A critical review of microbially induced carbonate precipitation for soil stabilization:The global experiences and future prospective
2023-10-16SafaaEZZAT
Safaa M.EZZAT
Microbiology Department,Central Laboratoryfor Environmental Quality Monitoring(CLEQM),National Water Research Center(NWRC),El-Kanater 13621/6(Egypt)
ABSTRACT Microbially induced carbonate precipitation (MICP) is a new technology having the potential to induce soil stabilization and provide a green and sustainable comprehensive solution to some geotechnical engineering problems in the environment.The present article is dedicated to present a critical review of this technology and discuss its mechanisms of action and the key factors influencing its performance.The global experiences and national participation from Egypt are demonstrated,in addition to attempts for real life applications.This review provides an insight into the practical steps taken to mitigate some of the current limitations of MICP application and the identified gaps in analogous studies.It was concluded that integrating MICP with existing technologies would favor both engineering needs and market requirements.In addition,providing effective solutions to MICP limitations would highlight this technology as an eco-friendly and cost-effective option to several engineering obstacles.Finally,recommendations focused on encouraging global collaboration for knowledge transfer about this technology among different countries,as well as positive financial support from industrial entities to aid in the progress of scientific research and achieving large-scale applications in the near future,are provided.
Key Words: biocementation,calcite,geotechnical engineering,urease activity,ureolytic bacteria
INTRODUCTION
In many parts of the world,the mechanical properties of soil are considered one of the most challenging factors in construction and engineering(Naveedet al.,2020).Soil may undergo unexpected collapse in response to environmental changes such as weathering effects and overload pressures,thus resulting in surface cracks,landslides,and ground settlement.Indeed,such circumstances may lead to failure of man-made infrastructures(Jaliliet al.,2018).Additionally,erosion of riverbanks and coastlines,as well as slope instability of embankments are among the issues affecting soil mechanical properties(Chaeet al.,2021).In Egypt,loose sandy soils cover a considerable land area.Their geotechnical behavior is mostly prone to low stability,bearing capacity,and shear strength and high permeability and seepage,thus significantly hindering the fulfillment of vital and feasible engineering objectives(Mohamedet al.,2021).Accordingly,soil stabilization is considered indispensable to overcome the aforementioned constraints.
Over the last century,soil stabilization has been attained through several mechanical,physical,and chemical methods.Mechanical stabilization usually involves densifying the soil mass by expelling air voids,whereas physical stabilization uses reinforcing bars,fibers,strips,grids,and sheets to achieve more strength and reduce soil settlements(Almajedet al.,2021).Chemical stabilization depends mainly on inducing reactions between the soil particles and the applied stabilizer.Portland cement,asphalt,fly ash,lime,slag,and xanthan gum are some of the most commonly used chemical stabilizers(Sharakyet al.,2018).
Traditional methods employed to address soil complications have proven several disadvantages,including single performance,high energy consumption,and secondary pollution(Liu and Zhao,2016).In the same context,Andrew(2018) reported that although cement utilization has led to recognizable engineering development,its production is energy-consuming and cement is regrettably responsible for releasing greenhouse gases such as carbon dioxide(CO2)(about 5%of global emissions).On the other hand,chemicals used as grouts and stabilizers are often cost-prohibitive and require many digging wells for injecting large volumes,thus rendering the treatment unfeasible.These chemicals may also hinder natural ground water flow and impact environmental balance due to the presence of toxic compounds such as polyurethanes,silicates,acrylamides,phenoplasts,epoxy,and lignosulfonates(Achal and Kawasaki,2016).
The inevitable demand for novel techniques that can improve soil stabilization with a minimal carbon footprint has compelled researchers to seek for non-conventional methods to meet infrastructural needs,while taking into consideration environmental concerns.Recently,microbiologists,geotechnical engineers,and chemists have proposed the new multidisciplinary microbially induced carbonate precipitation(MICP)technique,which can inducein situsoil strengthening and provide a sustainable and green alternative to traditional methods(Liu Jet al.,2021).
The technique MICP is a natural process facilitated by microbial metabolism and biogeochemical reactions producing calcium carbonate (CaCO3),mostly referred to as calcite.During the process,the precipitated CaCO3forms bridges that bind soil particles (Fig.1),hence improving the strength and stiffness of the soil,while reducing its permeability and erodibility.Currently,MICP is extensively applied to sandy soils,and is extending stepwise to clayey,silty,calcareous,and expansive soils (Krajewska,2018).Microorganisms constitute about 70%—85% of the living components within the soil system.Bacteria are the most widely distributed microorganisms as they have been present in nature for 3.5 billion years.Over 106bacterial cells can inhabit one gram of poorly graded soil as their physiological and genetical characteristics have allowed them to adapt to varying environmental conditions.The exponential growth rate(typically 1 h)of bacteria and their active participation in the natural carbon cycle make them ideal candidates for carbonate precipitation(Umaret al.,2016).
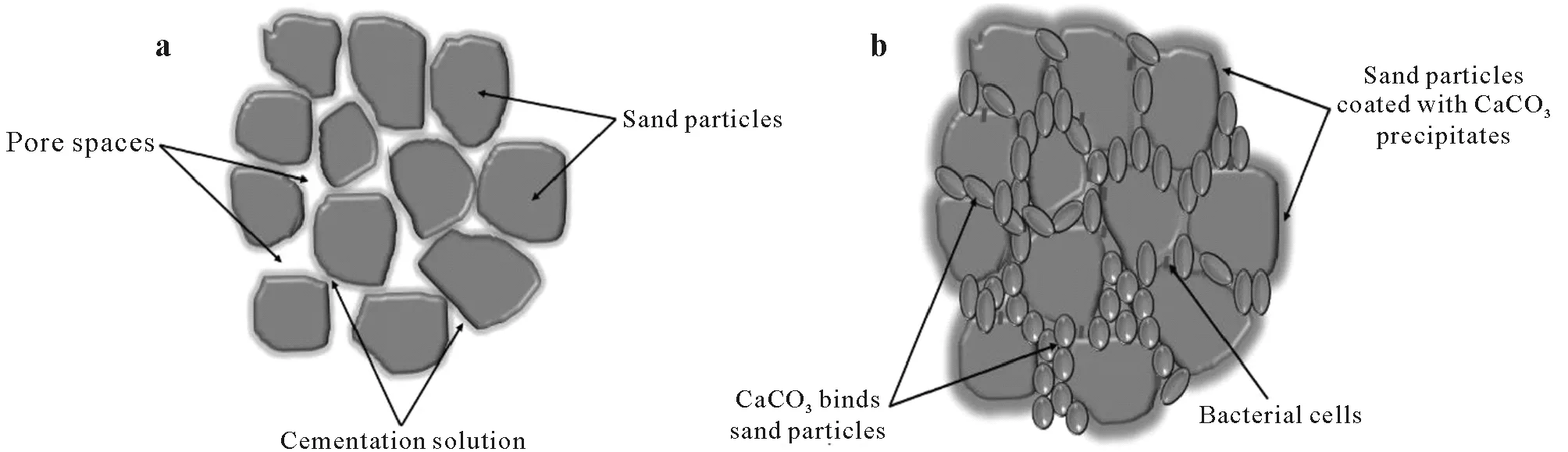
Fig.1 Conceptual diagram showing changes to soil particles during biocementation via microbially induced carbonate precipitation(MICP):soil particles and pore spaces at the beginning of MICP(a)and soil particles bound together by CaCO3 precipitates after MICP(b)(adapted from Naveed et al.,2020).
