Radiation therapy prior to a pancreaticoduodenectomy for adenocarcinoma is associated with longer operative times and higher blood loss
2023-10-21KristAploksMinhaKimStephanieStroeverAlexanderOstapenkoYoungBoSimAshwinkumarSooriyakumarArashRahimiArdabilyRamanathanSeshadriXiangDaDong
Krist Aploks, Minha Kim, Stephanie Stroever, Alexander Ostapenko, Young Bo Sim, Ashwinkumar Sooriyakumar, Arash Rahimi-Ardabily, Ramanathan Seshadri, Xiang Da Dong
Abstract
Key Words: Pancreaticoduodenectomy; Pancreatic adenocarcinoma; Neoadjuvant chemoradiation; National Surgery Quality Improvement Program; Whipple procedure; Operative time
INTRODUCTION
Pancreatic adenocarcinoma currently represents the fourth leading cause of cancer-related deaths in the United States. In patients suffering from the disease, an R0 resection is the primary and preferred method of treatment[1,2]. While this is often obtainable in patients with early-stage pancreatic malignancies, roughly 85% of patients present with disease that is not amenable to cure with surgical resection alone[3]. The tumors in many of these patients are considered borderline resectable, defined by National Comprehensive Cancer Network (NCCN) guidelines as contact with 180 degrees or less of the superior mesenteric artery or the celiac artery, contact with the common hepatic artery, and/or contact with the superior mesenteric vein/portal vein resulting in contour irregularity or vein thrombosis[4]. According to the NCCN guidelines, such cancers may benefit from treatment with neoadjuvant therapy in the form of chemoradiation[5].
When compared to adjuvant therapy, neoadjuvant chemoradiation has the theoretical benefit of testing for chemosensitivity, increasing the control of micrometastases and circulating tumor cells, ensuring a higher rate of systemic therapy completion, potentially downstaging a disease to make it amenable to surgical therapy and reducing pancreatic leak rates[6-8]. Multiple studies have demonstrated the morbidity benefits that comes with neoadjuvant treatment of patients with borderline resectable pancreatic adenocarcinoma prior to surgery compared with just surgical treatment alone. Among others, these benefits include a decrease in tumor size at the time of surgery, decreased number of positive lymph nodes, fewer organ space infections, and fewer post-operative pancreatic fistulae[9-12].
Despite the purported benefits, neoadjuvant therapy is still regarded with caution and its use remains low in the United States[13]. It is theorized that neoadjuvant therapy increases the rate of gastrointestinal toxicity and impairs postoperative wound healing, which can result in preoperative decompensation and subsequent post-operative morbidities.Neoadjuvant therapy can also theoretically delay definitive surgical resection, which can lead to the development of metastatic disease in non-responders. Radiotherapy, in particular, has been associated with disruption of the pancreatic tissue planes, which is thought to make eventual surgical resection more difficult[14]. Numerous studies have contradicted these ideas, showing that neoadjuvant therapy results in little to no intra- and post-operative increases in morbidity and mortality[9,10,15]. To date, however, many of these prior studies are plagued by single institution analysis with small sample sizes. These samples are even smaller when looking at the number of participants who received neoadjuvant radiotherapy, as chemotherapy remains the lion’s share of neoadjuvant therapy.
The aim of this study was to investigate the effects that neoadjuvant radiation therapy have on both intra-operative and 30-d postoperative morbidities using a nationwide dataset. We specifically hypothesize that among patients receiving a pancreaticoduodenectomy for pancreatic adenocarcinoma, those that also undergo neoadjuvant radiotherapy are more likely to have longer operative times and a perioperative transfusion compared with those who simply receive surgery alone.
MATERIALS AND METHODS
Study design and participants
We performed a cross sectional study utilizing data from the American College of Surgeons (ACS) National Surgery Quality Improvement Program (NSQIP) database from January 1, 2015 to December 31, 2019. Data from the standard public use file was merged with that from the NSQIP Targeted Pancreatectomy Participant Use Data Files (a separate collection of pancreas-specific variables) using the Case Identification Number (CASEID) variable. Patients older than 18 years old, who had a histological diagnosis of pancreatic adenocarcinoma and underwent a pancreaticoduodenectomy,were included in this study. Patients undergoing resection were identified by one or more of the following additional CPT codes: 48150, 48152, 48153, 48154. This study was exempt from our institutional review board review since the data was de-identified and obtained from a participant use data file.
