Root exclusion methods for partitioning of soil respiration:Review and methodological considerations
2023-10-16MeiYeeCHINSharonYuLingLAUFrazerMIDOTMuiSieJEEMeiLiengLOFaustinaSANGOKandLulieMELLING
Mei-Yee CHIN,Sharon Yu Ling LAU,Frazer MIDOT,Mui Sie JEE,Mei Lieng LO,Faustina E.SANGOK and Lulie MELLING
Sarawak Tropical Peat Research Institute,Lot 6035,Kuching-Kota Samarahan Expressway,Kota Samarahan,Sarawak 94300(Malaysia)
ABSTRACT Soil respiration is a vital process in all terrestrial ecosystems,through which the soil releases carbon dioxide(CO2)into the atmosphere at an estimated annual rate of 68—101 Pg carbon,making it the second highest terrestrial contributor to carbon fluxes.Since soil respiration consists of autotrophic and heterotrophic constituents,methods for accurately determining the contribution of each constituent to the total soil respiration are critical for understanding their differential responses to environmental factors and aiding the reduction of CO2 emissions.Owing to its low cost and simplicity,the root exclusion(RE)technique,combined with manual chamber measurements,is frequently used in field studies of soil respiration partitioning.Nevertheless,RE treatments alter the soil environment,leading to potential bias in respiration measurements.This review aims to elucidate the current understanding of RE,i.e.,trenching(Tr)and deep collar(DC)insertion techniques,by examining soil respiration partitioning studies performed in several ecosystems.Additionally,we discuss methodological considerations when using RE and the combinations of RE with stable isotopic and modeling approaches.Finally,future research directions for improving the Tr and DC insertion methods in RE are suggested.
Key Words: autotrophic respiration,deep collar insertion,heterotrophic respiration,microbial respiration,root trenching,soil microbial community,soil respiration component
INTRODUCTION
Global warming resulting from elevated levels of greenhouse gases,such as carbon dioxide(CO2),in the atmosphere has highlighted the importance of understanding ecosystem carbon(C)cycling to control C emissions from terrestrial ecosystems.Soil CO2emissions from soil respiration(Rs)are the second most significant source of terrestrial C fluxes,accounting for 68—101 Pg C year-1(Jianet al.,2021),a figure 10 times higher than anthropogenic CO2emissions(Friedlingsteinet al.,2020).
Soil CO2efflux,or soil respiration,is a crucial process in terrestrial ecosystems,through which CO2is released from the soil into the atmosphere(Luo and Zhou,2006).The process is commonly divided into autotrophic and heterotrophic components(Bond-Lambertyet al.,2004).Various terminologies have been used to denote the autotrophic component that comprises the respiration of plant roots(maintenance and growth)and that of rhizosphere microorganisms,such as autotrophic(Högberget al.,2001)and root and rhizosphere respirations(Hopkinset al.,2013).Hereafter,we use autotrophic respiration (Ra) to represent root respiration (Rr)and rhizosphere microbial respiration.
The heterotrophic component,which includes the respiration of microorganisms that decompose soil organic matter (SOM) and litter,is fascinating considering that SOM-derived CO2efflux from microbial decomposition contributes directly to the release of C into the atmosphere.Simultaneously,CO2efflux from autotrophic respiration is compensated for by fixation during photosynthesis (Aguset al.,2010).Consequently,separating soil respiration components is crucial for procuring accurate and reliable heterotrophic respiration (Rh) estimates,as it is a critical factor in determining soil C balance and ecosystem emission factors(Hergoualc’h and Verchot,2014;Murdiyarsoet al.,2019).
Generally,partitioning of soil respiration in the field into autotrophic and heterotrophic components is challenging because of the various biological and ecological processes by which they are regulated(Baggs,2006).Moreover,while plant activities and photosynthate supply to roots influence autotrophic respiration (Kuzyakov and Cheng,2001),heterotrophic respiration is influenced by soil temperature,moisture,and substrate quality and supply(Davidson and Janssens,2006).
Plant roots,mycorrhizae,and microbial decomposers contribute differently to soil respiration in ecosystems at varying soil temperatures and moisture levels,thereby allowing CO2efflux to respond to changes in these variables(Kechavarziet al.,2010;Hurshet al.,2017).For example,autotrophic respiration may contribute from 5%to over 90%of soil CO2efflux,depending on vegetation cover,season,and soil respiration partitioning approach (Hansonet al.,2000;Heinemeyeret al.,2007).Nonetheless,heterotrophic respiration is affected by changes in soil microbial biomass,metabolism,community composition,and bacteria-to-fungi ratio,resulting in variable decomposition rates (Classenet al.,2015).
Several methods have been developed for separating soil respiration into its constituents (Hansonet al.,2000;Kuzyakov,2006).These approaches can be broadly classified into isotopic(Patersonet al.,2009),such as13C natural abundance discrimination and isotopic14C mass balance,and nonisotopic,including component integration,root exclusion(RE)by trenching(Tr)or deep collar(DC)insertion,root removal,and gap analysis techniques.Several criteria are considered when determining the best approach to separate soil respiration components,including the extent of disturbance to the ecosystem,universality of application across ecosystems,reproducibility and reliability of results,ease of use,and cost-effectiveness(Kuzyakov,2006).
The RE method is one of the most widely used techniques for soil respiration partitioning,owing to its low cost and ease of application.Nevertheless,soil disturbance and root cutting from RE plot installation may increase CO2emissions in trenched plots(Hansonet al.,2000;Subkeet al.,2006).Conversely,the isotopic14C mass balance approach only results in minor soil disturbance(Vargaset al.,2011)but shows several disadvantages,including a complex experimental setup requiring specific14C sampling expertise and expensive,technically challenging radioactive isotope analysis(Carboneet al.,2016).The limitations of any single method can be solved by combining approaches,such as nonisotopic and isotopic (Kuzyakov,2006).Nonetheless,combined methods remain scarce(Biasiet al.,2012,2014;Carboneet al.,2016;Comeauet al.,2018).
Studies using RE for soil respiration partitioningviathe Tr approach in various ecosystems have gained increasing interest,thus allowing for more detailed analysis of experimental limitations.Further,numerous studies have reported higher soil CO2efflux in trenched plots than in nontrenched plots,despite a prolonged stabilization period after Tr,leading to inaccurate estimations of heterotrophic respiration.Although the causes for such inaccuracies have not been thoroughly investigated,the phenomenon is generally attributed to the effects of the RE method,such as severed root decomposition,differences in soil moisture,rhizosphere priming effect(RPE),and changes in microbial community structure(Lalonde and Prescott,2007;Comstedtet al.,2011;Drakeet al.,2012;Comeauet al.,2016;Kukumägiet al.,2017;Savageet al.,2018;Ishikuraet al.,2019).
The distinction between autotrophic and heterotrophic respiration is critical for understanding soil respirationrelated environmental changes and designing effective strategies for reducing CO2emissions through environmental management(Murdiyarsoet al.,2019).Consequently,this review aims to summarize our current understanding of RE using Tr and DC insertion methods(RE:Tr/DC insertion)by analyzing recent studies on soil respiration partitioning in various ecosystems.Furthermore,we discuss methodological considerations when using the RE:Tr/DC insertion technique and the potential of combining approaches(RE with stable isotope and RE with modeling approaches)to overcome the limitations of any single method.Finally,future research proposals for improving RE:Tr/DC insertion are outlined.
AN OVERVIEW OF RE METHODS FOR SOIL RESPIRATION PARTITIONING
As suggested by Hansonet al.(2000),an RE approach refers to any process that indirectly quantifies root respiration by measuring surface soil CO2efflux with or without involving roots(no direct root tissue evaluations).The method is divided into root removal,gap analysis,and Tr/DC insertion.This review describes the RE technique,with an emphasis on the Tr and DC insertion approaches.