Accordingly,in a wide range of ancient geological environments,carbonate precipitation has been governed and impacted by microorganisms.Carbonate rocks cemented by CaCO3precipitated by microbial community as an integral part of their natural metabolic activities have been identified in several environments(Sarayuet al.,2014).For instance,although the formation of CaCO3in caves was assumed to be an abiotic process,recently conducted studies suggested the active participation of microorganisms in the development of stalagmites and stalactites found in caves.The CO2,calcium (Ca),and urea introduced through the urine of mammals might have taken part in the bio-mineralization processes occurring in caves(Okyay and Rodrigues,2015).The precipitation of CaCO3in some freshwater lakes,commonly referred to as“whiting event”,is a common natural phenomenon in the summer season in Lake Ontario and Lake Erie.Picocyanobacteria have been suggested as the causative agents of this phenomenon (Zhu and Dittrich,2016).In geological timescale,CaCO3bio-mineralization is of great significance in the fossil records of marine environments.Formation of thrombolites,stromatolites,and carbonated sediments results from calcifications by photosynthetic cyanobacteria(Vasconceloset al.,2014).Travertine is a commonly deposited form of terrestrial limestone around hot springs.It is often formed due to continuous deposition of CaCO3by the combined action of metabolic activities of cyanobacteria and sulfate-reducing bacteria(Okumuraet al.,2013).As nature has always inspired humans to explore different possibilities to replicate natural phenomena with different scientific purposes,using bacteria to improve soil performance and induce soil stabilization can be considered as another attempt to mimic nature.
As reported in the literature,the expected lifetime of soils treated with MICP may extend to more than 50 years.This implies that MICP has the significant potential to compete with traditional methods and to present an economic and sustainable solution for several engineering problems.In this regard,the National Research Council of the USA has announced MICP technology as an important scientific topic for research in the 21st century(Rahmanet al.,2020).
At present,Egypt is experiencing an outstanding development of irrigation water systems through implementation of the“National Project for Rehabilitation of Canals”.This project aims to improve the efficiency of water management and distribution,ensure water rationalization and quality,and increase farmers’profitability.Accordingly,the introduction of non-conventional engineering technologies such as MICP would help ensuring the sustainability and efficiency of irrigation projects and reduce maintenance times and expenses.In addition,MICP technology may contribute actively in the“National Project for Development and Protection of Coastal Zones”through offering an eco-friendly and cost-effective technique to combat coastal erosion.
The present review aims to provide an in-depth understanding of the potential role of microbiological processes in offering comprehensive solutions to some geotechnical engineering problems through MICP technology,particularly in soil stabilization and cementation.Laboratory-scale studies and field-scale trials of different global and national experiences are discussed.Furthermore,the advantages and limitations of MICP as well as future prospective and potential applications will be clarified.
PRINCIPLE AND PROCESS OFMICP
Several bacteria are known to induce carbonate precipitation through different metabolic pathways.Six main pathways capable of mediating the process have been identified so far: photosynthesis by cyanobacteria and algae,ammonification by myxobacteria,ureolysis by ureolytic bacteria,denitrification by nitrate-reducing bacteria,sulfate reduction by sulfate-reducing bacteria,and methane oxidation by methanogens(Zhu and Dittrich,2016).Among these pathways,ureolysis(urea hydrolysis)is the most studied and well-developed process in geotechnical engineering,accounting for about 43%of metabolic pathways.It has low complexity,is easily controlled,and may precipitateca.90%of the required CaCO3in less than 24 h(El Mountassiret al.,2018).
Successful MICP is a straightforward biochemical reaction governed by bacteria producing active urease enzyme in the presence of sufficient urea and Ca2+.The main reactions involved in CaCO3crystallization by ureolysis are presented in Eqs.1—7(Naveedet al.,2020).
First,urease accelerates the hydrolysis of urea,producing ammonia(NH3)and carbamic acid(NH2COOH):
Then,NH2COOH undergoes spontaneous decomposition,forming carbonic acid(H2CO3)and one mole of NH3(Eq.2).Further hydrolysis of the generated NH3results in the production of ammoniumand hydroxide(OH-)ions(Eq.3).
The presence of OH-increases the pH of the medium,which in turn promotes the formation of bicarbonate(HCO-3)and carbonateions(Eqs.4 and 5).
The overall ureolysis-driven MICP reaction of CaCO3precipitation is given in Eq.7.
FACTORS INFLUENCING SOIL STABILIZATION BY MICP
Many researchers have conducted systematic studies concerning the factors affecting MICP and its results.In the present review,the major effective factors are discussed below.
Type of bacteria and cell concentration
The selection of an appropriate bacterial strain for certain MICP application is considered critical because different bacterial strains can result in distinct MICP outcomes according to their genotype and metabolic activities.Sporosarcina pasteurii(previously known asBacillus pasteurii)is considered the most widely used ureolytic bacterium in soil stabilization(Omoregieet al.,2017).Other bacterial genera with different urease activities(2.2—20 mmol urea min-1)have been utilized,such asMicrococcus yunnanensis,Lysinibacillus sphaericus,Bacillus megaterium,Parahodobactersp.,and others.These bacteria are active in moderate to high temperature regions(30—60°C),and Gowthamanet al.(2019)identifiedLysinibacillus xylanilyticusas a suitable candidate for MICP soil improvement in cold regions(15—25°C).
