A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits
2023-10-21RuiJinRanTingWangMengYingTaoYueQinGouMingZhang
Rui-Jin Ran, Ting Wang, Meng-Ying Tao, Yue-Qin Gou, Ming Zhang
1Department of Ophthalmology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province,China
2Department of Ophthalmology, Minda Hospital of Hubei Minzu University, Enshi 445000, Hubei Province, China
Abstract
● KEYWORDS: surgical technique; endotamponade model; vitreous substitute; three-port vitrectomy; rabbit
INTRODUCTION
The 25-gauge transconjunctival sutureless vitrectomy is now widely applied in ophthalmological practice[1-3].Owing to this, an imbalance between advanced surgical techniques and the lack of perfect vitreous substitutes has been seen at present in this field.Considerable structural and biochemical differences between human vitreous bodies and the present available vitreous substitutes deter their clinical application, even though a large amount of research has focused on artificial vitreous body (AVB)[4-7].Many sightthreatening complications, such as intraocular hypertension,cataract formation, intraocular inflammation, and toxicity, were the major issues facing vitreous substitutes[8-9].
Many researchers have appliedin vivorabbit ocular modeling for endotamponade evaluation at present, especially for assessing the biocompatibility of a vitreous substitute.But their reports reflected the extreme difficulty of a standard three-port vitrectomy in rabbit’s eyes.Rabbits have a higher volume ratio of the lens to the vitreous chamber (1.27) than that of humans(0.25), which may cause an iatrogenic trauma to the lens and retina during a standard three-port vitrectomy[10].Besides,surgical exposure of eyeballs during the procedure was an intractable operation due to horizontal narrowness of palpebral fissure and softness of tarsal plates; a thin sclera may fail to incarcerate the trocar[11-13].In addition, it is urgent to establish an appropriate intraocular tamponade model for evaluating the efficacy and safety of new vitreous substitutes.
The cost for the use of rabbits in experiments is relatively low,and the sizes of their eyeballs are bigger than that of other small animals[14].Thus, rabbits are the best option for the endotamponade model in the evaluation of a novel vitreous substitute.The endotamponade models were successfully established using the modified surgical technique in rabbits,and through the models, the safety and efficacy of eightarm polyethylene glycols (8-arm PEGs) hydrogel were also evaluated.The modified technique in this experiment appeared to have few complications, less operation time, and satisfactory healing of sclera wounds in contrast with previous reports[11].
MATERIALS AND METHODS
Ethical ApprovalOur protocol was approved by the Animal Care and Use Committee and the Ethics Committee in Animal Experimentation of West China Hospital of Sichuan University(No.2020016A).
Preparations for the Vitreous SubstituteThe novel vitreous substitute was provided by the National Engineering Research Center for Biomaterials at Sichuan University.The two raw materials were 8-arm PEG modified with thiol termini(MW 20 000 Da) and 8-arm PEG-modified with maleimide termini (MW 20 000 Da).The patent on thein situcrosslinking hyperbranched polyether-based hydrogel (as a vitreous substitute) and synthesis method thereof have been granted in China (No.201810417292.7).According to the previous methods[15], the two materials were prepared with balanced salt solution (BSS; Alcon Laboratories, USA) within 2h before surgery, and stored at room temperature for later use.After the fluid-air exchange, the hydrogel (10 mg/mL) was formed byin situcross-linking within 1min after injecting the two prepared materials into the vitreous cavity togetherviaa double-lumen syringe.
AnimalsA total of 44 chinchilla rabbits (22 male and 22 female) were randomized into three groups, BSS (Alcon Laboratories, USA) group (n=14), silicone oil (SO; S5.7570 FRA) group (n=14), and AVB group (n=16).The rabbits were reared for environmental adaptation for 1wk before surgery.The surgery was only assigned to the right eye, with the left non-operated eye serving as the normal control.Rabbits with lens opacity, intraocular pressure of over 21 mm Hg, or retinal diseases were excluded.
Surgical ProcedureAll animals received an injection of 3% sodium pentobarbital (1 mL/kg body weight) through the auricular marginal vein for general anesthesia and a single drop of 0.4% oxybuprocaine hydrochloride ophthalmic solution for topical anesthesia.Their pupils were dilated by separately dropping tropicamide and phenylephrine 20min before surgery.The handheld rigid infusion catheter with a connector was designed to link with the 25-gauge needle to support the fixation of an infusion cannula and meanwhile provide a stable injection of BSS (Figure 1A).The 25-gauge needle combined with the catheter can be immediately and easily inserted into an infusion cannula when its trocar blade is taken out.The assistant pressed the handheld infusion catheter connected with a 25-gauge needle into the infusion cannula and as a whole inward towards the optic nerve to make a relatively fixed position.