Root removal
The root removal method involves removing roots from the sampled plot soil and placing the soil horizons back in the reversed order of removal.During analysis,purposely installed barriers block root growth from the surroundings.Subsequently,root respiration is estimated by determining the difference between the measured CO2effluxes of the soil with and without roots(Hansonet al.,2000).For instance,a study reported not utilizing a barrier to prevent root regrowth in a pit of 0.5 m × 0.5 m and 30 cm deep as the soil CO2efflux was measured 2—4 weeks after root removal(Wiant,1967).Another soil respiration partitioning study performed inFitzroya cupressoidesforests in southern Chile also employed the root removal method,where a polyvinyl chloride(PVC)collar(40 cm long)was inserted 30 cm into the root-free soil to evaluate its heterotrophic respiration.The roots were manually removed before carefully reinstalling the collar into the root-free soil without compacting or mixing the soil horizons(Urrutia-Jalabertet al.,2017).
Although the root removal technique eliminates the contribution of dead roots to CO2production and allows root biomass measurements in research plots,it requires a long period of time and alters soil structure and environmental variables,such as soil temperature and moisture (Wiant,1967;Urrutia-Jalabert,2017).Furthermore,plant roots enable soil moisture reduction through evapotranspiration(Leunget al.,2015).Consequently,removing roots and employing a non-permeable PVC collar might lead to waterlogging,which inhibits heterotrophic respiration,resulting in useless data(Urrutia-Jalabertet al.,2017).Moreover,utilizing long PVC collars in peatlands and grasslands could lead to waterlogging after heavy rain(Heinemeyeret al.,2007),suggesting that wet ecosystems(forests,peatlands,and grasslands)with poorly drained soils(Urrutia-Jalabertet al.,2017)are more susceptible to this phenomenon.
Gap analysis
Gap analysis provides indirect root respiration estimations by comparing the gaps and forested areas under crown soil respiration measurements.Clear-cutting in forest stands and vegetation clippings in grasslands create gaps that reduce carbohydrate supply into the soil and thus affect soil respiration(Brumme,1995).For example,the soil respiration of a mature beech stand in Germany was compared to that of the 30-m gap in the stand installed two years earlier.The measurements were obtained in the gap center.The study documented that the respiration of living roots accounted for 40%of soil respiration(Brumme,1995).In another study,the estimated root respiration of a 10-year-old Japanese cedar wood forest two years after the formation of a relatively small gap(2.5 m×2.5 m)was approximately 50%of soil respiration.The soil respiration in the report represented the difference between the CO2effluxes in the gaps and the forest area(Ohashiet al.,2000).
The gap approach is labor-efficient when gaps have been established in the system or from individual tree death or windthrow.Nonetheless,similar to other RE methods,gap analysis presents the same challenges as Tr,such as increased CO2emissions due to dead root decomposition(Toland and Zak,1994) and soil temperature and moisture alterations(Mayeret al.,2017).Moreover,microenvironmental factors(e.g.,air and soil temperatures,solar radiation,and soil moisture) that enhance microbial activities were found to be different in gaps compared to neighboring closed canopy stands,suggesting that higher decomposition rates might potentially occur in gaps than in closed forests(Schliemann and Bockheim,2014).Further,Mayeret al.(2017)reported that soil temperature and moisture were considerably higher in the gaps within a mixed forest stand than in the control area,and that no changes in the potential soil enzyme activities were recorded.Consequently,the soil respiration levels in the gaps were not reduced due to increased decomposition rates(driven by warmer soil conditions),compensating for autotrophic respiration reduction.Furthermore,an increasing gap size might result in the rapid growth of understory vegetation due to improved microclimate conditions,such as light and moisture,which could offset the gap-induced reduction in soil respiration through respiration of the new roots of understory vegetation(Panget al.,2016).Accordingly,approaches to reduce any alterations in environmental conditions need to be considered.Techniques that employ net-covered frame boxes with clear-cut areas to maintain similar surroundings as the control plots (Nakaneet al.,1996),use smaller gaps (Ohashiet al.,2000),and apply correction approaches to account for the effects of altered soil temperature and moisture on soil respiration(Wan and Luo,2003)could be beneficial.
RE:Tr approach
The Tr method has been widely employed in various ecosystems,including grasslands(Fanget al.,2018;Zhanget al.,2019),tropical(Vijayanathanet al.,2021)and temperate forests(Savageet al.,2018;Jiao and Wang,2019),afforested temperate (Hermanset al.,2022) and tropical peatlands(Comeauet al.,2016;Hergoualc’het al.,2017;Itohet al.,2017;Ishikuraet al.,2018,2019) because of its ease of use.Nonetheless,the Tr approach reduces C supply to the roots and surrounding rhizospheres(Vogel and Valentine,2005).
Deep incisions,commonly 20 cm below the rooting zone,are manually made with a knife and shovel(Itohet al.,2017)or mechanically with a chainsaw(Comeauet al.,2016)to sever all roots.The trenched plots are then surrounded by physical barriers of non-permeable materials,such as plastic sheets,stainless steel plates,and plexiglass,or permeable mesh fabric(1-or 50-μm pore size)to prevent root and mycorrhiza ingrowth(Ishikuraet al.,2018;Ryhtiet al.,2021;Hermanset al.,2022).Aboveground vegetation in the plots is removed manually or by clipping at ground level.Herbicides are avoided,as they can alter the microbial community of the soils under study(Epron,2010).
Trenched plots in soil respiration partitioning studies are usually 30 to 100 cm deep and 0.4 m×0.4 m to 2.5 m×2.5 m in size(Table I).Figure 1a illustrates site installation of trenched plots.Soil CO2efflux measurements are typically obtainedviathe closed-chamber method,which involves attaching soil collars to infrared gas analyzers(Comeauet al.,2016;Itohet al.,2017;Hermanset al.,2022).
RE:DC insertion method
Another RE method at a smaller spatial scale is the DC insertion approach,which involves inserting rigid PVC or stainless steel cylinders into the soil to sever roots and cut offC supply (Buchmann,2000;Vogel and Valentine,2005;Bond-Lambertyet al.,2011;Heinemeyeret al.,2012;ArchMiller and Samuelson,2016;Jovani-Sanchoet al.,2018).The technique is considered a miniature version of the Tr method and causes only minor disturbances,as no soil digging is required(Subkeet al.,2006).
The cylinders function as soil collars,cutting roots,preventing root ingrowth,and providing soil surface contact to measure soil CO2fluxes(Fig.1b).Various diameters and heights of stainless steel cylinder have been used in soil respiration partitioning studies in tropical peatlands and forests(Takahashiet al.,2011;Mellinget al.,2013;Batubaraet al.,2019).Moreover,aluminum nets with 1-mm mesh size may be placed on the collar openings to prevent litter collection or soil disturbance by animals or mesofauna(Da Costaet al.,2018;Jovani-Sanchoet al.,2018).
