Retardant Epoxy Resin by ReaxFF Molecular Dynamics