Figure 1 The design of there-port vitrectomy in rabbits A: The structure of the handheld rigid infusion combination; B: The placement of three ports.
Three sclerotomies were transconjunctivally performed at the sites adjacent to the third eyelid of a rabbit and 3.5 mm from the corneal limbus using a 25-gauge blade trocar.The infusion cannula was first placed in the inferior nasal quadrant at the 5 o’clock position.BSS was continuously fed into the vitreous cavity through the handhold infusion catheter.The vitreous cutter and endoilluminator were then respectively placed in the superior nasal quadrant at 12 o’clock and 2 o’clock positions (Figure 1B), using a wideangle noncontact viewing system (Resight, Carl Zeiss,Germany) and the Constellation Vision System (Constellation,Alcon Laboratories, USA).Core vitrectomy was performed with a halogen light source without intravitreal injection of triamcinolone acetonide, to observe the biocompatibility after implantation of those vitreous substitutes (video of the surgical procedure is available at https://drive.google.com/file/d/1_LZ3mPyGUtveTqwthr4cMXIS8loyE1My/view?usp=drivesdk).There were no sutures for all incisions.The retinal breaks were made by gently touching the retina with the distal tip of a vitreous cutter blade and then were filled with SO or AVB about 0.8-1.0 mL.The operated eyes were administrated with tobramycin dexamethasone eye ointment(Alcon Laboratories, USA) following surgery.The 0.5%levofloxacin ophthalmic solution (Santen Pharmaceutical,Japan) was administered to the eyes three times a day after surgery.No other treatments were applied after surgery.
Postoperative ExaminationThe anterior segment of the eye,retina, and intraocular pressure were examinedviaslit-lamp microscope, ophthalmoscope, and rebound tonometer (iCare,Finland), respectively.The data or images were recorded at 1, 3, 7, 14, and 28d after surgery, and images of spectraldomain optical coherence tomography (SD-OCT; Heidelberg,Germany) were obtained at 1, 2, and 4wk after surgery by realtime tracking and scanning the eyes of rabbit models.Fundus photography (Carl Zeiss, Germany) was performed 4wk after surgery.
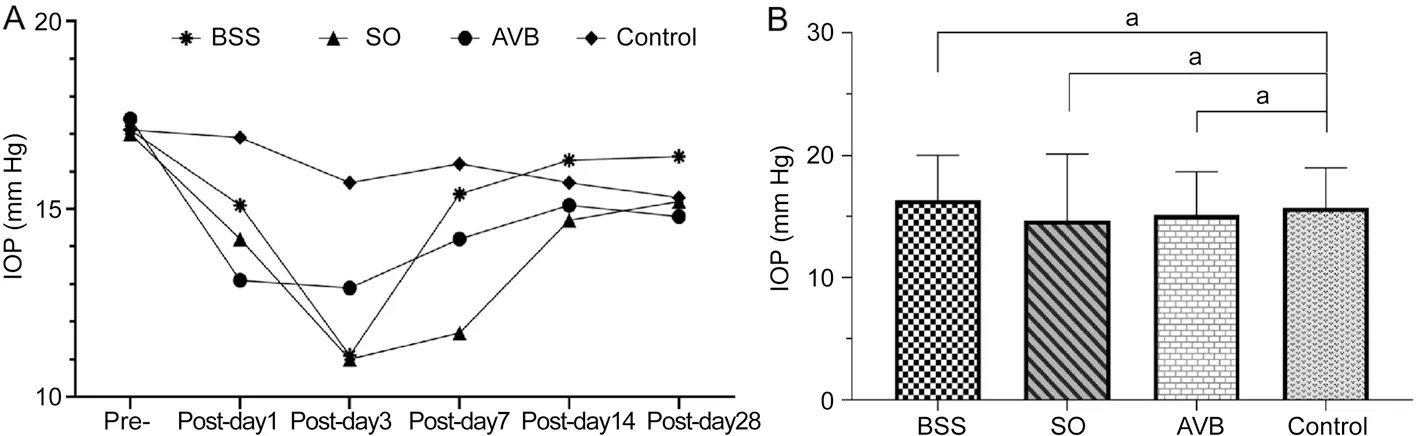
Figure 2 The changes of intraocular pressure A: Intraocular pressure at different times before and after surgery in the BSS, SO, and AVB groups;B: Intraocular pressure was analyzed between the operated groups and the normal control group 2wk after surgery (aP>0.05).BSS: Balanced salt solution; AVB: Artificial vitreous body; SO: Silicone oil.