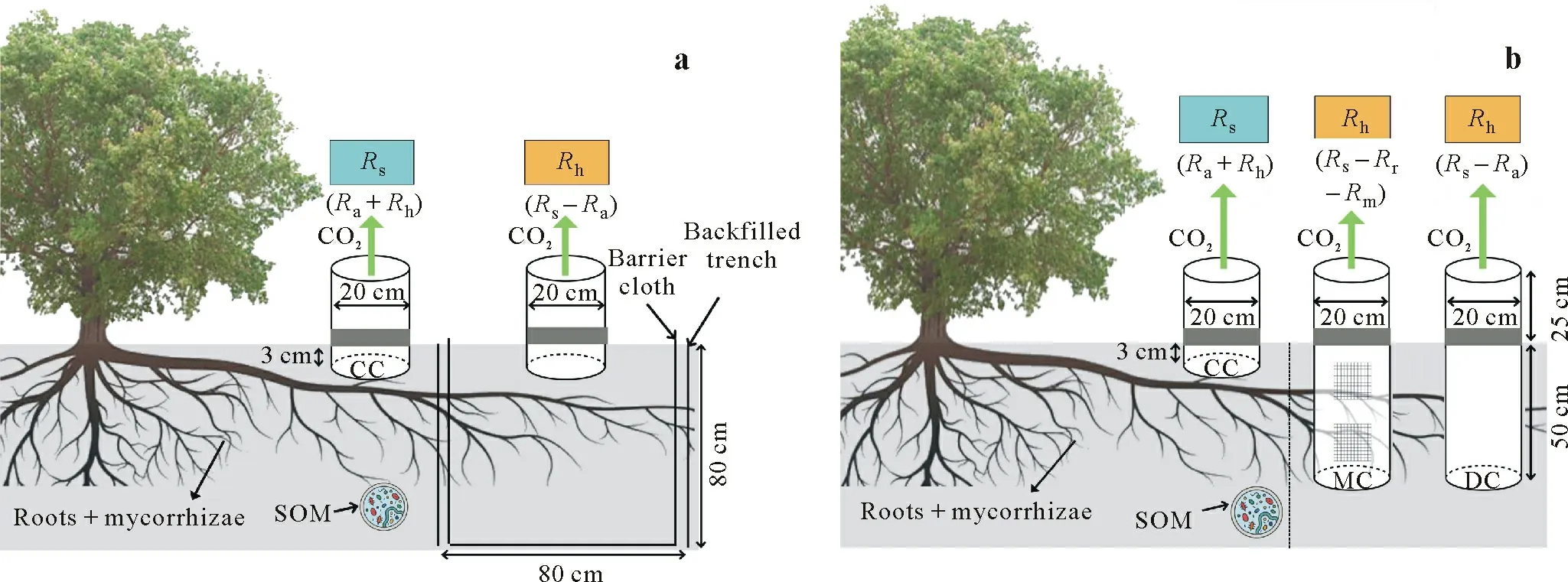
Fig.1 Schematic diagrams showing site installation in soil respiration(Rs)partitioning studies using the root exclusion method via trenching(a)or deep collar(DC)insertion(b).In a,an 80 cm×80 cm trenched plot is excavated to a depth of 80 cm and lined with a barrier cloth before being refilled with soil.Soil CO2 efflux is measured using a closed,manual or automated chamber within the trenched plot to represent heterotrophic respiration(Rh),whereas the control collar(CC)represents Rs.In b,a DC,i.e.,an open-ended cylinder(20-cm diameter,50-cm height),is driven into the soil profile to cut the roots and act as a barrier to root ingrowth.Another open-ended cylinder(20-cm diameter,25-cm height)is placed directly on the soil surface above the 50-cm open-ended cylinder.A mesh collar(MC)is an open-ended cylinder with nylon mesh windows to allow water to flow while preventing root and mycorrhiza ingrowth.The soil CO2 efflux measured from DC represents Rh,whereas the data obtained from CC represent Rs.Ra=root and rhizosphere respiration;Rr=root respiration;Rm=mycorrhiza respiration;SOM=soil organic matter.
A modified version of the DC approach,the RE:DC insertion(with root removal)method utilizes cylinders with perforated holes or wire mesh of different sizes to prevent root or root-associated mycorrhizal hyphae invasion,while allowing moisture flow(Heinemeyeret al.,2007;Wuet al.,2014;Hanet al.,2021).Nonetheless,soil structure is disturbed when this method is used,because of the cutting into the soil with a DC,removing the soil for manual root removal,and then replacing the soil,as described by the researchers from the Global Ecosystem Monitoring Network(Marthewset al.,2014).
The RE:DC insertion method is more laborious,as additional control treatments are necessary to account for potential soil handling and mixing disturbance effects.The first control treatment,termed‘disturbed’,consists of collars inserted into holes that are then refilled with the handled soil.Next,another row of collars are installed by hammering them into the soil.This step does not involve soil handling,thereby termed ‘undisturbed’ (Marthewset al.,2014).A detailed comparison of the RE methods(Tr and DC insertion with and without root removal)is listed in Table II.
RE:Tr/DC insertion coupled withautomated chamber measurements
Automated chambers are increasingly employed with the Tr technique to separate soil respiration into autotrophic and heterotrophic respirations.Continuous hourly measurements generated by the technique enable detection of the diurnal and seasonal flux variations due to environmental fluctuations,which would be overlooked when a manual chamber is used(Ishikuraet al.,2018,2019;Savageet al.,2018;Hoytet al.,2019).Hoytet al.(2019)recorded hourly CO2efflux from peat surfaces in conjunction with a trench that was up to 30 cm deep and found strong diurnal cycles in CO2flux and near-surface(<10 cm)peat temperature that peaked at midday.The study documented that the magnitude of diurnal oscillation was significantly affected by shading and water table depth(WTD),thus highlighting the limitation of relying on daytime assessments and/or a single correction factor,which would cause daytime bias in flux evaluations.The mean daily heterotrophic respiration also exhibited a strong linear association with WTD,whereas in flooded environments,heterotrophic respiration was small and constant(Hoytet al.,2019).
Several studies on tropical peatlands reported that heterotrophic respiration increased with decreasing WTD(Husnainet al.,2014;Itohet al.,2017;Wakhidet al.,2017).Nevertheless,one of the drawbacks of automated chambers is poor spatial representation due to power constraints(Savage and Davidson,2003;Savageet al.,2008)and missing data due to technical issues,such as power loss,which contributes 23%of overall data loss(Ishikuraet al.,2018).To solve these problems,Ganaet al.(2018)recommended using manual and automated chamber measurements to compensate for spatial and temporal discrepancies.
CONSIDERATIONS WHEN UTILIZING THE RE:TR/DC INSERTION TECHNIQUE
When utilizing the RE:Tr/DC insertion method,pre-RE treatment installation and measured data interpretation need to be paid attention to.As the implementation of RE method would incur soil disturbance and severed root decomposition,a stabilization period is necessary before measurements.Furthermore,the possibility of root ingrowth into RE plots must be considered,especially when the experiment is prolonged.The second set of challenges concerning the RE:Tr/DC approach is related to experimental data.The RE effects(Table III),such as soil disturbance,severed root decomposition,induction of RPE,soil water content change,and shifts in soil microbial communities,might contribute to increased CO2emissions in trenched plots compared to controls,leading to an overestimation of heterotrophic respiration.Consequently,this review discusses steps of heterotrophic respiration data correction in cases of severed root decomposition.A well-planned experimental design and understanding of the strengths and weaknesses of a method would allow correct interpretation of the generated data.
Assumptions
Several assumptions are required for the Tr/DC insertion approach to RE(Hansonet al.,2000;Kuzyakov,2006;Subkeet al.,2006;Epron,2010).First,it is assumed that Tr/DC insertion immediately eliminates root respiration(Leeet al.,2003).Therefore,the soil CO2efflux from Tr/DC insertion plots represents microbial heterotrophic respiration only,whereas that from control(root-intact)plots is attributable to soil respiration.Accordingly,autotrophic respiration is the respiration rate difference between the control and RE plots.Assessing the baseline soil CO2efflux prior to RE treatment would also ensure that there is no spatial variance between the Tr/DC insertion and control plots and allow soil CO2efflux in RE plots to be attributed to the absence of autotrophic respiration(Epron,2010).
