Analysis and Enlightenment of Big Data Platform for Adverse Drug Reaction Supervision in China and the United States
2023-09-26ChangSenhaoChangYingnanGongJingranChenYuwen
Chang Senhao,Chang Yingnan,Gong Jingran,Chen Yuwen
(1. School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China;2. Research Institute of Drug Regulatory Science, Shenyang Pharmaceutical University, Shenyang 110016, China)
Abstract Objective To introduce the relevant big data platforms of FDA regulatory sciences and to provide reference for the construction of big data platform for China’s regulatory science under the “14th five-year plan” to deepen the reform of medical and health system.Methods A comparative analysis was made on China’s big data for regulatory science after studying the development process,operation mode,practical significance and characteristics of the big data platform for FDA regulatory science,which would help China to establish a perfect big database.Results and Conclusion The construction of big data platform for China’s regulatory science is not comprehensive compared with that in the United States.It is necessary to build data platforms in line with China’s national conditions through efforts in law,talents,standards,and other aspects.
Keywords: adverse drug reaction;regulatory science;big data platform;US FDA;life cycle
By establishing and improving the system of big data to assist scientific decision-making and social governance,we can promote the innovation of government management and social operation mode as well as the efficiency of public services.As big data has risen from strategic resources to national resources,it becomes a major strategy to improve the quality and level of China’s development.
Nowadays,drug regulation is facing multiple challenges.Changes in the field of science promote the way of drug development at tremendous speed,challenge the traditional evidence model of regulators,and force regulators to obtain evidence that can well support the decision-making of the whole product life cycle.These new sources of evidence,commonly referred to as big data,not only provide opportunities to improve decision-making,but also bring uncertainty about data quality.Therefore,there are many potential opportunities for the evidence.
Big data strategy can provide insights into physiological and pathophysiological processes at the organ,cell,and even subcellular level[1].Meanwhile,the development of new pharmacological models can improve the efficiency and cost-effectiveness of model-informed drug discovery and development.Through these methods,we can better describe and predict adverse drug reactions (ADRs),but we can also identify biomarkers of disease and drug reactivity.
According to the sources of big data platform of drug regulatory science,they can be divided into three categories: ADRs,clinical trials,production and circulation[2].This paper focuses on the comparative analysis of the big data platform of ADRs between China and the United States.
1 Construction of big data platform for ADRs in the United States
1.1 Sentinel system
Origin: In 2008,U.S.Department of Health and Human Services (HHS) and Food and Drug Administration (FDA) jointly launched the sentinel system,which is a project for long-term electronic monitoring of drug safety.As a proactive system,it will supplement the system established by FDA for tracking ADR reports related to the use of regulated products.Therefore,it can obtain a wide range of medical and health data information from various channels[3].
Development: From 2000 to 2019,the distributed database collected information of more than 300 million patients,including 70 million members who are accumulating new data,and beneficiaries of federal and private insurance.The system adopts distributed database design,making it one of the largest multi-site and privacy protection medical product safety monitoring systems in the world,with highly solidified data[4].
In response to the “FDA Amendment Act” of 2007,the FDA launched the sentinel initiative in May 2008.
In September 2014,FDA began the transition from the mini sentinel phase to the full sentinel system that was officially launched in February 2016.Over time,sentinel system has developed the world’s largest multi website distributed database,which is committed to medical product safety,continuous growth,and improvement to meet the needs of FDA.
Latest progress: In September 2019,FDA announced that it would expand to three different coordination centers: Operation Center,Innovation Center and Community Construction and Outreach Center.
This new design will attract the participation of a wider range of scientists,promoting the development of new technologies in emerging fields such as translation data science and big data,enhancing the creation of laboratories,developing new methods for using electronic health records,and cultivating a strong scientific team to discover drug safety in advance by using the core capabilities of the system.
Formation: There are three important roles in the sentinel system,the first is FDA,and the other two are data partners and coordination centers,as shown in Fig.1.