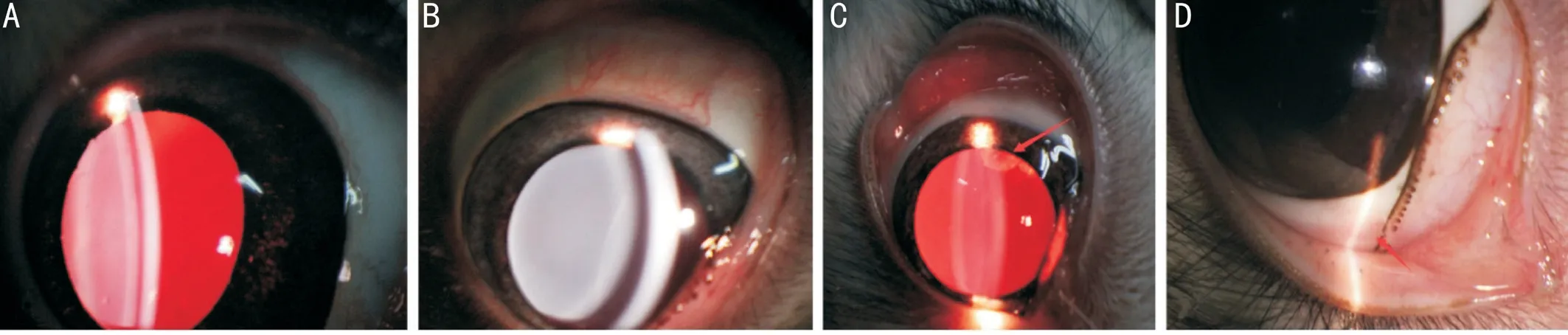
Figure 3 Images of the anterior segment after implantation of the artificial vitreous body A: Mild inflammation; B: Transparent cornea and lens; C: Traumatic crystal opacity; D: The healing of puncture sites.
Histopathological ExaminationThe enucleated eyeballs in all groups were subjected to 4% paraformaldehyde solution at 4℃ overnight 4wk after surgery.The eyeballs were then dehydrated, transparentized, and embedded in paraffin blocks.Sections were taken at 5 μm thickness with a microtome and transferred to triethoxysilane-coated glass slides.After being dewaxed and washed thoroughly, these sections were stained with hematoxylin-eosin (H&E), and then the sections were dehydrated and mounted with neutral resins.The images were obtained using a Leica IM50 microscope (Leica, Cambridge,UK).
Statistical AnalysisMeasurement data were presented as mean±standard deviation.GraphPad Prism (version 8.4.3) was used to analyze and graph a set of data.One-way analysis of variance followed by Bonferroni’s post hoc test was performed to analyze the differences between two or more independent groups.P-values<0.05 were considered statistically significant.
RESULTS
Evaluation of theOperative EffectThe modeling for the evaluation of intraocular tamponade was successfully performed in the 44 chinchilla rabbits.The mean operation time (calculated from the start of incision making to the end of the removal of a trocar) was 4.51±1.25min in all operated groups, and that of the BSS group was only 3.40±0.91min.Four eyes (9.1%) presented limited lens touch and two eyes(4.5%) showed retinal touch during surgery.Incision leakage was found in three eyes (6.8%) after surgery.There was no endophthalmitis, hemorrhage, or retinal detachment during the observation period.
Intraocular PressureLooking at the changes in intraocular pressure in all groups at different time points after surgery in Figure 2A, intraocular pressure presented a downward trend in the first week after surgery in all operated groups,but their average values were in the normal range.No significant difference between the operated eyes (BSS,P=0.62;SO,P=0.5; AVB,P=0.64) and the normal control eyes in intraocular pressure was shown 2wk after surgery (Figure 2B).
Images of the Anterior SegmentAs shown in Figure 3A,the anterior segment had a mild inflammation response after implantation of AVB 1d after surgery.The lens remained transparent (Figure 3B) and the traumatic crystal opacity(Figure 3C) was not aggravated 4wk after surgery in the AVB group.Satisfactory healing of the sclera wounds was seen without apparent scar formation 4wk after surgery(Figure 3D).