The second conjecture in the TR/Dc insert approach to RE is that the decomposition of severed roots affects and alters heterotrophic respiration in the treated plots.During the first few months after Tr,fine roots that decompose quickly would temporarily increase soil CO2efflux,whereas coarse roots that decompose more slowly would promote soil CO2efflux over a longer period.The time required for all rootsto degrade is unknown and depends on initial root biomass(Epron,2010).The RE:Tr/DC insertion approach also presumes that heterotrophic respiration would not be affected by the lack of C input from root exudates or litter/residue decomposition.
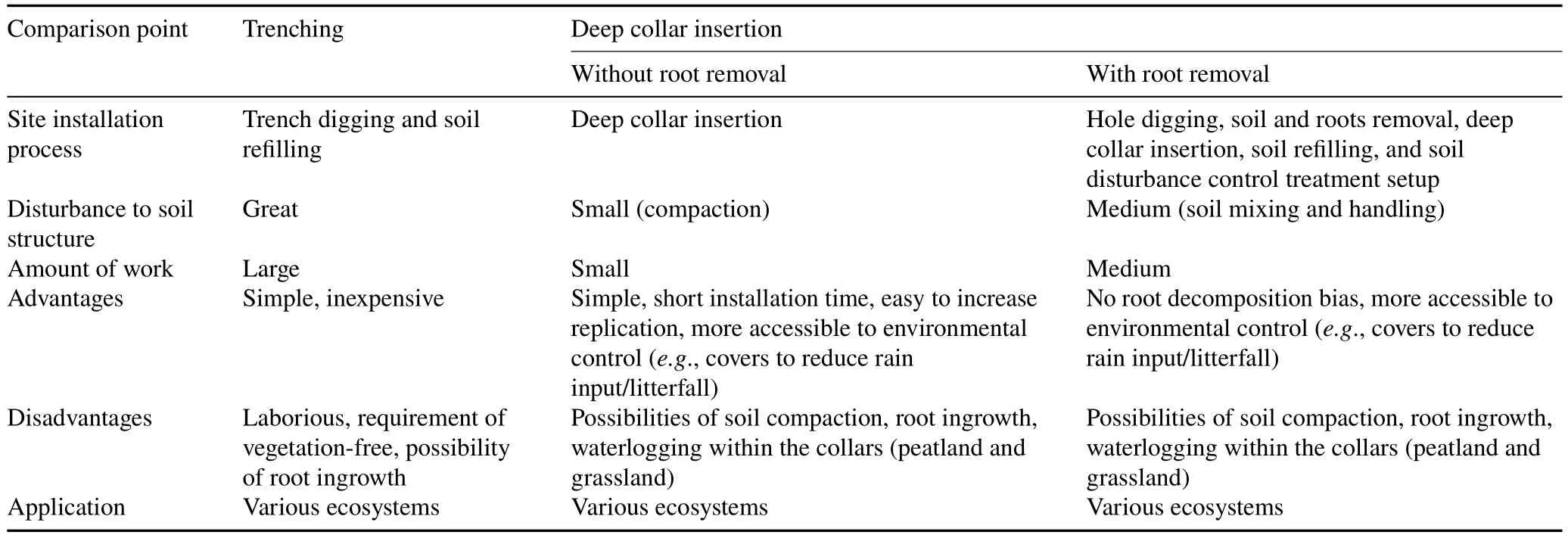
TABLE IIComparison of the root exclusion methods of trenching and deep collar insertion with and without root removal used in soil respiration partitioning studies
Soil disturbance
Inserting collars inevitably disturbs soil structure,leading to increased CO2emissions.In a 15-month RE study,the insertion of 10-cm diameter cylinders resulted in consistently higher mean respiration rates inside the cylinder than outside(Vogel and Valentine,2005).The elevated CO2emissions were attributed to a greater soil bulk density due to soil compaction (Lalonde and Prescott,2007).A field study demonstrated that mineral soil compaction increased soil CO2concentration(Conlin and Van den Driessche,2000),whereas a laboratory incubation study linked compacted tropical peat soil to a higher CO2efflux rate(Busmanet al.,2021).Accordingly,soil disturbance should be accounted for,or measurements must be postponed until the soil system has stabilized.Typically,Tr/DC insertion plots are left on the ground for one to two years to allow the soil to stabilize from the effects of soil disturbance and severed root decomposition before commencing soil CO2efflux measurements(Booneet al.,1998;Sulzmanet al.,2005;Sayer and Tanner,2010;Kukumägiet al.,2017;Jiao and Wang,2019).Nevertheless,data recorded years after Tr might underestimate theRh/Rsratio due to a lack of C input from litter(Subkeet al.,2006).Considering this potential bias,Langet al.(2021)established a disturbance plot similar to an RE plot but without root ingrowth barriers and control plots to account for the initial pulse of CO2from soil disturbance,root decomposition,and increased soil moisture.
Decomposition of severed roots
The time required for the severed roots from RE plot installation to stop respiration and start decomposition is uncertain(Epron,2010).Severed roots can continue respiring for several months to a year after Tr implementation by utilizing their carbohydrate reserves(Uchidaet al.,1998;Leeet al.,2003;Aubrey and Teskey,2018).For example,the severed roots in a montane forest were still respiring five months after Tr and stopped entirely until the tenth month(Díaz-Pinéset al.,2010).
Most of the available literature reported that the decomposition of recently severed roots,whether in temperate forests on mineral soil(Ewelet al.,1987;Keltinget al.,1998;Hansonet al.,2000;Leeet al.,2003;Fanet al.,2015,Arch-Miller and Samuelson,2016)or tropical peatlands(Mellinget al.,2013;Hergoualc’het al.,2017;Ishikuraet al.,2018),occurs in the first two months or up to a year.However,a few studies found that trenched plots showed significantly higher respiration rates than controls even after a stabilization period of one to two years,which was often attributed to severed root decomposition(Kukumägiet al.,2017;Savageet al.,2018;Ishikuraet al.,2019).Dead root respiration was still detected two years after a trench was installed in a temperate beech and oak forest and would have likely lasted longer if the study had been extended(Savageet al.,2018).Similarly,a one-year stabilization interval after Tr did not prevent the overestimation of heterotrophic respiration in an undrained tropical peatland forest due to high groundwater levels that hampered dead root breakdown.Decomposition of severed roots is site specific and depends on soil environmental conditions and root biomass(Subkeet al.,2006;Ishikuraet al.,2019).
An increasing number of reports have indicated that the CO2released from recently severed roots is substantial and can be corrected for by evaluating root biomass decomposition in trenched plots (Ngaoet al.,2007;Díaz-Pinéset al.,2010;Variket al.,2015;Kukumägiet al.,2017;Hermanset al.,2022).Methods for estimating root biomass decomposition include the mesh bag(Hermanset al.,2022),the root ingrowth core(Variket al.,2015),and the cellulose filter as a proxy(Vogel and Valentine,2005)approaches.The initial coarse(>2 mm)and fine(<2 mm)root biomasses obtained at the end of the experiment are required for CO2data correction(Hermanset al.,2022).The CO2efflux from severed root(different size classes)decomposition(RD,g C m-2year-1)can then be calculated as follows(Ngaoet al.,2007):
whereαRrepresents the microbial efficiency,which is the C decomposition constituent during total root C loss and the portion included in SOM,1-αRis the fraction lost as CO2,tis the time over which CO2efflux from severed root decomposition is measured (year),M0denotes the initial root biomass (g C m-2),andM1is the remaining root biomass(g C m-2),which can be measured or calculated by assuming a simple exponential decomposition function as follows:
wherekis the decomposition constant(year-1).Data fitting was performed separately for different root size classes.Accordingly,RDcan be subtracted from the CO2efflux from the trenched plots(Rh(trenched),g C m-2year-1)to obtain the corrected heterotrophic respiration(Rh(corrected),g C m-2year-1):
Hermanset al.(2022)documented that the correction ofRDreduced the heterotrophic contribution from 61%to 38%(a difference of 23%),suggesting that severed root biomass decay contributes significantly to soil CO2efflux even two years after Tr.In other studies,CO2efflux correction for fine root decomposition reduced heterotrophic respiration by up to 14%(Díaz-Pinéset al.,2010)and 15%—20%(Kukumägiet al.,2017).Nevertheless,further root turnover corrections increased heterotrophic respiration by 9%—13%,resulting in a 5%—8%net reduction.Table IV lists reports of higher soil CO2emissions in RE plots than in control plots due to methodological effects and the corrections applied.