Variables
From the patients included in the study, two groups were formed based on the independent variable of interest: those that received neoadjuvant radiotherapy prior to a pancreaticoduodenectomy, and those that had progressed directly to surgery. For the purposes of this study, neoadjuvant radiotherapy was defined as those receiving treatments within 90 d of the index operation. The primary outcomes of interest were perioperative blood transfusions (defined by the need for a blood transfusion within 72 h of surgery start time; OTHBLEED variable) and total operative time (defined by operative time in minutes; OPTIME variable). The secondary outcomes of interest were 30-d post-operative morbidities and mortality. Specific variables included rate of the following: wound dehiscence, ventilator dependence, stroke, myocardial infarction (MI), deep venous thrombosis (DVT), pulmonary embolism (PE), pneumonia, urinary tract infection, septic shock, superficial surgical site infection (SSI), organ space SSI, 30-d re-operation, 30-d mortality, 30-d readmission, renal failure, hospital length of stay, duration of pancreatic drain, pancreatic fistula, and delayed gastric emptying. Pancreatic fistulae were defined according to the International Study Group for Pancreatic Fistula grading scheme[16].
Statistical analyses
StataSE was used for the statistical analyses. Descriptive statistics including mean ± standard deviation for normally distributed continuous variables, median/interquartile range for skewed continuous variables, and number/percentage for categorical variables. We assessed bi-variable differences in outcomes between patients with upfront surgery and neoadjuvant radiotherapy with surgery using the χ2test, univariable logistic regression, and Fisher’s exact test for categorical variables. Two-tailed Student’s t-tests were used for continuous variables. Variables that were statistically associated (α < 0.05) with both the outcome and the independent variable, as well as those that were predicted theoretical confounders, were included in the multivariate regression analyses. Multivariable negative binomial regression was used for the total operative time variable, and multivariable logistic regression was used for the remaining secondary variables. We used stepwise, backward selection, and tested full/reduced models with the likelihood ratio test to determine the most parsimonious model. For 30-d outcome variables occurring less than 5% of the time, multivariable regression was not performed. For variables missing less than 5% of data, the listwise deletion method was used.Variables missing greater than 5% of data were reported as “unknown” in the tables.
RESULTS
Query of the ACS-NSQIP database identified a total of 11775 patients who underwent surgery for pancreatic adenocarcinoma from 2015 to 2019. Patients who had data missing with regards to neoadjuvant radiation therapy status, and patients in whom SSIs were reported upon admission, were excluded from the analyses. This left a final cohort of 11458 patients. The cohort was then split into two study groups based off neoadjuvant radiotherapy exposure: 1470 patients(12.8%) received neoadjuvant radiation therapy, compared with 9988 (87.2%) patients who proceeded directly to surgery(Figure 1).
Patients undergoing neoadjuvant radiotherapy were more likely to be younger, female, non-Hispanic white, diabetic,and of normal body weight (allP< 0.04). Conversely, patients undergoing surgery without radiotherapy were more likely to be Hispanic, overweight/obese, and jaundiced (allP< 0.002). They were also more likely to have chronic obstructive pulmonary disease, dyspnea, and hypertension (allP< 0.05) (Table 1).
With regard to tumor characteristics and operative approaches, patients receiving neoadjuvant radiotherapy were more likely to have a lower T-stage, lower N-stage, receive an elective surgery, have a higher wound class, and have a smaller pancreatic duct size (allP< 0.04). Such patients were also more likely to undergo an open surgical approach that involved resection of an artery or vein (allP< 0.001) (Table 2).
Within the first 30 d following surgery, bi-variable statistical analyses revealed that patients receiving surgical treatment only were more likely to experience an MI, PE, pneumonia, organ space infection, delayed gastric emptying,and pancreatic fistula (allP< 0.035). Such patients also had a higher 30-d mortality rate, longer hospital stay, a drain thatremained in place after 30 d, and a longer total operative time (allP< 0.041). Patients undergoing neoadjuvant radiation were more likely to receive a DVT and receive a perioperative transfusion (allP< 0.024). On multivariate analyses of the 30-d outcomes that occurred at a rate of greater than 5% in both study groups, neoadjuvant radiotherapy was associated with longer total operative times and the need for a perioperative transfusion. Patients undergoing neoadjuvant radiotherapy, however, were statistically less likely to acquire an organ space infection or a pancreatic fistula compared with patients who underwent surgery alone (Tables 3 and 4). While total hospital stay, presence of drain on postoperative day 30, and delayed gastric emptying occurred at significantly lower rates in the neoadjuvant therapy group upon univariate statistical analyses, this difference was not statistically significant on multivariable analyses.