Most studies on MICP application reported the strong relationship between the degree of soil stabilization and the concentration of bacterial cells used.It is essential to determine the time at which bacteria are in their log phase(maximum biomass yield).It has been reported that high concentrations of bacterial cells(106—108cells mL-1)provide high enzyme activity and thus increase the yield of precipitated CaCO3,which in turn promotes soil strength and stabilization(Soonet al.,2014).However,the role of bacterial cells is not limited to that mentioned above;it extends to provide nucleation sites for CaCO3deposition.Bacterial cells promote the binding of positively charged ions (Ca2+) to their negatively charged cell surfaces and catalyze the reaction between Ca2+andions to form CaCO3crystals that finally bind the soil particles as they grow in size(Osinubiet al.,2020).
pH
Given the significance of pH as a factor influencing MICP and soil stabilization,almost all investigations tend to determine the optimum pH that favors the performance of the selected bacterial strain.Most of urease-producing bacteria are optimally activated in slightly alkaline media;pH 8—9.5 is conductive to CaCO3precipitation and can actively influence the transport of bacteria within the soil and their attachment to the surface of soil particles.This scenario affects the uniformity of CaCO3crystals distribution along the biocemented soil,and hence the degree of stabilization(Stabnikovet al.,2013).However,a study on the use ofStaphylococcus saprophyticusto precipitate CaCO3and induce biocementation under neutral conditions found that the maximum precipitation of CaCO3crystals occurred at 30°C and pH 7 in urea-calcium chloride(CaCl2)medium.The precipitation potential ofS. saprophyticuswas five times higher than that ofS. pasteuriiunder the same operation conditions(Kimet al.,2018).A method using one-phase injection was examined under low and neutral pH values usingBacillussp.This approach proved able to provide a relatively uniform distribution of soil strength and eliminate about 90% of produced NH3gas when compared to classical MICP.This method was also effective in terms of mechanical properties,providing a net result of 2.5 MPa of unconfined compressive strength (UCS) (Chenget al.,2019).All previous attempts to examine the possibility of conducting MICP under neutral or lower pH conditions seem to depend on the characteristics and type of the organisms used.Interestingly,in a recent study,a large-scale bacterial culture in 3 000 L of medium was performed with no need for pH regulation.The authors indicated that pH was not critical during field-scale applications because ureolytic bacteria could adapt to unfavorable pH conditions.The growth performance of the used bacteria(S.pasteurii)was not affected and the production of sufficient CaCO3crystals needed for soil biocementation was not hindered(Omoregieet al.,2020).Table I summarizes the effects of urea-CaCl2medium pH on some vital factors of the MICP technology as reported from several studies.
Temperature
Like any other enzymatic reaction,urea hydrolysis by urease is temperature-dependent.The optimum temperature for most urease-mediated MICP processes ranges between 20 and 37°C(Anbuet al.,2016).In a study conducted by De Muyncket al.(2013),the authors reported an effective reduction in water absorption of limestone and the best improvement in strength at 20—28°C;poor performance was observed at low temperature(10°C).In another study,an increase in temperature up to 40°C could induce stabilization of silty clay with UCS of 92 kPa (Keykhaet al.,2017).In summary,the proper temperature for successful MICP outcomes seems to be similar to the optimum temperature for growth of the selected bacterial strain.
Soil particle size
Compatibility between the size of soil particles and that of bacterial cells is considered a crucial factor governing MICP results,as it affects the flow and transport of bacteria through the pore space,as well as adequate contact between the soil particles per unit volume.Particles too small hinder the normal bacterial movement and generate uneven distribution of CaCO3.As so,free passage of bacteria is not expected through a pore throat smaller than 0.4 μm.On the other hand,particles too large limit the number of available contact points among soil particles and prevent the proper deposition of CaCO3in pore spaces.Both cases result in poor soil stabilization.As shown in Table II,the most appropriate particle size for optimum soil consolidation is in the range of 50—400 μm(Zhaoet al.,2014;Amarakoon and Kawasaki,2016;Cuiet al.,2016).The geometric compatibility between ureolytic bacteria and the soil pore space is another factor governing the degree of consolidation strength(Osinubiet al.,2020).
Biocementation reagents
Cementation solutions used in MICP applications are mostly composed of urea and CaCl2.Urea is the source ofions while CaCl2provides Ca2+ions.The proper concentrations of both ions are critical for CaCO3precipitation and accordingly soil stabilization.Several concentrations and combinations of cementation solutions have been tested,and the MICP performances are different(Table III).The deposition of CaCO3increases with increasing concentration of the cementation solution.However,differences in optimum cementation schemes have been reported,with appropriate concentration ratios selected according to the required degree of reinforcement as well as the needed application (Liu Jet al.,2021).
Al Qabany and Soga(2013)reported the formation of CaCO3large crystals within the soil pore spaces when the concentrations of urea and CaCl2were increased from 0.1 to 0.5 mol L-1,which ultimately increased the soil compressive strength.However,several studies reported a concentration range of 0.5—1.0 mol L-1as being appropriate,whereas others reported that lower concentrations (0.05—0.25 mol L-1)were more effective(Osinubiet al.,2020).In a recent study,the retarding effect of increasing the concentration of cementation solution was investigated.The results showed that Ca2+concentration was a key factor.Concentrations higher than 1.0 mol L-1reduced the amount of precipitated CaCO3,and at 2.5 mol L-1,CaCO3was not formed(Lai H Jet al.,2021).Similarly,Iamchaturapatret al.(2022)found that increasing the concentration of Ca2+ions increased both the cohesion and the internal friction angle but to a limited extent.The optimum concentration of Ca2+ion was 0.25 mol L-1and the authors showed that using higher concentrations of Ca2+in the form of CaCl2(0.5 mol L-1)had a detrimental effect on the strength of biocemented sand.This was because high Cl-ions could possibly react with H+andions to form HCl and NH4Cl,respectively.This led to acidification rather than the desired alkalinity needed for biocementation(pH>8).Accordingly,the amount ofions would not be sufficient for CaCO3precipitation.Additional results from studies exploring optimal cementation schemes are given in Table III.
TREATMENT METHODS FOR BIOCEMENTATION
Successful soil stabilization depends on the method used for introducing bacterial suspension and cementation reagents into the soil column.The selected method is expected to provide homogeneous distribution of CaCO3crystals among the soil particles and prevent system clogging at the inlet point(Mahawishet al.,2018).The three main methods used for MICP treatment are as follows.