Sayer and Tanner(2010)recommended measuring soil respiration before and immediately after Tr (within seven days) for one-occasion measurements to obtain the most accurate estimate of heterotrophic respiration (when soil respiration was 38%lower in Tr than in control plots).Otherwise,soil respiration in Tr plots would rise to pre-Tr levels for the next seven months due to severed root decomposition.Moreover,high replication and staggered one-occasion soil respiration measurements of DC insertion treatments might help to obtain information on seasonal heterotrophic respiration variations and responses to management schemes(Sayer and Tanner,2010;ArchMiller and Samuelson,2016;Brown and Markewitz,2018).
Root ingrowth
Depending on the duration of the experiment,additional soil CO2efflux may arise from root invasion through underneath the trenches(Sayer and Tanner,2010).Nevertheless,the effect is influenced by root growth rate and is unlikely to lead to additional CO2efflux in short-term(1—2 years)experiment.For instance,DC inserts evaluated at the end of an RE experiment(20 months)found no evidence of root ingrowth(Jovani-Sanchoet al.,2018).Conversely,a 20-year RE study recorded root ingrowth in trenched plots(Bowdenet al.,2014).Ryhtiet al.(2021)argued that since pioneer pine roots grow 2—5 cm year-1(Dinget al.,2020)and the distance between the trench and the soil collar is over 30 cm,root ingrowths under the mesh fabric of the trenched plots of a boreal forest site in Finland were unlikely to enhance soil CO2emissions during the experiment.
Differences in soil moisture in REplots
The RE by Tr/DC insertion may result in differences in soil water content in RE plots due to the inability of severed roots to absorb moisture(Kuzyakov,2006;Subkeet al.,2006).This phenomenon may be exacerbated if the trenched plots are lined with non-permeable material to prevent root ingrowth(Bond-Lambertyet al.,2011).Increased soil moisture in trenched plots may also elevate heterotrophic respiration,as soil moisture is crucial for microbial respiration (Yanet al.,2018).Accordingly,the effect may be stronger in sandy soils and weaker in soils with high water retention and low oxygen diffusivity (Epron,2010).For example,trenched plots in a temperate beech forest in the summer and early autumn recorded up to twice the moisture content of control plots,resulting in overestimated heterotrophic respiration(Epronet al.,1999).
Soil moisture differences in trenched plots are more pronounced in dry ecosystems(Hansonet al.,2000;Baldocchiet al.,2006)or during drought events(Savageet al.,2018).For example,a study in which a DC was installed in a semi-arid grassland in Mongolia reported that soil moisturecontent increased by 37%compared to the moisture outside the collar(Zhanget al.,2019).Meanwhile,water-filled pore space values in the trenched and control plots remained consistent throughout a two-year study conducted in an oil palm plantation in a tropical peatland(Ishikuraet al.,2018).The soil respiration was also unaffected despite the higher soil water content in trenched plots compared to the control plot in afforested organic soil croplands(Mäkirantaet al.,2008)and a Japanese larch plantation(Quet al.,2018).Therefore,monitoring soil moisture content in trenched and control plots during Tr experiments is essential for developing empirical models to reliably account for the differences between the two groups(Epron,2010).Nonetheless,few studies have considered artifacts in soil respiration partitioning investigations(Ngaoet al.,2007;Comstedtet al.,2011;Drakeet al.,2012;Kukumägiet al.,2017;Zhanget al.,2019).
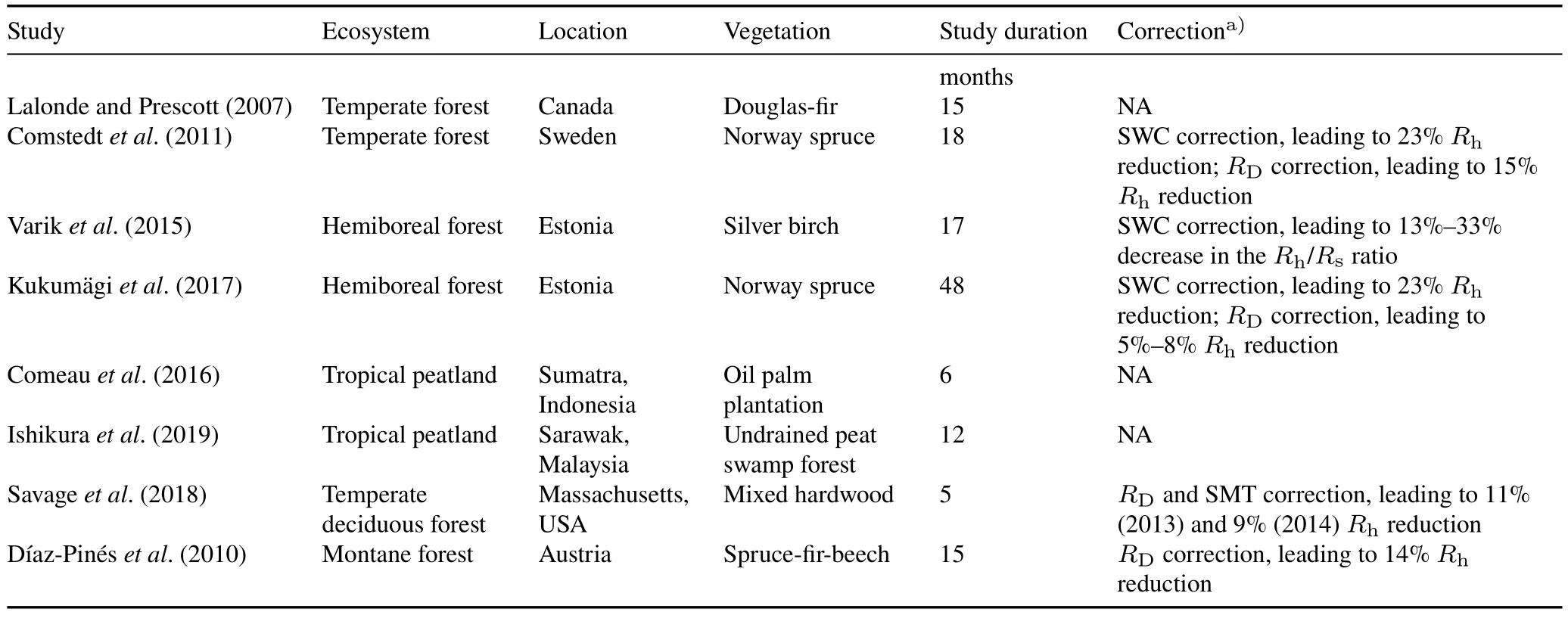
TABLE IVSummary of studies reporting higher soil CO2 emissions in root exclusion plots compared to control plots due to methodological effects and corrections applied
RPE
Priming effect(PE)is defined as significant short-term alterations in SOM turnover resulting from relatively mild soil treatments(Kuzyakovet al.,2000),including the addition of plant residues,dead microbial biomass,and organic and mineral nitrogen (N) fertilizers (Kuzyakovet al.,2000;Kuzyakov,2002,2010).The RPE describes the PE occurring in rhizosphere(Kuzyakov,2002).Root exclusionviaTr or DC insertion can induce a positive RPE by adding C input from decaying roots(Cheng,2009;Lajthaet al.,2018),leading to increased soil CO2efflux in Tr/DC insertion plots compared to control plots.In contrast,the absence of a root system in Tr/DC areas reduces heterotrophic respiration due to the lack of soil priming(Cheng,2009).