DISCUSSION
In the past, numerous studies have implicated single and multi-agent neoadjuvant chemotherapy with both tumor downstaging and an increased rate of R0 resections[17,18]. In comparison with neoadjuvant chemotherapy, however,evidence demonstrating similar advantages with neoadjuvant radiation therapy have been somewhat sparce. In 2019,Jianget al[19] used the NCDB database to show an increased R0 resection and overall survival rate among patients who utilized neoadjuvant stereotactic body radiation therapy in addition to neoadjuvant chemotherapy compared to just neoadjuvant chemotherapy alone[19]. A paper by Chunget al[20] showed that higher doses of radiation (i.e.intensitymodulate radiation therapy) corresponded to increased 1 year survival and progression-free survival with no significant increase in short or long-term side effects[20]. Upon initial analyses of our study data, we found that patients undergoing neoadjuvant radiotherapy prior to surgery had smaller tumors and less positive lymph nodes on pathologic staging compared with those undergoing surgery alone. This suggests that neoadjuvant therapy successfully worked to downstage the tumors prior to surgical re-section. Although this effect could be confounded by the substantial difference in baseline demographic data between the two study groups, these results are nearly identical to those found in similarly designed NSQIP studies comparing neoadjuvant chemoradiation to surgery alone[9].
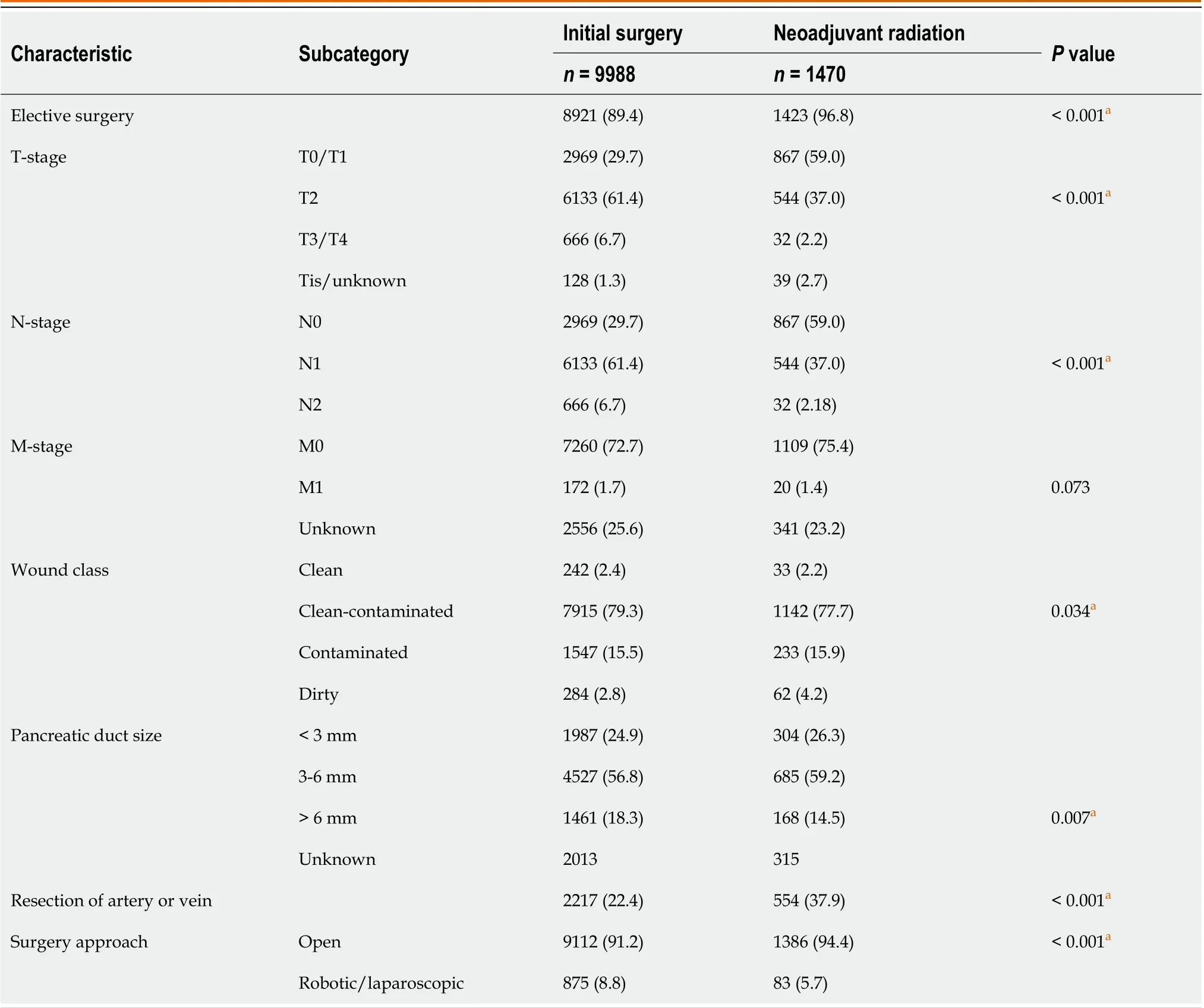
Table 2 Operative characteristics of patients with adenocarcinoma of the pancreas undergoing a pancreaticoduodenectomy from 2015 to 2019, n (%)
When examining our two primary outcome variables, we found a statistically significant increase in total operative time and perioperative transfusion requirements among patients receiving neoadjuvant radiation therapy compared to just surgery alone. This is the first time that such associations have been reported using multivariable analyses with patients receiving only neoadjuvant radiotherapy (vsneoadjuvant radiotherapy and/or chemotherapy). An analysis of 2005-2010 NSQIP data from Choet al[14] found similar results, but with bi-variable analysis only[14]. Similarly, a