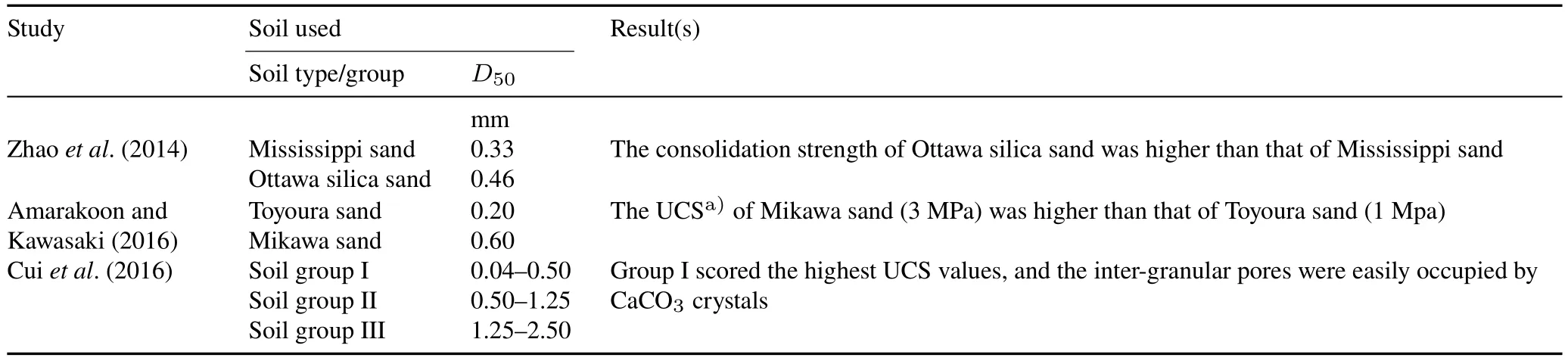
TABLE IISome studies that investigated the influence of soil particle effective diameter(D50)on the performance of microbially induced carbonate precipitation technology and the results obtained
Injection method
This method has been used frequently for soil stabilization but with some drawbacks.It is relatively complex and needs to be run in a separate room to control pressure flow and the hydraulic gradient.In addition,injection from both directions (vertical and horizontal) is also required (Mujahet al.,2017).In most cases,bioclogging occurs around the injection points,which prevents the even distribution of bacterial and cementation solutions,leading to the nonhomogenous distribution of CaCO3(Rowshanbakhtet al.,2016).Recently,a one-phase injection method under low pH was proposed to replace the existing multiple injection.This approach prevented bioclogging and reduced the generated NH3gas by 90%(Chenget al.,2019).
Surface percolation method
This method is considered one of the most preferable in MICP applications for soil consolidation and stabilization.It is technologically simple,doesn’t require heavy machinery,and offers a relatively cost-effective path for soil improvement as compared to other methods(Almajedet al.,2018).The bacterial suspension is vertically introduced into the sand column,followed by the cementation solution,in which they move freely and self-adjusted by gravity and capillarity.Cheng and Cord-Ruwisch(2014)treated a 2-m column of coarse sand using this method and successfully achieved UCS values of 850—2 067 kPa.Similarly,in two 0.6-m sand columns(fine and coarse)treated using the same method,the UCS values reached 920—1 250 kPa(coarse sand)and 410—570 kPa (fine sand).Similar levels of CaCO3were precipitated in both sand columns (Chenget al.,2017).Although this method has proven remarkably successful,some limitations have been reported for very fine-grained soils,most probably because of the lower infiltration rate and permeability of such soils as compared to those of coarser soils.
Premixing method
This method was suggested to overcome the problem of non-homogenous distribution of CaCO3crystals.In this method,the bacterial suspension is mechanically premixed with soil to guarantee homogeneity.Limitations of this method include the disturbance of local soil due to repeated mechanical mixing,which leads to pseudo stress of the treated soil,eventually resulting in problems during the assessment of engineering properties(Mujahet al.,2017).Moreover,the resulting UCS of biocemented soil is relatively low compared to that obtained using other methods.It is worth mentioning that regardless of the biocementation method used,its optimum performance should be evaluated to ensure that the product could meet the required application.Numerous tests have been applied in different research studies.While some monitored the conditions governing the process progress,such as urease activity,biomass concentration,pH,and CaCO3content (Omoregieet al.,2021),others measured some important engineering properties,such as UCS,stiffness,liquefaction resistance,hydraulic conductivity(permeability),bulk density,shear strength,and slake durability index(SDI)(Liu X Jet al.,2021).
GLOBAL EXPERIENCES IN MICP TECHNOLOGY
Before discussing the details of laboratory-scale and field-scale studies carried out globally,a brief chronological overview of the development of MICP applications in geotechnical engineering is presented.In 1973,a Spanish group of researchers demonstrated for the first time the potential of soil bacteria to precipitate CaCO3under laboratory conditions.Ten years later,the different mechanisms governing this ability were investigated,and the microbial origin of limestone was evidenced.In 1990,a French research group applied for the European patent (90400G97.0) of surface coating(biocalcin)fabricated by microorganisms.This was followed by the establishment of the company“Calcite Biocement”,and in 1993,the firstin situapplication of biocalcin was carried out on the tower of a church in Paris,France,covering an area of 50 m2.This treatment is renewed every 10 years to restore the protective action of biocalcin (De Muyncket al.,2010).
Laboratory-scale studies
The improvement of soil strength and stiffness was first studied in Australia.The authors reported increases in the UCS values of MICP-treated soils within 5 h of the experiment(Ismailet al.,2002).Also in Australia,Whiffin(2004)proposed the“Whiffin’s conductivity method”for predicting changes in urease activity and CaCO3precipitation and developed the use of MICP technology to cement loose sand particles.Two years later,the author,together with a group of researchers in Australia,was awarded the world patent(066326)as they could convert a bag of sand into calcareous sandstone (Kucharskiet al.,2006).In the same year,an Indian researcher applied for a patent usingShewenellasp.bacteria to improve concrete strength(Saroj,2006).After one year,the method of biomineralization was patented in China(Qianet al.,2007).