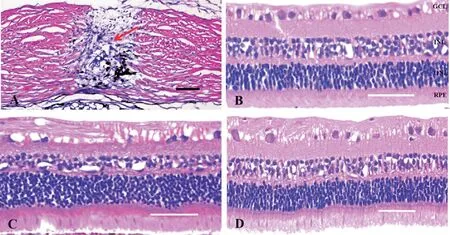
Figure 4 Hematoxylin-eosin-stained images A: The scleral puncture wounds (the red arrow) 4wk after surgery; B: The retinal tissues 4wk after filling with BSS; C: The retinal tissues 4wk after filling with SO; D: The retinal tissues 4wk after filling with AVB.Scale bars are 100 μm in (A) and 50 μm in (B-D).BSS: Balanced salt solution; AVB: Artificial vitreous body; SO: Silicone oil; GCL: Ganglion cell layer; INL: Inner nuclear layer; ONL:Outer nuclear layer; RPE: Retinal pigment epithelium.
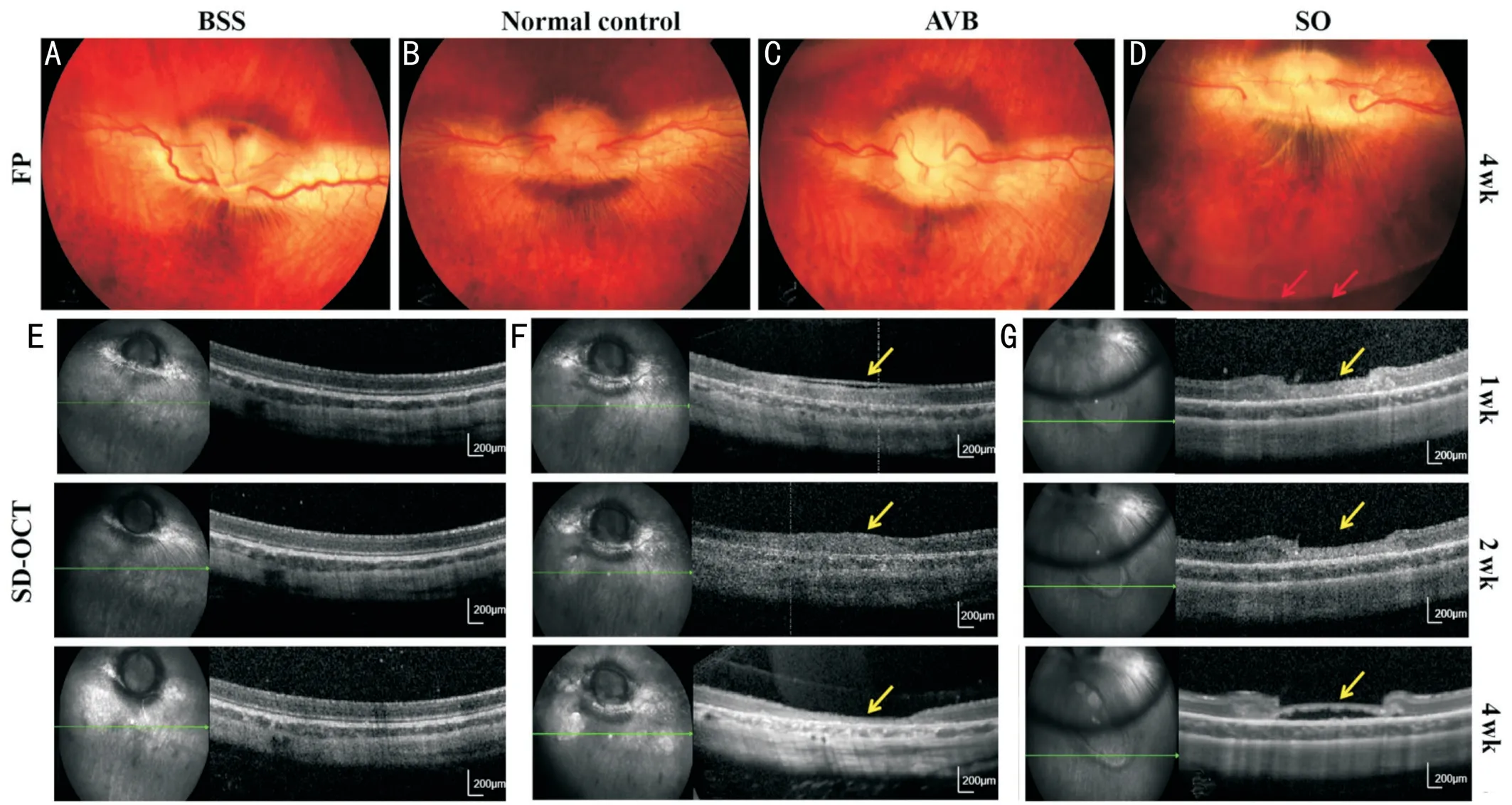
Figure 5 Fundus photographs and SD-OCT images A, C, D: Fundus photographs after implantation of BSS (A), AVB (C), and SO (D) 4wk after surgery; B: Image of the normal control eye; E, F, G: The images obtained by SD-OCT 4wk after surgery showed retinal breaks (the yellow arrows) after implantation of AVB (E, F) and SO (G).The red arrows in D point to the SO-fluid interface.Scale bars were 200 μm in E-G.SD-OCT:Spectral-domain optical coherence tomography; BSS: Balanced salt solution; AVB: Artificial vitreous body; SO: Silicone oil.