study using NSQIP data from 2014 to 2015 showed that the perioperative transfusion requirement rate among patients receiving neoadjuvant therapy (chemotherapy and/or radiotherapy) was significantly higher than the rate in patients who progressed directly to surgery[21]. In 2021, Krellet al[22] showed that (with propensity score matching) there were increased total operative times and more frequent perioperative blood transfusions among those receiving neoadjuvant therapy (chemotherapy and/or radiotherapy) prior to surgery compared to those undergoing surgery alone[22].
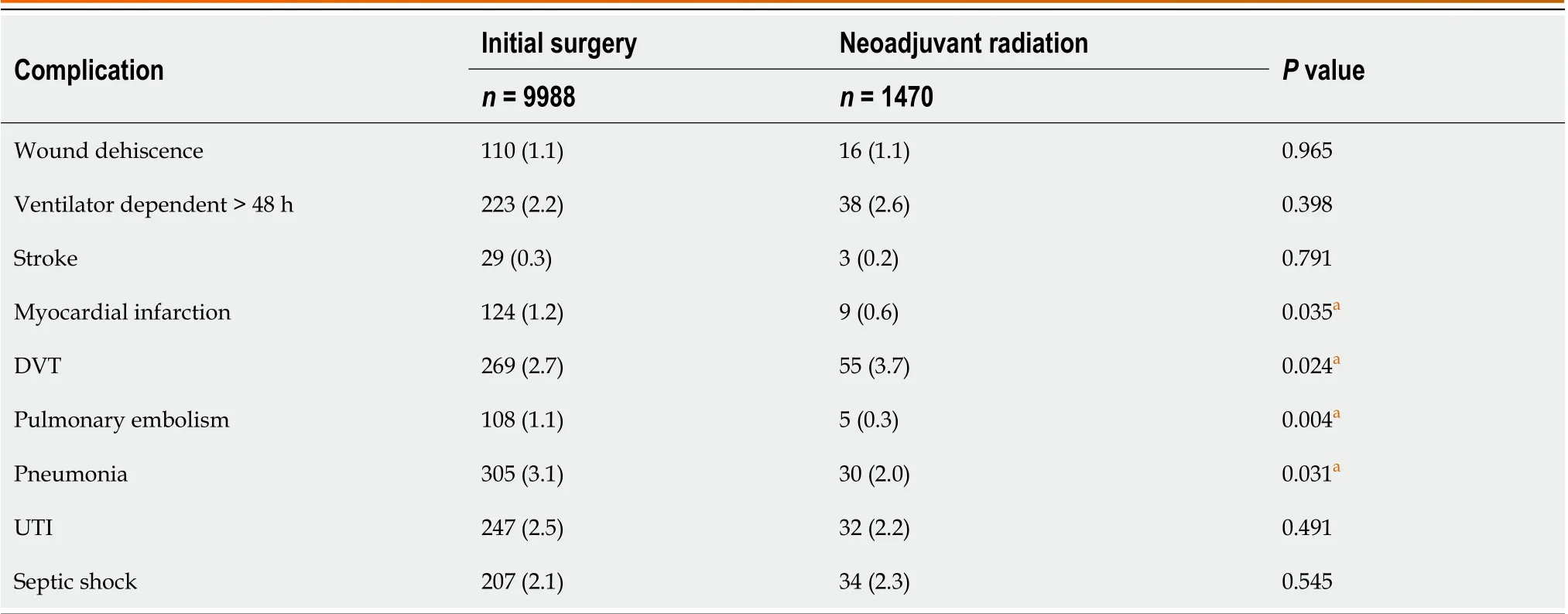
Table 3 Thirty-day postoperative complications for patients with adenocarcinoma of the pancreas undergoing a pancreaticoduodenectomy from 2015 to 2019, n (%)
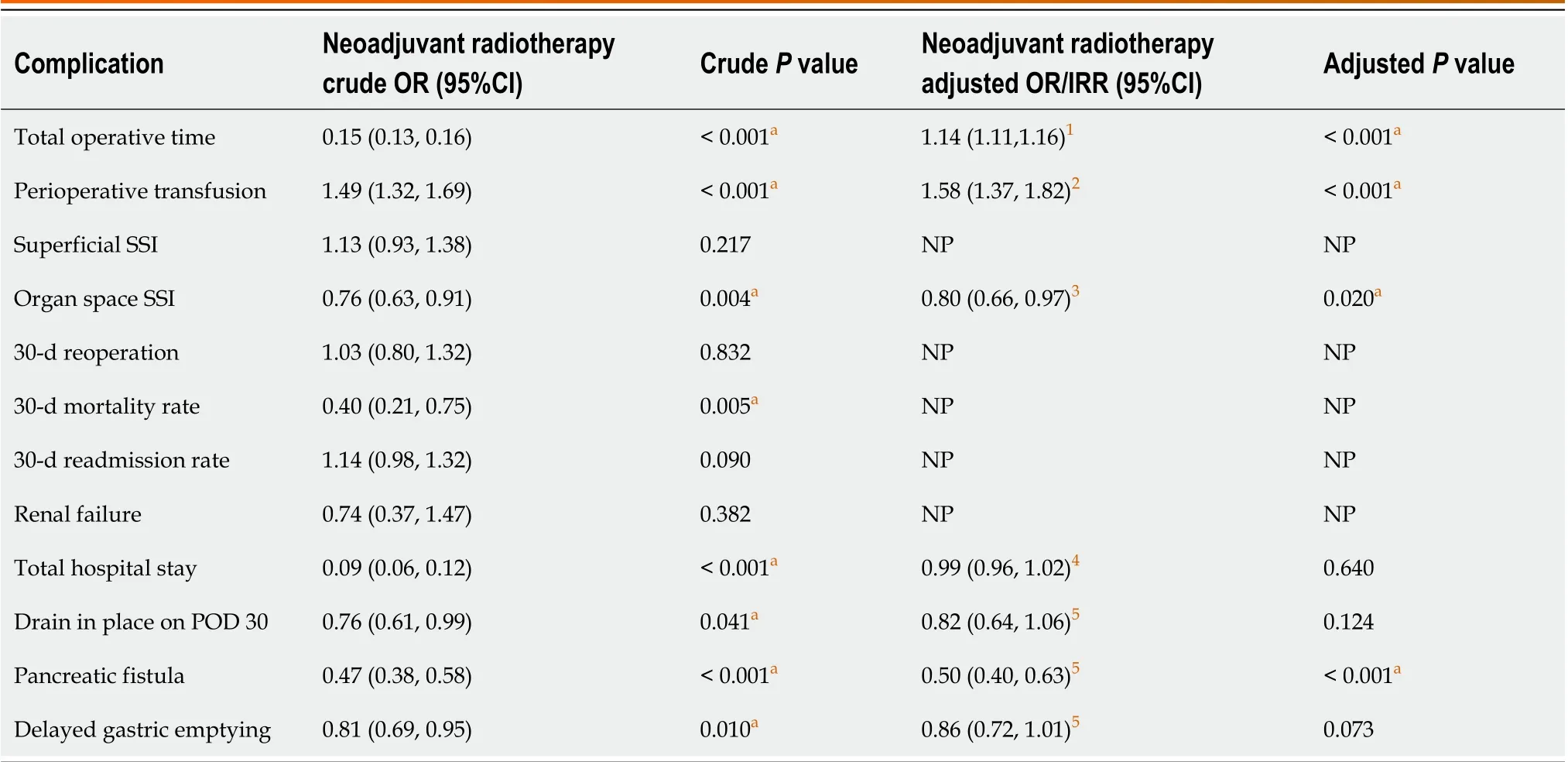
Table 4 Odds ratios for 30-day postoperative complications for patients with adenocarcinoma of the pancreas undergoing a pancreaticoduodenectomy from 2015 to 2019

Figure 1 CONSORT diagram of study cohort. ACS-NSQIP: American College of Surgeons-National Surgery Quality Improvement Program.