According to a short-term study to quantify heterotrophic respiration in an oil palm plantation in a tropical peatland after N fertilization,soil CO2efflux in the N-fertilized Tr plot was higher than the total soil respiration of the control plot(non-trenched).The higher respiration rates observed were attributed to positive RPE.The excess inorganic N in the root-excluded Tr plots stimulated SOM decomposition and mineralization(Comeauet al.,2016).These results are consistent with previous reports of more prominent RPEs in C-rich,N-deficient environments,such as peatlands(Hartet al.,1986;Kuzyakovet al.,2000).
Nitrogen fertilization in tropical peatlands significantly increased CO2emissions(Hatano,2019),considering that N fertilizers not only influence the soil N pool but might also impact the C equilibrium in agricultural ecosystems by directly influencing plant and microbial elements(Russellet al.,2009;Mahalet al.,2019).Moreover,some soil CO2efflux measurements were higher in N fertilizer-treated Tr plots than in a non-trenched plot in a temperate loblolly pine forest,resulting in a negative autotrophic respiration during drought.Nonetheless,these unexpected findings were attributed to spatial and temporal variabilities,whereby occasional low soil CO2efflux from an untrenched location paired with high soil CO2efflux from a trenched location was recorded(Drakeet al.,2012).
While RPE was demonstrated in RE plots with N fertilization,the effect lasted only a few days following fertilization(Comeauet al.,2016).In the short term (days to weeks),RPE is primarily relevant to dynamic changes in labile SOM.Nevertheless,the long-term(months to decades)impact of RPE on stabilized SOM remains uncertain owing to the scarcity of long-term experiments(Huoet al.,2017).
Effects of REon soil microbial communities
Despite the importance of soil microorganisms in SOM decomposition and CO2production(Clevelandet al.,2007),the effect of the RE approach on soil microbial populations has received little attention (Díaz-Pinéset al.,2010;Weiet al.,2016).The RE technique increases C supply through severed root decomposition,with potential impacts on the structure of the microbial communities and,in turn,heterotrophic respiration(Brantet al.,2006;Wuet al.,2019).
A few studies have attempted to elucidate heterotrophic respiration connections with soil microorganisms by correlating field-measured CO2emissions with soil microbial parameters(Kutschet al.,2010),such as microbial biomass,extracellular enzyme activities,and microbial diversity and community structure(abundance and compositions)(Weiet al.,2016;Bordenet al.,2021).Traditionally,soil microbial biomass has been adopted as a simple means to quantify soil microorganisms as a single entity(Gonzalez-Quiñoneset al.,2011) that plays an essential role in organic matter decomposition and nutrient mineralization.In other words,microbial biomass is the main catalyst in soil biogeochemical processes and an energy and nutrient source(Tate,2020).Nonetheless,the black-box approach does not allow the differentiation of microbial species(Nannipieriet al.,2020).
Extracellular enzymes catalyze organic matter breakdown and mineralization and are commonly analyzed as a response to changing environmental conditions(Acosta-Martínezet al.,2010;Kotroczóet al.,2014).Furthermore,microbial diversity and community structure are essential regulators of various ecosystem functions(Wagget al.,2014;Delgado-Baquerizoet al.,2016).Changes in microbial diversity (Tardyet al.,2015) and community composition significantly influence C mineralization in soil(Riekeet al.,2022).Empirical data have revealed that microbial taxonomic and functional attributes can predict soil CO2fluxes(Liuet al.,2018,2020;Riekeet al.,2022).
A positive correlation was observed between soil CO2emission and soil microbial biomass in urban woody and grassy soils in Russia(Sushkoet al.,2019)and a forest after fire disturbance(Holden and Treseder,2013).Nevertheless,different studies have reported that soil microbial biomass may increase,remain unaffected,or decrease after RE.For instance,Weiet al.(2016) observed enhanced microbial respiration in trenched plots with high soil microbial biomass in subtropical forests in China but only during the warm season.In contrast,RE by Tr in a montane forest exhibited minor effects on microbial biomass after one year,with a slight increase in bacteria five months later,which might be attributed to the beginning of fine root decomposition(Díaz-Pinéset al.,2010).In a study,detritus removal treatment revealed that soil microbial biomass diminished 1—3 years after Tr,depending on the region (Bluhmet al.,2019).Compared to the control,the RE treatment showed lower biomasses of Gram-positive and-negative bacteria and fungi,enzyme activities,and soil C and N contents (Zhuet al.,2021).A recent soil respiration partitioning investigation of a riparian agrosystem showed that microbial diversity was strongly correlated with heterotrophic respiration;however,no changes were observed in the abundance or activity of total bacteria and fungi between the trenched and control plots(Bordenet al.,2021).
The soil microbial community structure,determined by phospholipid fatty acid(PLFA)analysis,showed alterations after a year of RE.Nevertheless,no difference between the trenched and control plots was observed after three years(Bluhmet al.,2019).Another study found that variations in CO2effluxes between short-term(nine months)DC insertion treatments(RE and root+mycorrhiza exclusion)of a temperate grassland were not associated with microbial diversity or community structure.Both reports suggest that microbial communities at the study sites were resilient to short-term soil disturbances (Allison and Martiny,2008;Heinemeyeret al.,2012).
The effects of Tr on soil microbial communities get stronger over time.For instance,soil dehydrogenase activity and soil pH of a hemiboreal forest in Estonia were significantly higher in the trenched plots than in the control plots after four years of Tr,which affected microbial activity and community composition(Wanget al.,2019).Consistently,Kukumägiet al.(2017) reported that the O-horizon was more decomposed in the trenched areas than in control areas.Consequently,long-term(over two years)RE by Tr/DC insertion is not recommended due to a structural shift in the microbial communities,leading to an enhanced heterotrophic respiration reduction(Kukumägiet al.,2017).A summary of the effects of the RE method on soil microbial properties relevant to CO2emissions is shown in Table V.
Overall,the low correlation between soil microbial properties and increased CO2emissions in RE:Tr/DC insertion plots might be due to the scarcity of studies that employ coarse-scale methods for profiling microbial communities.Accordingly,more investigations that utilize available modern molecular approaches(e.g.,metatranscriptomics and mRNA-stable isotope probing)are necessary to unveil the direct link between CO2flux in RE plots and active soil microbial activities at a higher taxonomic resolution than the total microbial community PLFA(Kwonet al.,2019;Nannipieriet al.,2020).