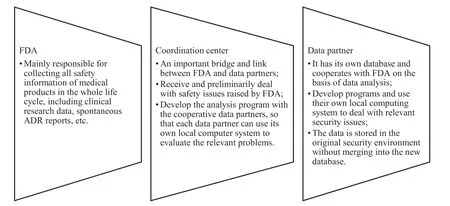
Fig.1 Roles of sentinel system
Sentinel system is an active monitoring system that enables FDA to actively and realtime obtain information from data partners.FDA can apply existing electronic medical and health data in real time,which is the key to understand and evaluate safety problems faster[5].The operation mode of sentinel system is shown in Fig.2.

Fig.2 FDA Sentinel system operation mode
1.2 Voluntary reporting system - MedWatch
Origin: In 1993,FDA proposed a voluntary reporting system -MedWatch,which aimed to improve FDA’s ability to supervise drug safety and encourage medical personnel to timely and actively submit ADR reports and drug safety issues[6].It streamlines the reporting process of medical institutions to shorten the time.It also sets up an ADR handling mechanism to evaluate ADRs.At the same time,it takes reporting ADRs as one of the responsibilities of medical personnel,so that FDA can take timely and effective measures for drug safety events.The operation mode and contents of MedWatch are shown in Table 1[7].
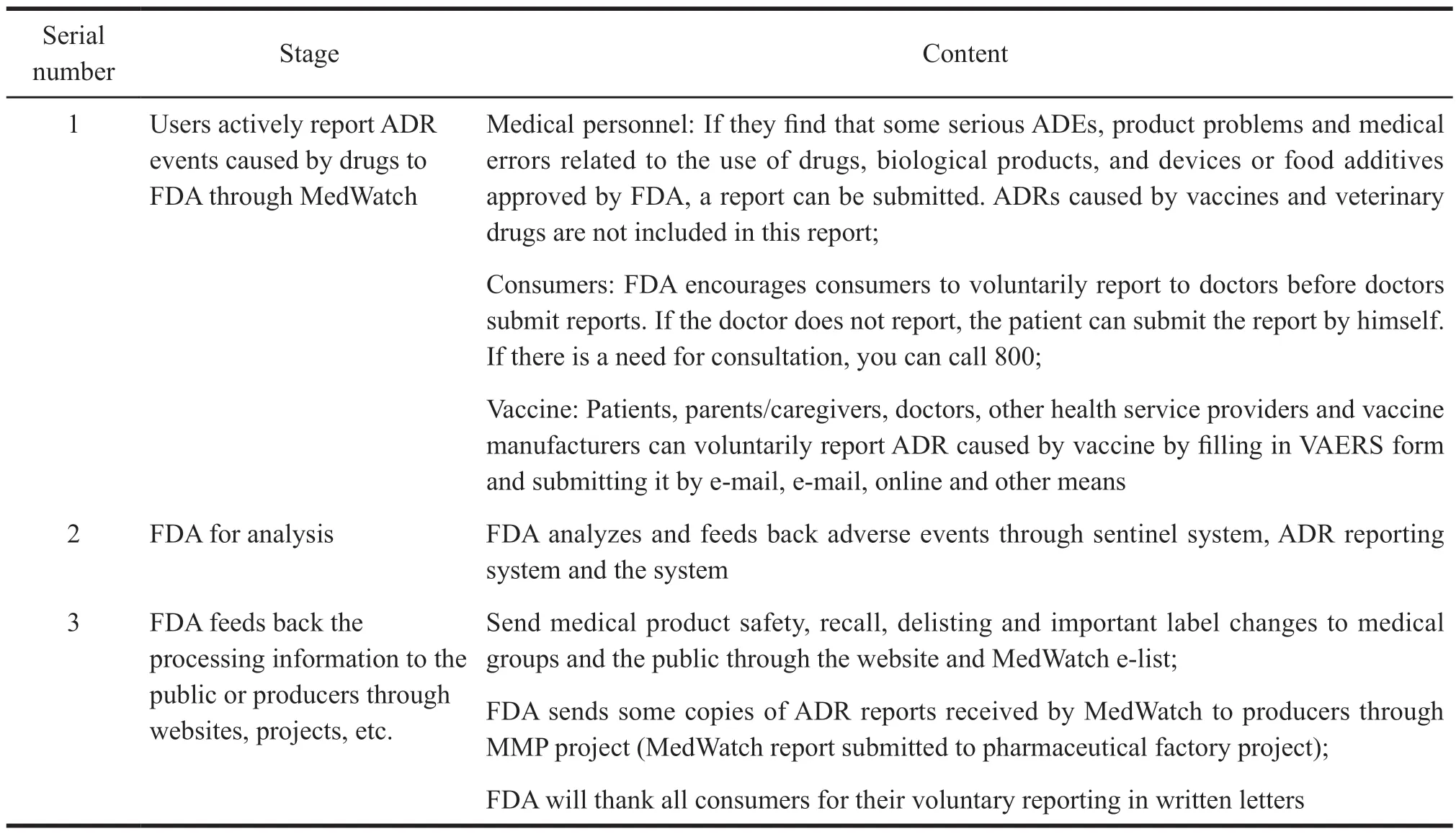
Table 1 MedWatch operation mode and content
Function: Corresponding to the sentinel system,MedWatch is a passive monitoring system.The public can actively report ADRs caused by drugs to FDA and receive online ADR events and reporting system.MedWatch provides the latest warnings and recalls of drugs and medical devices[8].