Overall, the 30-d post-operative complication and morbidity rates were similar between both study groups. Of the variables that were analyzed using multivariable regression, only organ space infections and pancreatic fistula rates were significantly different between the two study groups. Numerous studies have detailed the decreased rate of both variables in patients receiving neoadjuvant therapy, suggesting that neoadjuvant radiotherapy may have a small positive effect on short term post-operative morbidity[9,10,14,21,25].
Although the data provided in this study lend credence to the safety and efficacy of neoadjuvant radiotherapy, there are some limitations to keep in mind. Data regarding the specific details of neoadjuvant radiotherapy regimen used(duration, intensity, timing, prior to surgery) is not available within the NSQIP database, making it impossible to account for related confounding factors in our data analyses. Additionally, NSQIP only collects data on post-operative outcomes that occur within 30 d of the index operation, making the results of this study difficult to extrapolate over a longer-termed period. Facility and surgeon data are also not reported in NSQIP, which again may represent confounding factors that our analyses did not consider. Finally, it is impossible to determine whether a patient’s tumor is resectable or borderline resectable (per NCCN guidelines) based on the information reported in NSQIP. As surgeries may be more difficult in borderline resectable patients, this represents a further confounding factor that could not be completely controlled for.
CONCLUSION
The results of this study contribute to the notion that neoadjuvant radiotherapy is both safe and effective to use prior to a pancreaticoduodenectomy for pancreatic adenocarcinoma. The study does, however, suggest that adverse intraoperative outcomes like total operative time and perioperative transfusion requirements may be increased among patients receiving surgery for cancer resection after neoadjuvant radiotherapy. Surgeons are encouraged to keep in mind the potential positive and negative effects that neoadjuvant radiotherapy has on the complexity of the eventual surgery when it is performed.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Aploks K, Kim M, Ostapenko A, Dong XD, and Seshadri R contributed to the conceptualization of the project;Aploks K, Stroever S, Kim M, Ostapenko A, Dong XD, and Seshadri R contributed to the methodology and validation of the data;Stroever S conducted the formal statistical analyses; Aploks K, Kim M, Sim YB, and Sooriyakumar A prepared the original manuscript;Aploks K, Kim M, Ostapenko A, Sim YB, Sooriyakumar A, Rahimi-Ardabily A, Dong XD, and Seshadri R contributed to the final draft revision and editing; Dong XD and Seshadri R supervised the project.
Institutional review board statement:Ethical review and approval were waived for this study since the data used was de-identified and obtained from a participant use data file.
Informed consent statement:This study was a retrospective review that utilized only de-identified patient data from National Cancer Database. Given this fact, no signed informed consent is needed.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Data sharing statement:Data was obtained with permission from the American College of Surgeon's National Cancer Database. NSQIP data can be obtained by visiting https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Krist Aploks 0000-0003-3775-1775; Xiang Da Dong 0000-0001-9324-1281.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
6Lambert A, Schwarz L, Borbath I, Henry A, Van Laethem JL, Malka D, Ducreux M, Conroy T. An update on treatment options for pancreatic adenocarcinoma.Ther Adv Med Oncol2019; 11: 1758835919875568 [PMID: 31598142 DOI: 10.1177/1758835919875568]
11Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer.Ann Surg2015; 261: 12-17 [PMID: 25599322 DOI: 10.1097/SLA.0000000000000867]
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Is endoscopic mucosal resection-precutting superior to conventional methods for removing sessile colorectal polyps?
- Knowledge, attitude, and practice of monitoring early gastric cancer after endoscopic submucosal dissection
- Changing trends in gastric and colorectal cancer among surgical patients over 85 years old: A multicenter retrospective study, 2001-2021
- Enhanced recovery nursing and mental health education on postoperative recovery and mental health of laparoscopic liver resection
- Effects of ultrasound monitoring of gastric residual volume on feeding complications, caloric intake and prognosis of patients with severe mechanical ventilation
- Risk factors and their interactive effects on severe acute pancreatitis complicated with acute gastrointestinal injury