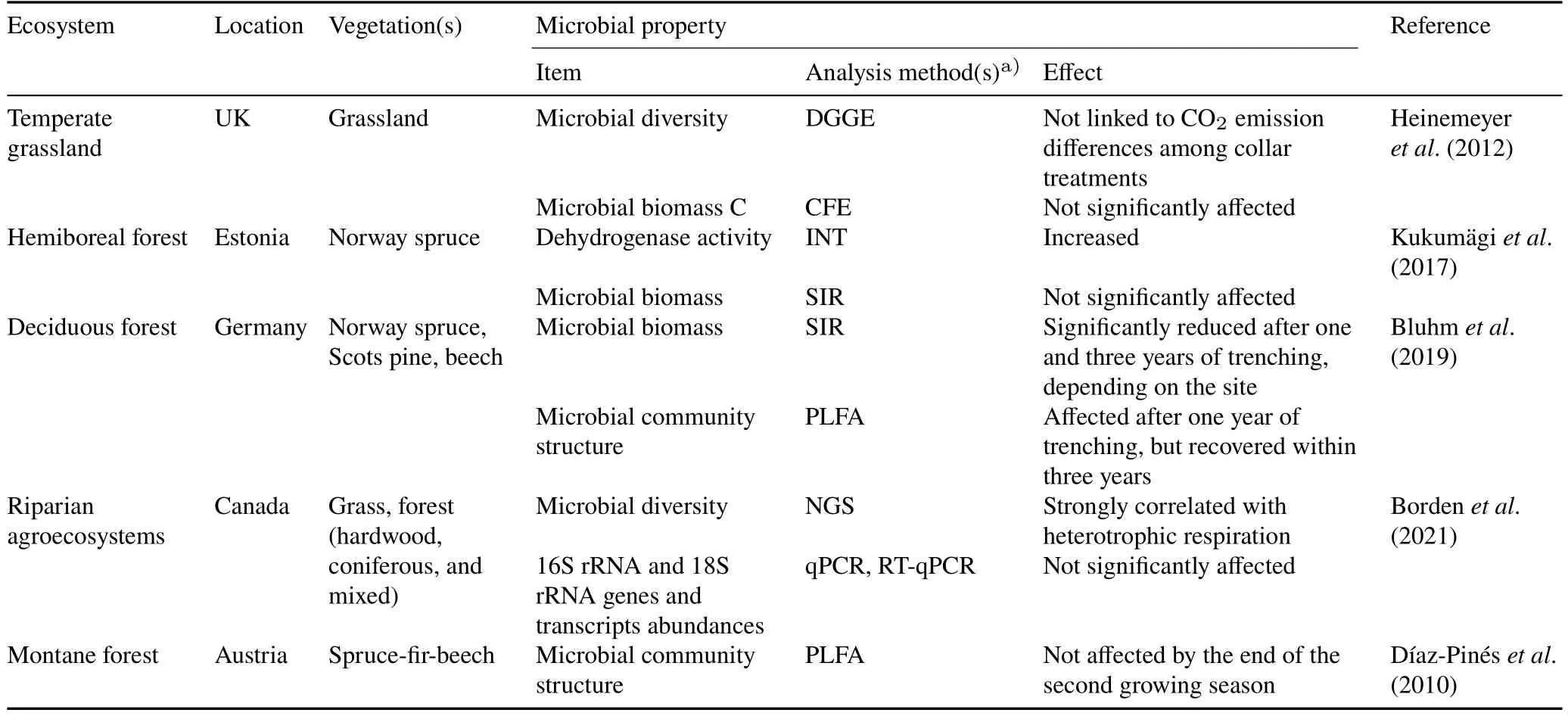
TABLE VEffects of root exclusion on soil microbial properties relevant to CO2 emissions
Microbial C use efficiency(CUE)is becoming a critical microbial parameter reflecting the proportions of mineralized C respired as CO2and assimilated into microbial biomass(Manzoniet al.,2012;Blagodatskayaet al.,2014;Geyeret al.,2016).Since soil microbes with lower CUEs are less efficient in incorporating C into biomass,they might exhibit higher heterotrophic respiration levels.Alternatively,a higher CUE indicates that more plant C is retained as SOM(Manzoniet al.,2012).Thus,further exploration of microbial community composition combined with microbial CUE might help us to better interpret the higher CO2emissions in trenched plots compared to the control plots,as reported in some studies.
COMBINATIONS OF METHODS FOR SOIL RESPIRATION PARTITIONING
Earlier reviews concluded that no perfect method is available for soil respiration partitioning,as each has its own strengths and weaknesses(Hansonet al.,2000;Kuzyakov,2006;Subkeet al.,2006).A previous study supported this conclusion by comparing five different techniques used for soil respiration partitioning in a subtropical secondary forest and revealing that no individual approach was completely satisfactory(Comeauet al.,2018).
Combining two or more methods has been suggested to assess the autotrophic and heterotrophic components of total soil respiration to overcome measurement-specific limitations.Isotopic approaches(13C-labeling and13C-natural abundance)are considered the most reliable for estimating CO2emissions from SOM decomposition(Hansonet al.,2000;Baggs,2006;Kuzyakov,2006),given that they result in minimal disturbance to soil structure.Nonetheless,only a few investigations have attempted to combine RE and isotopic methods for evaluating soil respiration partitioning(Biasiet al.,2012;Risket al.,2012;Baloghet al.,2016;Zhaoet al.,2017).
Modeling techniques have been combined with RE approaches to simulate soil respiration and its components.Modeling methods include statistical models of soil respiration component responses to environmental variables(Brown and Markewitz,2018)and process-based biogeochemical models(Wanget al.,2011;Dondiniet al.,2017;Brown and Markewitz,2018).In the sections that follow,stable isotope-based approaches and their combination with RE approaches are briefly described.
Isotopic methods
Quantifying belowground C due to translocation between plant and soil is challenging and easily leads to underestimating the total C input to the soil(Bromandet al.,2001;Kuzyakovet al.,2001).For decades,isotopic techniques have been used to estimate the relative contributions of plant roots and SOM decomposition to soil respiration.Radioactive14C or stable13C is used as a tracer for the origin of soil CO2efflux in isotopic methods.Several isotopic techniques incorporating various principles were introduced by Kuzyakov(2006),who also highlighted the influence of environmental conditions on the selected methods.
Separation of heterotrophic respiration from autotrophic respirationin situthrough isotopic approaches provides quantitative data with minimum disturbance to the soils and roots and avoids the assumptions of equilibrium in the soil C pool(Hansonet al.,2000;Sakataet al.,2007).Nonetheless,the primary disadvantage of this method is the high cost and complexity of the experimental setup,which involves complex analytical instrumentation for radioactive or stable C isotopes(Hansonet al.,2000;Biasiet al.,2012).Furthermore,partitioning of some soil-plant systems through isotopic methods is limited to the transition of C3to C4ecosystem orvice versa(Rochetteet al.,1999).
Isotopic techniques are classified into pulse,repeated pulse,and continuous labelings.Depending on the degree of mass balance,the methods would yield variations in plant C allocations and contribution of root respiration to soil CO2efflux(Hansonet al.,2000).Pulse labeling or repeated pulse labeling can be used to assess the influence of recent incorporation of C on total soil CO2efflux(Kuzyakovet al.,2001).However,Kuzyakov and Bol(2004)questioned the accuracy of data obtained through pulse labeling with13C natural abundance(δ13C).They raised concerns with respect to various issues including:background variation ofδ13C values with 10%—15% errors,choice of substances,such as slurry and sugar,with appropriate decomposition rates,isotopic discrimination by CO2production from different sources,which was assumed to be negligible,and the correspondence ofδ13C values between two C3/C4sources to calculate the contribution of the third C3/C4source.
Kuzyakov and Larionova(2005)evaluated four methods for partitioning root-derived CO2effluxes using the pulse labeling techniques: isotope dilution,model rhizodeposition,14CO2efflux dynamics modeling,and exudate elution.They found that the isotope dilution and14CO2efflux dynamics modeling techniques recorded similar root respiration and heterotrophic respiration estimations,whereas the model rhizodeposition and exudate elution approaches overand underestimated root respiration and heterotrophic respiration,respectively.They concluded that CO2δ13C and microbial biomass were the best methods when plants were continuously labeled in13CO2or14CO2atmosphere.
Continuous labeling with14CO2or13CO2is commonly used under laboratory or field conditions for durations comparable to the life span of a plant (Hansonet al.,2000)because plants are continuously exposed to the atmosphere at a fixed14C or13C ratio.Consequently,with a known value of the newly assimilated amount of C from the atmosphere,continuous labeling is more suitable for estimating the total C transferred by plants to soil than for belowground C pools labeling and investigating the soil organic C losses,as CO2is compensated by root C input(Meharg,1994;Kuzyakov,2006).Continuous labeling under field conditions was deemed unsuitable,as the requirement for maintaining a constant isotope ratio over an extended period and controlling the temperature and moisture within the labeling chamber is complex and costly(Kuzyakov,2006).