Recently,several studies on MICP showed successful results in improving the geotechnical engineering properties of treated soils.A group of scientists from University of Idaho,USA used indigenous soil bacteria for CaCO3precipitation and reported their effectiveness in increasing the sand resistance for liquefaction,and the process was considered economically feasible(Burbanket al.,2011).In a study in China,the feasibility ofS.pasteuriito strengthen the surface of Aeolian sandy soil was studied.The obtained UCS reached 3.20 MPa after MICP treatment(Tianet al.,2018).In another study,the use of some additives along with MICP treatment to improve soil strength was examined by a team of five researchers from China.Using fiber felt scrap waste material,they observed a recognizable improvement in the UCS and tensile strength of the treated sand(Zhaoet al.,2020).Lai Y Met al.(2021)investigated the change in permeability of two types of sand using MICP technology.They found that the samples showing a marked decrease in permeability contained more deposited CaCO3.The authors concluded that CaCO3precipitation is a detrimental factor influencing soil permeability and stability.They recommended using MICP technology for the anti-seepage of reservoirs,dams,and embankments.
Iamchaturapatret al.(2022) studied biocementation based on enzyme-induced carbonate precipitation using urease combined with hemp fibers for improving sandy soil properties.It was found that the maximum amount of natural hemp fibers for mixing in biocemented sand should be 2.5%by volume,and the results of wave velocity and initial shear modulus revealed that the process of biocementation requires a few days for the reaction to start.The ammonification reaction reached its maximum at day 4 and finished at day 6.
As the durability of MICP-treated soil depends on the formed CaCO3crystals,its long-term performance and deterioration under various environmental conditions remain a critical question.A group of researchers from Hokkaido University,Japan investigated the freeze-thaw(FT)effect on MICP specimens through a series of experiments.Cycles of FT can influence the stability in regions experiencing seasonal frost,thus impacting the runoffand erosion of slopes.Results from FT tests revealed the resistance of MICPtreated slope soils are to FT-induced erosion.Specimens cemented with 20%—23%CaCO3by mass showed only minor tendency to erosion(2%mass loss)when subjected to 25 FT cycles(Gowthamanet al.,2020).The authors attributed this high resistance to the adequate cementation levels,which facilitate effective particle contact capable of resisting the emergence of ruptures and crystal-soil detachment during pore water frosting.Similar studies have investigated the influence of other natural phenomena on MICP-treated soil durability and properties including tidal wave(Salifuet al.,2016)and rainfall(Jianget al.,2019)effects.The obtained results showed that erosion resistance was recognizable in MICP-treated slopes.
As recognizable from the above overview,there is a leap in the number of publications on MICP studies and applications since 1973,which reflects the exponential growth of interest in this research area.An additional snapshot of these publications is given in Table IV,which also shows the diversity of countries that have stepped in to contribute to the knowledge base using different bacterial strains and types of soils.Indeed,the differences in these laboratory-scale studies led to the enrichment of the results obtained and paved the way for field-scale studies and applications.
Field-scale studies and attempts for applications
The novel MICP technique will be much appreciated if the results of laboratory-scale studies are geared to up-scaled field applications.Below we review some of the attempts towards solving some real-life engineering problems using MICP.
The ability of MICP to induce cementation over a long distance was first examined using a 5-m sand column.The authors observed cementation throughout the entire column length and reported significant CaCO3precipitation and improvement in soil stiffness and strength (Whiffinet al.,2007).In attempts for applications,the Netherlands was among the pioneers;two distinguished large-scale studies were carried out.In the first,a 100-m3sand box was treated over a distance of 5 m using three injection wells and three extraction wells for the biocementation solution(Van Paassen,2011).After 16 d of treatment,a clearly well-developed cemented sand body (40 m3) was obtained,with UCS ofca.12 MPa and CaCO3content of 110 kg m-3.In the second study,MICP was applied at two different locations along the River Waal,eastern Netherlands (Van der Staret al.,2011).Approximately 1 000 m3of gravel was stabilized with biocementation solution.The gravel boreholes improved significantly,and two gas pipelines(1.22 m)were successfully installed.
In Singapore,Chuet al.(2013)used MICP to construct an anti-seepage reservoir(aquaculture pond).After treatment,the permeability of sand was reduced from 10-4to 10-7m s-1and the UCS of the side walls reached 932 kPa.In China,Tanet al.(2018)applied MICP in the bioclogging of three bank sections of the Dawa reservoir to prevent seepage and ensure clay soil consolidation.Soil permeability was reduced by two orders of magnitude in a short time,and the soil strength was increased by 13%.
Application of MICP treatment for resistance to wind and hydraulic erosion has also been performed worldwide.In Canada,a field-scale study conducted by Gomezet al.(2015)evaluated the ability of this biotechnology to increase wind erosion resistance and surface stability of loose sandy soil.The obtained results demonstrated a recognizable improvement in the treated soil up to 28 cm depth,and the measured CaCO3content reached up to 2.1%.Recently,a field-scale study was conducted in the USA to evaluate the resistance of biocemented coastal dunes(poorly graded sand)againstwave erosion(Montoyaet al.,2021).Both treated and untreated(control)dunes were subjected to approximately 300 waves in each trial (a total of 19 trials).In each trial,the wave period,height,and depth were increased sequentially.Results revealed that all treated dunes displayed recognizable improvement in erodibility parameters and surface strength under inclement weather conditions as compared to untreated dunes.