2 China’s scientific platform for ADR supervision
2.1 Development process of scientific platform for ADR supervision in China
Since China is in the primary stage of new infrastructure construction,the development of data platform is still slow.Therefore,it does not have the characteristics of large data set.Compared with the relatively perfect big data platform of regulatory science in the United States,there is still a large gap,especially in terms of database content,implementation standards,availability,funds,talents,and achievements.
In 1985,China’s first “Drug Administration Law” was promulgated and implemented.Since then,ADR monitoring has been carried out for more than 30 years,and China’s ADR reporting system has become more mature.In 1989,an ADR testing center was established in China.The online reporting system of national ADR monitoring network was officially put into operation in December 2004.In 2019,China’s newly revised “Drug Administration Law” and “Vaccine Administration Law” were promulgated and implemented officially.China’s government planned to promote the full coverage of drug supervision through a series of innovative systems such as the listing Licensor system,pharmacovigilance system,drug traceability system and drug sales filing system for online third-party platform.
Drug abuse monitoring posts were established in 2020.By December 2020,there were 100 drug abuse monitoring posts in China,5 in Sichuan,Hubei and Jiangsu respectively,and 1 to 4 in other provinces,reflecting the different drug abuse situation and work strategy in different provinces (Table 2).

Table 2 Annual report of ADRs in China from 2014 to 2020
It can be seen from Table 2 that the number of ADR reports in China is increasing,and the proportion of serious ADRs is on the rise too.At the same time,the report per million population in 2020 made great progress compared with the reports of 991 in 2014.In the statistics of report sources,medical institutions have always been the main force of ADR reports,but the proportion of reports from drug manufacturers and holders is low.
The enthusiasm of responsible persons of all drug parties to report ADRs has increased year by year.So,the number of ADR reports has been rising.The increase in the number of reports does not mean the decline of drug safety level,it shows that China’s drug regulatory authorities have continuously improved their collection of drug response information.Therefore,their ability to control risks has been increased,and they can make more reliable drug evaluation and more accurate decision-making.
2.2 Comparison of scientific platform for ADR supervision between the United States and China
At present,China mainly adopts passive monitoring,which is the safety monitoring system of spontaneous reporting ADRs.Medical institutions at all levels are more active in reporting.Therefore,the main data of ADR reports are made by medical institutions.At the same time,it can be found that the reporting willingness of drug marketing licensors or manufacturers is low,and the probability of missing reports of ADR is relatively high.The specific comparison contents are shown in the Table 3.

Table 3 Comparison of ADR monitoring systems between the United States and China
Through comparison,we can see that there are many differences in the monitoring systems between China and the United States.The United States designs reports for different subjects and clearly defines the scope of the report,laying the foundation for a wide range of drug safety information.The crossreporting system promotes the flow of information and the improvement of information in the database.In comparison,China only reports to the Food and Drug Administration step by step at present,and there are obstacles in the communication of information.
Since China’s ADR monitoring starts late,the monitoring system is not perfect,and the reporting quality is uneven.For example,there are many problems in different regions such as missing reports or narrow reporting scope.Medical institutions have always been the main force of ADR reports,and the proportion of ADR reports from drug manufacturers and holders is low.