Histopathological ExaminationThe H&E-stained whole slide images showed that the retinal tissues presented no apparent inflammation cells in the three groups treated by filling with BSS (Figure 4B), SO (Figure 4C), and AVB(Figure 4D) separately 4wk after surgery.Normal structure and morphology were well maintained.No disordered arrangement of cells was found in the ganglion cell layer, inner nuclear layer, outer nuclear layer, and retinal pigment epithelium in each group.In addition, the puncture sites healed well in the sclera and were filled with fibroblasts and no significant inflammatory cells 4wk after surgery (Figure 4A).BSS is a commonly used agent of intraocular endotamponade in clinics, and it causes no structural damage to the retina and no serious inflammatory reactions after implantation.No obvious structural changes and inflammatory cell infiltration were found in the H&E-stained images of the BBS-filled rabbit eyes in our study.
Fundus Photographs and SD-OCT ImagesFundus photographs showed that the morphology of the optic disk,retina, and vessels thereof was clear after implantation of the vitreous substitutes, along with no hemorrhage or edema(Figure 5A-5D).The AVB maintained good transparency without clustered opacities.According to SD-OCT, the AVB showed intimate adherence to the retina, with no presence of edema, vacuoles, subretinal fluid, or other lesions in the eyes 4wk after surgery (Figure 5E).The retinal breaks (the yellow arrows) were repaired well without retinal detachment, which was successively recorded by SD-OCT 4wk after implantation of the AVB (Figure 5F).Nevertheless, the retinal breaks (the yellow arrows) appeared to worsen after filling with SO when receiving no laser treatment (Figure 5G).
DISCUSSION
To our knowledge, there are rare reports on the surgical techniques of vitrectomy in animals.In practice, it is considerably difficult to apply the standard three-port pars plana vitrectomy in rabbits[4,11,16]; while in humans, the surgically induced corneal astigmatic changes were minimum but insignificant following standard 25-gauge transconjunctival sutureless vitrectomy[17].As reported by Barthet al[11], major surgical complications appeared in 14/60 (23.3%) rabbit eyes with the standard vitrectomy, and traumatic cataracts and retinal breaks had exceeded 20% severally among treated eyes.Huet al[18]extended the scleral incision and sutured infusion cannula to reduce the complications of vitrectomy.But the operation time is prolonged, along with an increased possibility of aggravated inflammatory response and even the presence of endophthalmitis after surgery.Consequently,it is necessary to minimize ocular trauma, and there is a hint here that the tamponade materials could induce an intraocular inflammatory response.In our work, the modified technique could successfully establish the endotamponade model in rabbits, with only four eyes (9.1%) presenting lens touch, two eyes (4.5%) presenting retinal breaks, and no eyes presenting endophthalmitis, vitreous hemorrhage or retinal detachment.In addition, our surgical procedureviathe modified approach did not lead to the removal of any lens of the treated eyes,which is beneficial for the evaluation of the effect of vitreous substitutes.As manifested in clinical studies, removal of the lens could increase the risk of secondary glaucoma and corneal endothelial injury[19], which supports the benefit.