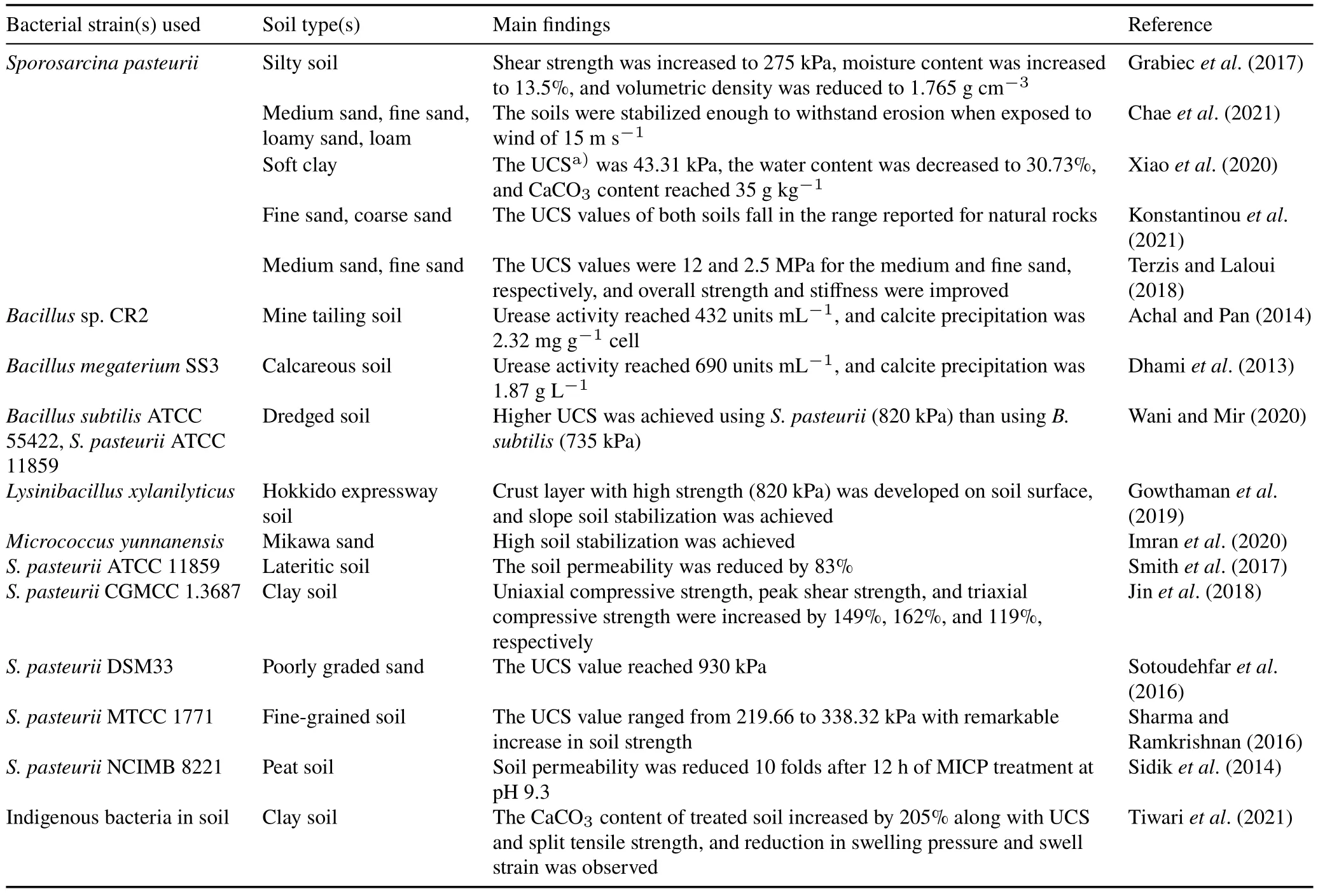
TABLE IVSome laboratory-scale studies on soil stabilization using the microbially induced carbonate precipitation(MICP)technology worldwide
APPLICATION OFMICP TECHNOLOGY IN EGYPT
In Egypt,MICP technology is still in its infancy and the available studies are so far limited to laboratory-scale efforts.However,the obtained results are considered promising and encouraging.Table V represents an inventory of all studies that have been conducted in Egypt on MICP and the main outcomes of each study.These studies were performed by various scientific research institutions in cooperation,including the Housing and Building National Research Center,Faculty of Science-Ain Shams University,National Research Center,Faculty of Science-Helwan University,Faculty of African Postgraduate Studies-Cairo University,and National Water Research Center (NWRC).A variety of soil types,such as poorly graded,siliceous,and calcareous sands were tested.Soil stabilization results were basically monitored through measuring the UCS,which reached 120 kg cm-2(Abo-El-Eneinet al.,2012),and the SDI,which was 55%(Elmashad and Hafez,2017),as well as the CaCO3content(93.5%)in the study conducted by Mohamedet al.(2021).
In parallel to attempts for improving soil properties and achieving satisfactory consolidating results,some researchers examined the MICP ability of bacteria on synthetic media to assay maximum urease activity and CaCO3precipitation(Hammadet al.,2013).Several microscopic images showed six different polymorphs for CaCO3crystals that could influence the process of soil stabilization.Moreover,Sharakyet al.(2018) selected the mixing method as a successful candidate for MICP under the conditions of their experiments.In summary,variations in the generated results depended mainly on the applied testing conditions,including soil type,bacterial biomass,aeration time,casting time,and the strength of cementing solution.It is worth noting that all reported studies herein selectedS.pasteuriias a source for bacterial suspensions.Although this trend may haveachieved satisfactory results,it may not attain the desired economic feasibility.Collectively,studies conducted so far in Egypt pointed out the importance of MICP in geotechnical engineering,particularly soil stabilization and cementation,and recommended that scale-up studies should be encouraged in Egypt for future application and commercialization.
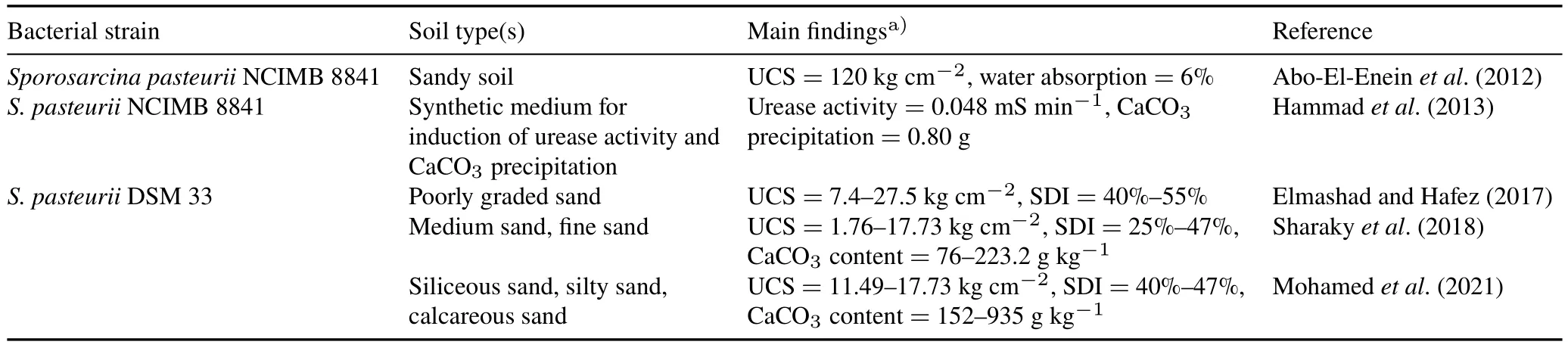
TABLE VSome laboratory-scale studies on soil stabilization using the microbially induced carbonate precipitation technology in Egypt
A CASE STUDY FROM EGYPT AND THE STEPS TAKEN TO MITIGATE MICP LIMITATIONS
Based on the literature reviewed in the present article,MICP has shown various achievements at both laboratory and field scales.However,the expansion of this technology in real-life applications has some limitations that should be addressed carefully to guarantee its successful implementation at a commercial scale.From our point of view,the most challenging gaps include environmental biosafety,acclimation of bacteria to environmental conditions,homogeneity of CaCO3distribution in stabilized soils,and economic feasibility.A recent case study conducted in Egypt addressed these challenges and proposed some practical solutions(Ezzat and Ewida,2021).The study was conducted in the Microbiology Department,Central Laboratory for Environmental Quality Monitoring(CLEQM),NWRC,Egypt.The aim was focused on the isolation of a native bacterial strain capable of inducing CaCO3precipitation in soil samples from three different coastal areas in Egypt(Alexandria,Ain Sokhna,and Marsa Matruh)and improving their mechanical properties for geotechnical engineering needs.Bacterial strain,reagent grade,and treatment method were subjects of investigation to achieve environmental safety,economic feasibility,and quality results.