3 Optimization strategy
Big data includes real-world data,such as electronic health records,insurance claim data,the collection of clinical trial data,data sets from spontaneous reports of suspected ADRs,genomics,metabolomics,and proteomics data sets.Big data can supplement some data missing from clinical trials and provide important opportunities to improve the evidence for us to make drug decisions.
3.1 Improving relevant laws and regulations and playing the role of a third-party platform
In recent years,the regulatory science of drugs in China has developed rapidly.The academic circles and scholars have paid more attention to the construction of big data platform of drug regulatory science.All kinds of big data platforms of drug regulatory science also help the progress and development of drug supervision.However,the information sharing of various regulatory platforms is not sufficient.Because there is no one-stop comprehensive regulatory platform,it is impossible to fully integrate the scientific information resources of drug supervision.Combining with the specific requirements of the construction of big data platform for drug regulatory science in the post epidemic era,we can use the comprehensive resources of third-party data platform to create a one-stop regulatory service platform.
3.2 Cultivating digital talents and building a sustainable construction model
The development of drug regulatory science is inseparable from the support of excellent talents.In the process of setting up big data platform for drug regulatory science,we need to actively cultivate talents and build a sustainable development model.Combining with the requirements of platform construction,we can actively cultivate excellent talents from the following aspects.
In terms of the colleges and universities,professional courses on the construction of big data platform of drug regulatory science should be added to relevant majors.In the post epidemic era,drug regulatory science is an important support for the development of China’s pharmaceutical industry.Increasing the professional knowledge in colleges and universities enables talents to understand the specific direction of the construction and development of big data platform of drug regulatory science.We can also conduct targeted training of talents through schoolenterprise cooperation.By strengthening the training of regulatory methods,knowledge and tools,we can turn them into practical guidance.
In terms of the government,the successful experience of the United States shows that the adjustment and optimization of talent access system and the government’s comprehensive strengthening of talent training will significantly help the development of drug regulatory science.In order to fully cultivate talents,the Chinese government should set up a professional qualification certificate system,which can improve the professional quality of talents in the drug regulatory science.Then,it can provide help and support for the establishment and development of big data platform for drug regulatory science.
3.3 Building a modern drug risk control system around the whole life cycle of drugs
The government should make full use of emerging information technologies such as big data and artificial intelligence to establish a drug information collection and tracking platform based on the concept and method of drug life cycle risk management.It takes long time to establish big data platform for drug regulatory science.The establishment of the big data platform for drug supervision can not only guide the development of China’s medical and health undertakings,but also help the integration of drug inspection,ADR notification and warning information for medical workers and drug holders.In this process,it is crucial to comprehensively gather social information and create a modern information feedback model.
3.4 Making good standards and giving play to the guiding function of supervising big data
According to FDA’s successful experience,during the construction of big data platform for China’s drug regulatory science,various clinical experiment databases should be integrated to form the support for the development of drug regulatory science through the full integration of different system resources,technologies,and information.By enriching the platform database,we can use the data information to improve the fairness of the development of drug supervision and offer support for drug regulatory science.Besides,we should specify international standards based on scientific data.
China should build a comprehensive data base.In addition to the scientific data and information of domestic drug supervision,China should also strengthen cooperation with FDA and other national drug safety supervision and administration laboratories and government agencies.Through the integration of data resources and various data bases,we can jointly carry out the construction and development of drug regulatory science.China’s initiative of one belt,one road is developing rapidly.The drug data platform should also form a good cooperation mechanism with the drug regulatory science institutions along the route of one belt.It will strengthen the exchange of drug regulatory science through the integration of resource sharing and data integration.It can not only help to further enrich the clinical experiment information,but also help the fast development of drug regulatory science.
杂志排行
亚洲社会药学杂志的其它文章
- Supervision of Public Opinion under the Background of Social Co-governance-Take the Changsheng Vaccine Incident as an Example
- Suggestions for Promoting China’s Drug Regulatory Agency to Join Pharmaceutical Inspection Co-operation Scheme -PIC/S
- An Empirical Study on Organizational Efficiency of Drug Review lnstitutions
- The Impact of Population Aging on the Expenditure of Medical Insurance Fund for Urban Workers in China
- Exploration and Research on the Integrated Development of “Internet Plus Medical Treatment”
- A Systematic Review of Patient-Reported Outcome Measurement for Psoriasis in Chinese Population