The13C natural abundance technique is another isotopic approach that might be more advantageous than other methods as,in this case,all C pools in plants are labeled.Moreover,this method is non-invasive and does not involve handling radioactive materials(Rochetteet al.,1999).The13C natural abundance technique is commonly applied to growing C4plants on C3soils,orvice versa,and is estimated based on theδ13C value of soil CO2(Kuzyakov,2006).
Free-air CO2enrichment (FACE) is another isotopic technique coupled to a shifted C isotopic composition of added CO2that generates differences in theδ13C of rootand SOM-evolved CO2(Søeet al.,2004;Kuzyakov,2006).Nevertheless,FACE experiments are only suitable for plants grown at CO2concentrations higher than those of the present atmosphere.
REand stable isotopic methods
Biasiet al.(2012)examined an isotope technique based on13C pulse-chase labeling with the conventional root Tr approach to assess the fractional contribution of heterotrophic respiration to the overall soil respiration in Finland boreal peatlands cultivated with reed canary grass.The two-pool isotope mixing model (Hansonet al.,2000) used to separate respiration sources revealed that 50%of the total CO2originated from heterotrophic respiration,compared to 70%obtained from a root Tr experiment,which was possibly overestimated.Nevertheless,the study cautioned that,as root cutting was necessary to obtain theδ13C values of autotrophic respiration,the method was not entirely non-invasive.Consequently,further studies are required to demonstrate the potential of13C pulse labeling.
Using more than one experimental technique to evaluate soil C loss from grasslands is preferable.For example,a study employed Tr and isotopic methods based on13C natural abundance to separate soil respiration into autotrophic and heterotrophic fractions in aRobinia pseudoacaciaL.plantation in the southern Taihang Mountains,China and reported that the contributions of autotrophic respiration in both approaches were similar.Surprisingly,the report suggested that the experimental error produced by the Tr approach was insignificant compared to the isotopic technique(Zhaoet al.,2017).
A comparative study of RE and isotopic approach in dry grassland by Baloghet al.(2016)found that the measured CO2effluxes and isotopic signals showed similar results regarding component responses,with autotrophic respiration displaying the most significant decrease in response to drought and the relative contribution of heterotrophic respiration to soil respiration rising during soil drying and being the highest during drought.A combination of various approaches might reduce the uncertainties in estimating soil respiration component contributions(Risket al.,2012).Accordingly,δ13C stable isotopic signal and soil CO2efflux in RE plot measurements to estimate the relative contributions of soil respiration constituents require more attention in future investigations.
REand process-based biogeochemical models
Wanget al.(2011)combined autotrophic respiration and heterotrophic respiration field observations obtainedviathe RE method with a process-based FOREST-DNDC model to predict soil respiration and its constituents in a subtropical conifer forest in China.The findings demonstrated that although there was a slight underestimation of the simulated autotrophic respiration value due to overestimation of the field-measured autotrophic respiration values,the simulated soil respiration and components were comparable to those obtained using the RE method.Dondiniet al.(2017)compared monthly heterotrophic respiration values simulated by the ECOSSE model with those obtained from automated chamber measurements in RE plots and discovered a strong association between them,with the correlation coefficients ranging from 0.81 to 0.96 for three vegetations.Nonetheless,the model underestimated the flux values during spring and summer(warm weather).The study concluded that the ECOSSE model combined with continuous measurements of heterotrophic respiration in RE plots aids in evaluating the performance of models that simulate heterotrophic respiration at site levels.
A process-based biogeochemical model,DAYCENT,combined with RE,was used to quantify the proportion of heterotrophic respiration to soil respiration in loblolly pine plantations over an annual cycle(Brown and Markewitz,2018).The findings revealed that the technique performed poorly compared to statistical models that utilized soil characteristics,such as microbial biomass,temperature,and moisture,which explained 75%of heterotrophic respiration variability.Furthermore,the DAYCENT model only simulated CO2efflux from the topsoil(0—20 cm)without accounting for autotrophic respiration from the roots in subsoil,which is a drawback of this approach.Therefore,although the model might work in an agricultural system,it would perform rather poorly in pine plantations and larger forest ecosystems.
Combining RE with different modeling approaches has demonstrated comparable or significantly different simulated and measured heterotrophic respiration values.Nevertheless,RE and modeling combination offer the most precise method for estimating soil respiration and its components,as isotopic methods are costly,time-consuming,and not appliable to all ecosystems and study sites.
CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE RESEARCH
Although the RE method is simple,its methodological concerns are frequently overlooked,resulting in biased estimates of autotrophic and heterotrophic soil respiration.Methodological issues also influence the Tr and DC insertion procedures.Nonetheless,the DC insertion technique is advantageous in terms of its smaller soil-disturbance effects and greater control of environmental variation due to the small collars.However,DC insertion increases the risk of soil compaction,thus leading to increased respiration rates.
The higher overall CO2emissions in trenched plots compared to control plots are attributable to residual root decomposition,soil moisture differences,and RPE resulting from enhanced C inputs from dead roots.Although a few studies have revealed a weak correlation between soil microbial properties and increased CO2emissions,changes in microbial communities should not be ruled out.Nevertheless,the RE method is still preferred for soil respiration partitioning studies because of its simplicity,provided that the methodological effects are accounted for to acquire more accurate data.Therefore,this review recommends the following improvements to the soil respiration partitioning method.
First,studies on soil respiration partitioning utilizing the RE:Tr/DC insertion method should consider methodological effects,such as the decomposition of cut roots and differences in soil moisture content in trenched plots,to accurately estimate soil respiration components(Savageet al.,2018).
Second,the soil respiration component results need to be interpreted with caution due to the possibility of positive RPE influence when employing the Tr method with N addition.More long-term studies are required to determine the combined effects of Tr and N fertilization on soil CO2emissions,especially in ecosystems with low N levels(Comeauet al.,2016).
Third,long-term(i.e.,over two years)RE:Tr/DC insertion is not recommended due to the structural shift of soil microbial community,which results in an increase or decrease in heterotrophic respiration(Kukumägiet al.,2017).Furthermore,the possibility of root reinvasion from the outside into the trenched plot through the root barrier or below the trenched plot may lead to an overestimation of heterotrophic respiration(Bowdenet al.,2014;Savageet al.,2018).
Fourth,more studies on the response of the active soil microbial community to Tr effects/plant input removal,microbial species composition in various ecosystems,and vegetation cover are necessary.In particular,assays of microbial community composition with CUE are needed to explain the higher heterotrophic respiration levels in trenched plots than in non-trenched plots.
Fifth,the combination of at least two methodological approaches to soil respiration partitioning investigations(such as combining the RE method with isotopic modeling approaches)is preferable to compensate for the methodological bias of any single strategy and,thus,improve confidence in result interpretations.Furthermore,heterotrophic respiration measurements over RE plots with automated and manual chambers enable the detection of diurnal and seasonal flux variations while accounting for spatial differences.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the Sarawak State Government and the Federal Government of Malaysia for their support.
杂志排行
Pedosphere的其它文章
- Carbon farming by recarbonization of agroecosystems
- Utilization of lignocellulosic plant residues for compost formation and its role in improving soil fertility
- A critical review of microbially induced carbonate precipitation for soil stabilization:The global experiences and future prospective
- Hand-feel soil texture observations to evaluate the accuracy of digital soil maps for local prediction of soil particle size distribution:A casestudy in Central France
- Influence of soil physicochemical properties,particle size fractions and mineralogy on the leaching potentials of arsenic and antimony in abandoned mine soils
- Viscoelasticity and shear resistance at the aggregate scale of structured and organic carbon-free Chernozems