Environmental biosafety
The biological safety of the surrounding environment is the first priority of MICP application attempts.Unusually,some studies used bacterial strains other thanS.pasteuriiwhose biosafety is questionable.Species ofProteus,Pseudomonas,Klebsiella,andCorynbacteriumare known causative agents of lung and urinary tract infections,as well as of long-lasting diseases(Koniecznaet al.,2012).Another concern with MICP biosafety is the probability of uncontrolled growth of the used bacteria.Spore-forming species like those ofBacilluscan survive longer and multiply in the environment.This is definitely undesirable and requires environmental impact assessment studies.
Ezzat and Ewida(2021)dealt with biosafety issues by isolating a native bacterial strain from agricultural fields in Egypt,identified asAlkalibacterium iburiensestrain EE1 and given the accession number MF355369.1 in the National Center for Biotechnology Information GenBank database.The bacterial strainA.iburienseEE1 showed recognizable efficiency in urea hydrolysis and subsequent MICP activity and soil stabilization,with urease activity of 20 mmol urea min-1,UCS of 2.11 kg cm-2,and CaCO3content of 385 g kg-1.According to the Technical Rules for Biological Agents that classify bacteria into risk groups,A.iburiensewas assigned into group 1,which contains bacteria unlikely causing infectious diseases to humans or inducing hazards to the environment(CBA,2015).In addition,the morphological and biochemical characteristics ofA.iburienseconfirmed that it is a non-spore-forming bacterium(Nakajimaet al.,2005).This eliminates the drawback of using spore-forming bacteria that lead to probable uncontrolled growth in the environment.Accordingly,A.iburienseEE1 is considered eco-friendly with significant biosafety.To the best of our knowledge,A.iburiensewas first reported for its biogrouting activity and ability of inducing stabilization of sandy soils in this study.
Acclimation of bacteria to environmental conditions
In practical applications,bacteria are often exposed to several harsh environmental conditions which may affect their efficiency for MICP treatments.Among these critical conditions are oxygen and nutrient deficiency,high pH and salinity,and unsuitable temperature changes(Zhu and Dittrich,2016).To mitigate the adverse effects of such extreme conditions,Ezzat and Ewida(2021)isolated and used a native bacterial strain(A.iburiense)rather than using the classical patent strain examined by most studies(S.pasteurii).Their study showed thatA.iburiensecould withstand pH up to 9.56 and salinity up to 38.36 g L-1.The biogrouted soil samples showed high stability in water(only 0.11%loss in weight),indicating that soil treated withA.iburiensecould experience high durability against both salinity and hydraulic erosion.Additionally,the study reported the detection of urease activity in the filtrate of cultivation medium,which indicated that urease might be extracellular rather than cell-associated.This feature pointed out the possibility of using the enzyme itself instead of the bacterial cells.Actually,bacteria isolated from natural environments are expected to acclimate better to local field conditions than alien bacteria,and using bacteria native to the environment has been considered as a main factor ultimately influencing MICP efficiency.In this respect,Burbanket al.(2013)demonstrated that indigenous bacteria can be stimulated to induce CaCO3precipitation with measurable changes in geotechnical properties.The authors concluded that employing indigenous bacteria for modification of soil properties is a significant step that renders biomodification processes economically practical and feasible.In another study,a comparison was made between the amounts of CaCO3precipitated by exogenous bacteria(S.pasteuriiATCC 11859)and four indigenous isolates from calcareous sand and limestone cave soils(Kim and Youn,2016).The four indigenous isolates were competitive to the exogenous strain and could precipitate CaCO3successfully.
Homogeneityof CaCO3 distribution
Obtaining a homogenous distribution of CaCO3along sand columns in MICP applications is a crucial technical step for the success of the entire process.Choosing an unsuitable treatment method may result in bioclogging around injection points and prevent the proper distribution and adherence of bacterial strains and cementation solution to the soil particles.The resulting biocemented product will therefore not fulfill the targeted characteristics.Ezzat and Ewida(2021)managed to overcome this technical limitation through applying the surface percolation method using staged injection instead of parallel injection.To ensure the steady distribution of reagents,five consecutive batches of cementation solution followed bacterial injection.Moreover,the study used a fixation solution(50 mmol L-1CaCl2)to immobilize the injected bacteria and reduce the electrostatic repulsive forces between bacteria and sand grains.This strategy was translated into uniform strengthened soil and mature and stable CaCO3crystals,as evidenced in scanning electron micrograph images.This methodology was supported by several earlier studies(Tobleret al.,2012;Cheng and Cord-Ruwisch,2014).