由于疾病缠身,患者可有易感因素,总疑心自己是否身患重症,诊断是否明确,治疗是否有效等,患者集中心思于疾病上,使精神更加紧张,症状反而加重,症状的恶化更加重了患者焦虑和多疑,易形成恶性循环[3]。所以要告诉患者神经衰弱不是说患者心理不健康或者更严重解释为精神病、精神分裂症,它只是由于患者因长期的情绪紧张和精神压力,使个体精神活动能力减弱的状态。造成这个病症的原因有恋爱受挫、人际交往中的困顿,家庭、婚姻或工作中的重重压力等等。让患者明确这个病在当今社会上很常见,并非难以治愈,只要积极配合治疗,很快就能出院。
Three incisions near the third eyelid of the rabbits allied with the handheld infusion combination have not been described before.Although the “all-nasal” approach in stage 4B retinopathy of prematurity was adopted by Dograet al[20], the infusion catheter could not place at the center of the paranasal sclerotomy because of the hypertrophic third eyelid in rabbits.For further research, we also attempted to set the handheld infusion combination at the opposite place of the third eyelid(outer canthus) and the other two incisions were placed on both sides of the third eyelid but failed for the vitrectomy.Surgical exposure of the eyeball in rabbits was harder than that in humans, and the inserted soft infusion catheter would easily slip out due to the thinness of the sclera.Therefore,we designed a handheld rigid infusion catheter combined with a connector to link with a 25-gauge needle and thereby together fix the infusion cannula as well as the eyeball when the other two sclerotomies were being performed.Compared with the standard three-port vitrectomy, the distance between the vitreous cutter and illuminator could be too close in our approach, whereas it had little impact if only the core vitreous was removed.
The sclerotomies healed rapidly with less leakage or mild inflammatory response in our experiment, which could probably attribute to the covering of the hypertrophic third eyelid on the sclerotomy sites.Four weeks after surgery, H&Estained whole slide images indicated that the sclerotomies were completely healed with no presence of abnormal inflammation cells.Sclerotomies were made at 1-4 mm from the corneal limbus in previous studies[4,11,21], while Ahnet al[21]held the view that the anterior vitreous could be removed easily at 4 mm from the corneal limbus.But the latter could incur retinal breaks and vitreous hemorrhage.Our model demonstrated that 3.5 mm from the corneal limbus was a proper distance for sclerotomiesviathe modified approach.
Less time (<1min) was used forin situforming of the novel hydrogel, which was significantly shorter than forming time of the hydrogel (about 10min) fabricated by Hayashiet al[4].The novel AVB exhibited good transparency and better ability to help repair the retinal breaks than the clinically used vitreous substitutes such as SO[5,7,22-24].Hyaluronic acidbased cross-linked (with N-vinyl-pyrrolidone) biopolymers from the study of Gonget al[25]have good biocompatibility in rabbit eyes and could be used as vitreous substitutes.In our previous research[15], the novel material showed excellent biocompatibility.
In conclusion, this work provided a practical surgical technique for improving the standard three-port vitrectomy in rabbits to establish proper models for the evaluation of an endotamponade substitute.The 8-arm PEGs might serve as a promising candidate in the treatment of vitreoretinal diseases.The modified surgical approach appeared to be efficient and had few complications, which is worthy of application in establishing animal endotamponade models.
ACKNOWLEDGEMENTS
The authors thank Prof.Jun Cao who provided and synthesized the AVB in National Engineering Research Center for Biomaterials, Sichuan University.
Foundations:Supported by the Project of National Key Research and Development (No.2018YFC1106103); the Health Commission of Hubei Province Scientific Research Project (No.WJ2019Q024).
Conflicts of Interest: Ran RJ,None;Wang T,None;Tao MY,None;Gou YQ,None;Zhang M,None.
猜你喜欢
杂志排行
International Journal of Ophthalmology的其它文章
- A novel pathogenic splicing mutation of RPGR in a Chinese family with X-linked retinitis pigmentosa verified by minigene splicing assay
- Vault predicting after implantable collamer lens implantation using random forest network based on different features in ultrasound biomicroscopy images
- Multiple evanescent white dot syndrome relapse following BNT162b2 mRNA COVID-19 vaccination
- Acute micro-macular hole associating with extensive intraoperative rotation of implantable collamer lens without ophthalmic viscosurgical device assistance: a case report
- Effectiveness of conjunctival bleb scarring by knockdown of heat shock protein 47 in rat model
- Effect of miR-27b-3p and Nrf2 in human retinal pigment epithelial cell induced by high-glucose