Economic feasibility
According to the information available in literature,opinions are consensual regarding the MICP technology potential to invade the commercial market.This is due to its eco-friendly orientation,nearly negligible consumption of energy,and low operational and machinery requirements(Myhret al.,2019).Ivanov and Chu(2008)compared the cost of raw materials used in MICP treatments and those used in traditional chemical grouting.They estimated the costs for a cubic meter of soil were US$0.5—9.0 and US$2—72 for biogrouts and chemical grouts,respectively.However,Wanget al.(2017)proposed a total cost of US$25—75 per cubic soil,including materials and equipment as well as installation,for soil stabilization using MICP technology.The authors agreed that the main cost factor in the process is associated with the mass production of bacteria and reagents needed for the cementation solution.From this standpoint,it is recognized that most MICP studies used analytical-grade ingredients for both cultivation of bacteria and preparation of cementation solution.These are reagents with high purity intended for laboratory-scale use.In fact,the cost of analytical-grade reagents hinders MICP scale-up implementation.
Ezzat and Ewida (2021) were able to reduce the cost by two main ways without causing adverse effects on the quality of soil stabilization results.First,the authors used a native bacterial strain(A.iburiense)rather than purchasing the patentedS.pasteuriiATCC®11859™,which costs about US$402.0 according to current global market price.Second,they replaced the analytical-grade reagents with technicalgrade reagents that are simple,cost-effective,and commercially available.The costs of technical-grade yeast extract and urea are estimated to be around US$18.6 and US$1.03 per kilogram,compared to US$180.0 and US$111.25 per kilogram of analytical-grade ones,respectively.Interestingly,the starting biomass of bacteria used in the case study(optical density at 600 nm:2.04)was sufficient to induce the desirable soil strength and reduce the number of injection times by 50%under natural non-sterile conditions.This reduction tackles the challenge of cost and extends the feasibility of large-scale application (Ezzat and Ewida,2021).Similar attempts to reduce the cost were made by using industrial wastes.In this direction,Yoosathapornet al.(2016)tested the use of chicken manure effluent for cultivation ofS.pasteuriias an alternative source of nutrients to make biocemented cubes.The authors reported higher compressive strength(30.27%)and higher density(5.38%)than those of traditional cement.The chicken manure effluent was 88.2%cheaper than nutrient broth commonly used as cultivation medium.The uses of lactose mother liquor and corn steep liquor(Achalet al.,2009),food-grade media(Omoregieet al.,2019),sugarcane molasses(Kiasariet al.,2019),and tofu wastewater(Fanget al.,2019) were also reported in literature.The authors concluded that these materials are inexpensive and exert lower risk to the geoenvironment compared to analytical-grade chemicals without hindering the process of biogrouting.Application costs of MICP can be further reduced when using animal and plant wastes,urea fertilizers,and eggshells as nutrient sources for the bacteria.Gowthamanet al.(2019)explored the use of urea fertilizers,beer yeast,and snow melting agents as substitutes for analytical-grade chemicals in slope soil stabilization using MICP.The authors could achieve significant soil strength along with 96%reduction in treatment costs.This replacement approach could ensure the process sustainability and speed up bacteria self-healing materials delivery to the market(Naveedet al.,2020).
OTHER EMERGING APPLICATIONS
The MICP technology has clearly shown its merits in improving soil mechanical properties to cope with various geotechnical engineering needs.However,other biotechnological applications of MICP have been examined and recommended by many researchers worldwide(Castro-Alonsoet al.,2019;Brasileiroet al.,2021).These include remediation of concrete cracks,bioconcrete,and self-healing concrete(Castro-Alonsoet al.,2019;Pungrasmiet al.,2019;Brasileiroet al.,2021).These new prospective can solve the disadvantages of traditional methods,which include thermal expansion of treated layers,degradation over time,constant maintenance,high cost,and contribution to environmental threats.
Currently,several companies worldwide have implemented MICP technology for commercial engineering applications,such as Biomason in North Carolina,USA,Biocement Technologies in Washington,USA,and Bachy Solentanche in Singapore.In the Middle East,the Kingdom of Saudi Arabia is the exclusive agent for bioconcrete products.These products promote the self-healing of cracked concrete through MICP technology,thus improving the mechanical properties and durability of concrete structures.No doubt,expanded knowledge and research on these topics will represent an added value to MICP real-life applications.
CONCLUSIONS AND RECOMMENDATIONS
As an emerging soil biostabilization technology,MICP is evidenced in natural environmental phenomena observed in caves,freshwater lakes,hot springs,and oceans.Laboratoryand field-scale studies worldwide have shown its effectiveness in soil consolidation,slope stabilization,seepage control,and crack healing.Soil stabilization through MICP is influenced by the type and concentration of bacteria,pH and temperature of working medium,soil particle size,and concentration of the cementation solution.The urea hydrolysis pathway showed optimum efficiency for inducing CaCO3precipitation (up to 90%),S. pasteuriiis among the most widely investigated bacteria worldwide,and the surface percolation method with staged injection along with fixation solution is the most successful method for soil stabilization.The limitations of MICP technology,concerning its biosafety,homogeneity of stabilized soil,and economic feasibility,can be addressed by ways such as using native bacterial strains,staged injection of cementation solution,and technical-grade reagents.Currently,MICP is implemented commercially through several international and regional companies.
Future investigations should focus on proposing practical solutions for reducing the undesirable byproducts to ensure environmental biosafety.Investing in the repurposing of the NH3generated during the process of ureolysis as fertilizer is recommended.Minimizing the cost of MICP through using native bacteria and technical-grade reagents should be encouraged.At the global level,the exchange of practical experiences between different countries would expand the scope of actual applications.At the national level,industrial entities should adopt MICP technology and support continuous scientific research to achieve a distinct product capable of competing at the regional level.From our point of view,MICP is expected to be selected as an eco-friendly and cost-effective technology in the near future due to the global interest in improving conditions for its application.The integration of MICP with existing traditional methods in proportion to engineering needs as well as market requirements will favor the spread of this technology.
ACKNOWLEDGEMENT
We acknowledge the support of the Microbiology Department,Central Laboratory for Environmental Quality Monitoring (CLEQM),National Water Research Center(NWRC),Egypt.
杂志排行
Pedosphere的其它文章
- Carbon farming by recarbonization of agroecosystems
- Root exclusion methods for partitioning of soil respiration:Review and methodological considerations
- Utilization of lignocellulosic plant residues for compost formation and its role in improving soil fertility
- Hand-feel soil texture observations to evaluate the accuracy of digital soil maps for local prediction of soil particle size distribution:A casestudy in Central France
- Influence of soil physicochemical properties,particle size fractions and mineralogy on the leaching potentials of arsenic and antimony in abandoned mine soils
- Viscoelasticity and shear resistance at the aggregate scale of structured and organic carbon-free Chernozems
